Abstract
Background and objectives
Rapid ultrafiltration rates are associated with adverse outcomes among patients on hemodialysis. The Centers for Medicare and Medicaid Services is considering an ultrafiltration rate quality measure for the ESRD Quality Incentive Program. Two measure developers proposed ultrafiltration rate measures with different selection criteria and specifications. We aimed to compare the proposed ultrafiltration rate measures and quantify dialysis facility operational burden if treatment times were extended to lower ultrafiltration rates.
Design, setting, participants, & measurements
Data were taken from the 2012 database of a large dialysis organization. Analyses of the Centers for Medicare and Medicaid Services measure considered 148,950 patients on hemodialysis, and analyses of the Kidney Care Quality Alliance measure considered 151,937 patients. We described monthly patient and facility ultrafiltration rates and examined differences in patient characteristics across ultrafiltration rate thresholds and differences in facilities across ultrafiltration rate measure scores. We computed the additional treatment time required to lower ultrafiltration rates <13 ml/h per kilogram.
Results
Ultrafiltration rates peaked in winter and nadired in summer. Patients with higher ultrafiltration rates were younger; more likely to be women, nonblack, Hispanic, and lighter in weight; and more likely to have histories of heart failure compared with patients with lower ultrafiltration rates. Facilities had, on average, 20.8%±10.3% (July) to 22.8%±10.6% (February) of patients with ultrafiltration rates >13 ml/h per kilogram by the Centers for Medicare and Medicaid Services monthly measure. Facilities had, on average, 15.8%±8.2% of patients with ultrafiltration rates ≥13 ml/h per kilogram by the Kidney Care Quality Alliance annual measure. Larger facilities (>100 patients) would require, on average, 33 additional treatment hours per week to lower all facility ultrafiltration rates <13 ml/h per kilogram when total treatment time is capped at 4 hours.
Conclusions
Ultrafiltration rates vary seasonally and across clinical subgroups. Extension of treatment time as a strategy to lower ultrafiltration rates may pose facility operational challenges. Prospective studies of ultrafiltration rate threshold implementation are needed.
Keywords: hemodialysis; ultrafiltration; Epidemiology and outcomes; heart failure; Humans; Kidney Failure, Chronic; Medicaid; Medicare; Prospective Studies; renal dialysis
Introduction
Growing evidence supports fluid management as a critical contributor to outcomes for patients on hemodialysis (HD). Suboptimal fluid management leads to increased hospitalizations, patient discomfort, and substantial cost (1–3). However, evidence-based guidelines are currently lacking. Accurate target weight estimation and interdialytic weight gain (IDWG) reduction have proven elusive (3). Fluid removal during dialysis (ultrafiltration [UF]) is a potentially modifiable, facility–controlled aspect of fluid management. Some experts have advocated for implementation of UF rate ceiling thresholds to reduce complications from fluid removal practices (4).
The US Centers for Medicare and Medicaid Services (CMS) is considering a UF rate measure for inclusion in the 2019 ESRD Quality Incentive Program (QIP). Before the QIP incorporation, proposed measures are vetted by technical expert panels and considered for endorsement by the National Quality Forum (NQF) (5). In May of 2015, the NQF considered two UF rate measures proposed by CMS (measure 2700) and the Kidney Care Quality Alliance (KCQA; measure 2701) (6). The NQF endorsed the KCQA measure, but CMS has not finalized the measure (7). Both UF rate measures assess facility fluid management by calculating the proportion of patients with delivered UF rates above specified thresholds (>13 ml/h per kilogram for the CMS measure and ≥13 ml/h per kilogram for the KCQA measure). Although similar in construction and interpretation, the measures differ in patient selection criteria, time windows, and number of contributing HD treatments. Neither measure has provisions for patient subpopulations with plausibly different UF rate risk thresholds.
To lower UF rates, dialysis facilities can extend treatment time (TT) or decrease UF volumes. Both strategies have important patient and facility implications, and both will likely be necessary to adequately reduce UF rates. The least operationally disruptive strategy is intensive dietary counseling to minimize weight gains and thus, obligate UF volumes. However, dietary counseling is standard in most nutritional programs, and high weight gains remain common (8). TT extension will likely be necessary to lower UF rates for many patients. Using 2012 data from a single large dialysis organization (LDO), we sought to (1) describe UF rate distributions and patterns, (2) compare facilities with higher (versus lower) percentages of patients with elevated UF rates, and (3) investigate the amount of additional facility TT required to meet specified UF rate thresholds.
Materials and Methods
UF Rate Measures
We evaluated the CMS and the KCQA proposed UF rate measures submitted to the NQF May 2015 multistakeholder Standing Committee meeting (6). The CMS-proposed measure was developed in consultation with nephrologists and measure development experts at the University of Michigan under the ESRD Quality Measure Development, Maintenance, and Support contract. The KCQA-proposed measure was developed by the KCQA, a dialysis measure development body supported by Kidney Care Partners, a multistakeholder coalition that aims to improve care through legislative, regulatory, and quality initiatives (9).
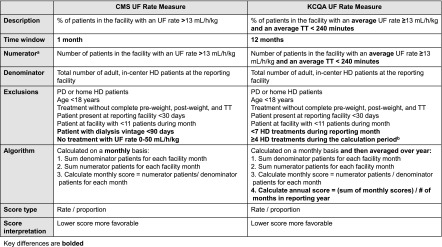
We analyzed UF rates according to the CMS and the KCQA measure specifications at the patient and facility levels using 2012 data from a single LDO (Figure 1, Supplemental Table 1) (6). The UF rate measures reflect the percentage of patients at a dialysis facility with elevated UF rates (>13 ml/h per kilogram across all delivered TTs for the CMS measure and ≥13 ml/h per kilogram across delivered TTs <240 minutes for the KCQA measure). The measures are reported as proportions (percentages of facility patients with UF rates above specified thresholds). Lower scores are more favorable. We also evaluated a modified KCQA measure, in which we omitted the TT<240 minutes requirement. In all analyses, delivered UF rate (milliliters per hour per kilogram) was calculated by (predialysis weight − postdialysis weight [milliliters])/delivered TT (hours)/postdialysis weight (kilograms).
Figure 1.
The Centers for Medicare and Medicaid Services (CMS) and the Kidney Care Quality Alliance (KCQA) ultrafiltration (UF) rate measure specifications and selection criteria. aFor both measures, the UF rate is calculated as UF rate (milliliters per hour per kilogram) = (predialysis weight − postdialysis weight [milliliters])/delivered TT (hours)/postdialysis weight (kilograms). The CMS UF rate measure numerator details are as follows. For the hemodialysis (HD) treatment that meets selection criteria, (1) calculate the UF rate (milliliters per hour per kilogram), and (2) sum the number of patients in each facility with a UF rate >13 ml/h per kilogram. The KCQA UF rate measure numerator details are as follows. For the HD treatments that meet selection criteria, (1) calculate the UF rate (milliliters per hour per kilogram), (2) calculate each patient’s average UF rate for all HD treatments during the week that the monthly Kt/V is drawn, (3) calculate each patient’s average treatment time (TT) for all HD treatments during the week that monthly Kt/V is drawn, and (4) sum the number of patients in each facility with an average UF rate ≥13 ml/h per kilogram and an average TT<240 minutes. bThe National Quality Forum reported an exclusion of more than four HD treatments during the calculation period in their documentation of the KCQA measure (6); however, the KCQA measure developers tested an exclusion of four or more HD treatments during the calculation period (21). Because an exclusion of four or more HD treatments represents developer intent and is congruent with the standard practice of three weekly treatments, we evaluated the measure using an exclusion of four or more HD treatments. PD, peritoneal dialysis.
In primary analyses, monthly CMS UF rate measures were calculated by dividing the number of facility patients with UF rates >13 ml/h per kilogram by the total number of facility patients on HD on a monthly basis. The UF rate was calculated from the pre- and postdialysis weights and delivered TT from the HD treatment corresponding to the day of the monthly clearance assessment. The KCQA measure was calculated by dividing the total number of facility patients with an average UF rate ≥13 ml/h per kilogram and an average delivered TT <240 minutes by the total number of facility patients on HD on a monthly basis. The KCQA monthly UF rate was computed as a mean of UF rates from the treatments during the monthly clearance assessment week. Facility monthly UF rate measure values were averaged to create an annual measure per the KCQA specifications. In secondary analyses, we considered alternative UF rate calculations by varying data collection days and the number of treatments contributing to UF rate calculations.
Monthly (CMS) and annual (KCQA) facility UF rate measure scores were dichotomized at the 75th percentile to mirror the CMS facility performance evaluation. Facilities in the highest score quartile had the largest proportion of patients with UF rates above the specified threshold compared with lower quartile facilities.
Study Design and Selection Criteria
This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board. Data were taken from 196,635 patients from 2449 facilities with available data in a single LDO’s 2012 database. Cohort entry was dynamic and could occur between January 1 and November 30, 2012. Patients entered and exited the cohort and contributed to different facility monthly cohorts according to selection criteria application on a rolling monthly basis. This design mirrors real world measure implementation and facilitates calculation of monthly proportions of facility patients with UF rates above proposed thresholds.
Patient eligibility criteria were assessed monthly (Figure 1, Supplemental Table 1). Inclusion criteria for both measures were patients on HD ages ≥18 years old and present at the reporting facility for ≥30 days. Exclusion criteria for both measures were patients on peritoneal dialysis or home HD, patients dialyzing at units with <11 patients, and patients with incomplete UF rate data. For the CMS measure, we excluded patients on dialysis for <90 days and patients with reported UF rates <0 or >50 ml/h per kilogram. For the KCQA measure, we excluded patients with less than seven treatments at the facility in the reporting month and patients with four or more treatments during the weekly calculation period.
Data Collection
Study data were obtained from the LDO’s medical record. Demographic data were documented at admission to one of the organization’s facilities. Comorbid conditions were assessed by the nephrologist at facility admission and updated on the basis of clinical course. Dialysis adequacy (Kt/V) was measured at least monthly. When more than one Kt/V measurement was available, the last monthly value was selected. Treatment variables, including pre- and postdialysis weights, delivered TTs, and vascular access type, were recorded every treatment. BP was measured pre- and postdialysis and at least every 30 minutes during HD. Intradialytic hypotension was defined as nadir intradialytic systolic BP <90 mmHg in >30% of treatments in the prior month. Low pre–HD systolic BP was defined as pre–HD systolic BP <100 mmHg in >30% of treatments in the prior month. Facility geographic region was assigned using US Census designations.
Statistical Analyses
Analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). Patient and facility characteristics were described as numbers (proportions) for categorical variables and means±SDs or medians (quartile 1, quartile 3) for continuous variables. UF rate thresholds were summarized across the full cohort and across key subgroups. Facility characteristics were stratified by region (western versus nonwestern region). Differences in the percentage of patients at a dialysis facility with elevated UF rates across the CMS and the KCQA criteria were compared using paired t tests. Two–tailed P values <0.05 indicated statistical significance.
To show facility burden resulting from implementation of the proposed KCQA UF rate threshold, we computed the additional TT required to lower prescribed UF rates to <13 ml/h per kilogram. Total TT was capped at 4 hours per the KCQA specifications. For these analyses, prescribed UF rate (milliliters per kilogram) was calculated using the formula (predialysis weight from current treatment − postdialysis weight from previous treatment [milliliters])/prescribed TT (hours)/postdialysis weight from previous treatment (kilograms). Supplemental Table 2 shows detailed methods.
Results
Cohort Characteristics
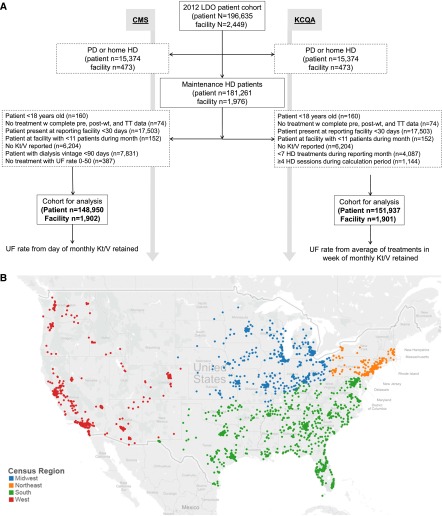
Figure 2 displays patient and facility selection (Figure 2A) and facility locations (Figure 2B). Analyses of the CMS measure considered 148,950 unique patients from 1902 facilities, and analyses of the KCQA measure considered 151,937 unique patients from 1901 facilities. In the CMS cohort, the mean age was 62 years old, 36.7% were black, 25.9% had heart failure, and 25.6% dialyzed at facilities in the western United States as of the first reporting month. In the KCQA cohort, the mean age was 62 years old, 36.4% were black, 24.8% had heart failure, and 26.2% dialyzed at facilities in the western United States as of the first reporting month. The cohorts were similar to the broader United States HD population in terms of age, sex, race, and comorbidities (10).
Figure 2.
The flow diagram shows selection of unique patient Centers for Medicare and Medicaid (CMS) and the Kidney Care Quaility Alliance (KCQA) cohorts by application of selection criteria on a rolling monthly basis, and the map illustrates the diverse locations of dialysis facilities included in the analytic cohorts classified by census region. (A) Flow diagram of patient and facility selection on the basis of the CMS and KCQA ultrafiltration (UF) rate measure specifications and (B) census regions of facilities included in the analytical cohort. HD, hemodialysis; LDO, large dialysis organization; PD, peritoneal dialysis; TT, treatment time; wt, weight.
UF Rate Operational Definitions
Patient–level mean UF rates peaked in winter months and nadired in summer months, ranging from 9.5±5.3 ml/h per kilogram (July) to 9.9±5.5 ml/h per kilogram (February) by the CMS criteria and from 9.5±5.4 ml/h per kilogram (July) to 10.0±5.9 ml/h per kilogram (February) by the KCQA criteria (Table 1, Supplemental Figure 1). Facilities had, on average, 20.8%±10.3% (July) to 22.8%±10.6% (February) of patients with UF rates >13 ml/h per kilogram by the CMS monthly measure. Facilities had, on average, 15.8%±8.2% of patients with UF rates ≥13 ml/h per kilogram by the KCQA annual measure. The proportions of facility patients with monthly UF rates above measure thresholds differed significantly comparing the CMS and the KCQA criteria (monthly P values <0.001) (Table 1).
Table 1.
Patient– and facility–level ultrafiltration rates as calculated by the Centers for Medicare and Medicaid Services and the Kidney Care Quality Alliance measure criteria on a monthly basis
| Calculation Period | CMS Measure | KCQA Measure | KCQA Measure without TT Restriction | |||
|---|---|---|---|---|---|---|
| Patient Level; UF Rate, ml/h per kg; mean±SD; median [Q1, Q3] | Facility Level; Percentage of Facility Patients with UF Rates >13 ml/h per kg, mean±SD | Patient Level; UF Rate, ml/h per kg; mean±SD; median [Q1, Q3] | Facility Level; Percentage of Facility Patients with UF Rates ≥13 ml/h per kg and TT<240 min, mean±SD | Patient Level; UF Rate, ml/h per kg; mean±SD; median [Q1, Q3] | Facility Level; Percentage of Facility Patients with UF Rates ≥13 ml/h per kg, mean±SD | |
| January | 9.8±5.5; 9.2 [6.1, 12.6] | 22.3±10.8 | 9.9±5.9; 9.4 [6.6, 12.6] | 17.4±9.7 | 9.6±5.5; 9.0 [6.3, 12.1] | 19.4±10.1 |
| February | 9.9±5.5; 9.2 [6.2, 12.7] | 22.8±10.6 | 10.0±5.8; 9.4 [6.6, 12.6] | 17.3±9.5 | 9.6±5.6; 9.0 [6.4, 12.1] | 19.5±9.7 |
| March | 9.7±5.3; 9.2 [6.1, 12.5] | 22.1±10.7 | 9.8±5.6; 9.3 [6.5, 12.4] | 16.3±9.3 | 9.4±5.3; 8.9 [6.3, 11.9] | 18.2±9.5 |
| April | 9.7±5.3; 9.1 [6.1, 12.6] | 22.0±10.7 | 9.8±5.4; 9.3 [6.5, 12.4] | 16.1±9.4 | 9.4±5.1; 8.9 [6.3, 11.9] | 18.1±9.6 |
| May | 9.7±5.2; 9.1 [6.1, 12.5] | 21.6±10.6 | 9.7±5.4; 9.2 [6.5, 12.3] | 15.8±9.2 | 9.3±5.2; 8.8 [6.2, 11.8] | 17.7±9.4 |
| June | 9.6±5.3; 9.1 [6.1, 12.4] | 21.4±10.6 | 9.6±5.4; 9.1 [6.4, 12.2] | 15.5±9.0 | 9.2±5.1; 8.7 [6.1, 11.7] | 17.3±9.2 |
| July | 9.5±5.3; 8.9 [5.9, 12.3] | 20.8±10.3 | 9.5±5.4; 9.0 [6.2, 12.1] | 14.7±8.7 | 9.1±5.2; 8.6 [6.0, 11.6] | 16.5±8.9 |
| August | 9.5±5.2; 9.0 [5.9, 12.3] | 21.0±10.1 | 9.6±5.4; 9.0 [6.3, 12.1] | 15.2±8.8 | 9.2±5.2; 8.7 [6.1, 11.6] | 17.1±9.0 |
| September | 9.5±5.2; 9.0 [6.0, 12.3] | 21.1±10.0 | 9.6±5.4; 9.1 [6.4, 12.2] | 15.5±9.1 | 9.3±5.1; 8.8 [6.2, 11.7] | 17.5±9.3 |
| October | 9.7±5.3; 9.1 [6.1, 12.6] | 22.1±10.6 | 9.7±5.3; 9.2 [6.5, 12.3] | 15.7±9.0 | 9.4±5.1; 8.8 [6.3, 11.8] | 17.7±9.2 |
| November | 9.8±5.3; 9.2 [6.2, 12.6] | 22.3±10.9 | 9.8±5.5; 9.3 [6.6, 12.4] | 16.2±9.6 | 9.4±5.2; 8.9 [6.3, 11.9] | 18.1±9.7 |
| December | 9.7±5.2; 9.1 [6.1, 12.6] | 21.9±10.6 | 9.7±5.4; 9.2 [6.5, 12.3] | 15.6±9.1 | 9.3±5.2; 8.8 [6.2, 11.8] | 17.5±9.4 |
| Annual | — | — | 9.5±4.3; 9.1 [6.8, 11.7] | 15.8±8.2 | 9.2±4.1; 8.8 [6.6, 11.4] | 17.7±8.2 |
Comparisons of percentage of facility patients with UF rates >13 ml/h per kilogram by the CMS criteria versus percentage of facility patients with UF rates ≥13 ml/h per kilogram by the KCQA criteria were made using paired t tests. P values for all comparisons were <0.001. CMS, Centers for Medicare and Medicaid Services; KCQA, Kidney Care Quality Alliance; TT, treatment time; UF, ultrafiltration; Q1, quartile 1, Q3, quartile 3; —, no annual calculation per CMS measure criteria.
Because the CMS measure relies on a single UF rate value, we performed secondary analyses varying the UF rate collection day. UF rates calculated from the last nonmissing monthly UF rate value had the greatest variation, ranging from 8.7±5.2 ml/h per kilogram (June) to 10.3±5.6 ml/h per kilogram (January). Months ending in Mondays or Tuesdays (treatment days after the long interdialytic break) had the highest UF rates. Months ending in Fridays and Saturdays (treatment days corresponding to the third weekly HD treatment) had the lowest UF rates. Supplemental Table 3 displays differences across UF rate operational definitions.
Patient and Facility Characteristics across UF Rate Groups and Thresholds
Patients with UF rates >13 ml/h per kilogram in the first reporting month were younger; more likely to be women, nonblack, and Hispanic; and more likely to have histories of heart failure, lower body weight, greater dialysis vintage, higher predialysis BP, greater IDWG, and shorter TT compared with patients with lower UF rates. Findings were consistent across measures and months (Table 2). Facilities falling in the highest UF measure quartile (facilities with >28.3% of patients with UF rates >13 ml/h per kilogram by the CMS criteria and facilities with >20.3% of patients with UF rates ≥13 ml/h per kilogram by the KCQA criteria) were more likely to be located in the western United States, have <25 patients, have fewer black patients, and have more Hispanic patients compared with facilities in lower quartiles (Table 3).
Table 2.
Patient characteristics by ultrafiltration rate group as of the first reporting month in 2012
| Characteristic | Patients included in the CMS Measure Denominator, n=148,950 | Patients included in the KCQA Measure Denominator, n=151,937 | ||
|---|---|---|---|---|
| ≤13 ml/h per kg, n=117,231 | >13 ml/h per kg, n=31,719 | <13 ml/h per kg, n=124,639 | ≥13 ml/h per kg, n=27,298 | |
| UF rate, ml/h per kg | ||||
| Mean±SD | 7.3±3.3 | 17.3±4.8 | 7.4±3.5 | 17.4±6.3 |
| Median [Q1, Q3] | 7.6 [5.0, 10.0] | 15.9 [14.2, 18.7] | 7.6 [5.3, 9.9] | 15.6 [14.1, 18.3] |
| Age, yr | 63±15 | 60±16 | 63±15 | 58.8±16.2 |
| Women | 51,935 (44.3) | 15,138 (47.7) | 55,232 (44.3) | 13,095 (48.0) |
| Black race | 44,273 (37.8) | 10,385 (32.8) | 46,398 (37.3) | 8907 (32.6) |
| Hispanic ethnicity | 18,993 (16.2) | 6793 (21.4) | 20,262 (16.3) | 6007 (22.0) |
| Median income, dollars | 47,716 [37,275, 61,992] | 47,788 [37,324, 61,629] | 47,716 [37,280, 61,985] | 47,895 [37,227, 61,629] |
| Time on dialysis, yr | ||||
| <1 | 37,214 (31.7) | 7427 (23.4) | 44,249 (35.5) | 6337 (23.2) |
| 1–5 | 53,192 (45.4) | 14,588 (46.0) | 53,326 (42.8) | 12,447 (45.6) |
| >5 | 26,825 (22.9) | 9704 (30.6) | 27,063 (21.7) | 8513 (31.2) |
| Access type | ||||
| Graft | 21,744 (18.6) | 6412 (20.2) | 21,421 (17.2) | 5536 (20.3) |
| Fistula | 66,671 (56.9) | 19,175 (60.5) | 66,101 (53.2) | 16,047 (58.9) |
| Catheter | 28,678 (24.5) | 6100 (19.3) | 36,827 (29.6) | 5672 (20.8) |
| Average pre–HD SBP, mmHg | 148±27 | 152±27 | 148±23 | 152±24 |
| Pre-HD SBP, mmHg | ||||
| <100 | 3086 (2.6) | 631 (2.0) | 1751 (1.4) | 294 (1.1) |
| 100–129 | 26,042 (22.3) | 5827 (18.4) | 24,302 (19.9) | 4405 (16.4) |
| 130–159 | 49,376 (42.2) | 12,971 (41.0) | 58,698 (48.0) | 12,310 (45.8) |
| ≥160 | 38,487 (32.9) | 12,209 (38.6) | 37,657 (30.8) | 9845 (36.7) |
| History of heart failure | 28,971 (24.7) | 9535 (30.1) | 29,129 (23.4) | 8482 (31.1) |
| History of diabetes | 50,224 (42.8) | 13,883 (43.8) | 50,796 (40.8) | 12,008 (44.0) |
| Post-HD weight, kg | 83.5±23.3 | 68.5±16.2 | 83.1±23.1 | 67.6±15.9 |
| BMI, kg/m2 | 28.8±7.6 | 24.3±5.2 | 28.7±7.5 | 24.0±5.1 |
| Prescribed TT <240 min | 79,249 (67.6) | 25,964 (81.9) | 84,099 (67.5) | 22,593 (82.8) |
| Delivered TT <240 min | 82,405 (70.3) | 26,977 (85.0) | 92,785 (74.4) | 24,325 (89.1) |
| IDWG, kg | 2.3±1.9 | 3.7±1.9 | 2.2±1.4 | 3.6±1.5 |
| UF volume, L | 2.2±1.2 | 3.9±1.4 | 2.2±1.2 | 3.8±1.5 |
| eKt/V | 1.6±0.3 | 1.7±0.3 | 1.6±0.3 | 1.7±0.3 |
Values are presented as means±SDs, medians [Q1, Q3], or numbers (percentages). Because patients entered and exited the cohort according to selection criteria application on a rolling monthly basis, the first reporting month varied across patients. The KCQA measure and the KCQA measure without TT restriction denominators reflect the same patient population. CMS, Centers for Medicare and Medicaid Services; KCQA, Kidney Care Quality Alliance; UF, ultrafiltration; Q1, quartile 1; Q3, quartile 3; HD, hemodialysis; SBP, systolic BP; BMI, body mass index; TT, treatment time; IDWG, interdialytic weight gain; eKt/V, equilibriated Kt/V.
Table 3.
Facility characteristics as of January of 2012 by facility ultrafiltration rate measure score
| Characteristic | CMS Measurea | KCQA Measureb | KCQA Measure without TT Restrictionc | |||
|---|---|---|---|---|---|---|
| ≤75th Measure Percentile | >75th Measure Percentile | ≤75th Measure Percentile | >75th Measure Percentile | ≤75th Measure Percentile | >75th Measure Percentile | |
| Geographic region | ||||||
| Northeast | 154 (11.7) | 21 (4.8) | 145 (12.5) | 30 (5.1) | 142 (12.4) | 33 (5.5) |
| Midwest | 301 (22.9) | 111 (25.5) | 267 (23.1) | 145 (24.4) | 265 (23.1) | 147 (24.4) |
| South | 616 (46.9) | 188 (43.1) | 537 (46.5) | 265 (44.6) | 529 (46.1) | 273 (45.3) |
| West | 243 (18.5) | 116 (26.6) | 205 (17.7) | 154 (25.9) | 209 (18.2) | 150 (24.9) |
| Average monthly unit size, no. of patients | 71±39 | 70±44 | 70±39 | 72±43 | 71±39 | 71±42 |
| Monthly unit size, no. of patients | ||||||
| ≤25 | 126 (9.6) | 56 (12.8) | 117 (10.1) | 65 (10.9) | 115 (10.0) | 67 (11.1) |
| 26–50 | 328 (25.0) | 113 (25.9) | 303 (26.2) | 138 (23.2) | 294 (25.6) | 147 (24.4) |
| 51–100 | 599 (45.6) | 179 (41.1) | 510 (44.1) | 268 (45.1) | 508 (44.3) | 270 (44.8) |
| >100 | 261 (19.9) | 88 (20.2) | 226 (19.6) | 123 (20.7) | 230 (20.1) | 119 (19.7) |
| Zip code income, dollarsd | 52,061±20,419 | 51,657±19,006 | 52,011±20,419 | 51,820±19,386 | 52,109±20,364 | 51,635±19,508 |
| Age <50 yr | 19.0±7.7 | 19.5±7.3 | 18.6±8.2 | 19.0±7.5 | 18.5±8.2 | 19.3±7.5 |
| Black race | 37.4±30.8 | 28.8±27.3 | 37.9±31.6 | 31.8±28.9 | 37.3±31.6 | 33.1±29.2 |
| Black race >50% of facility patients | 444 (33.8) | 94 (21.6) | 408 (35.3) | 154 (25.9) | 395 (34.4) | 167 (27.7) |
| Hispanic ethnicity | 12.7±19.4 | 16.7±22.5 | 12.3±19.3 | 17.2±23.2 | 12.4±19.6 | 17.0±22.7 |
| Hispanic ethnicity >25% of facility patients | 222 (16.9) | 108 (24.8) | 186 (16.1) | 151 (25.4) | 182 (15.9) | 155 (25.7) |
| Heart failure balance >25% of facility patients | 761 (57.9) | 251 (57.6) | 662 (57.3) | 367 (61.8) | 660 (57.5) | 369 (61.2) |
| Prescribed TT <240 min | 69.3±19.1 | 78.9±15.3 | 66.8±19.5 | 81.5±11.9 | 68.4±19.5 | 78.2±14.9 |
| Prescribed TT <240 min for >75% of facility patients | 598 (45.5) | 297 (68.1) | 465 (40.2) | 438 (73.7) | 508 (44.3) | 395 (65.5) |
| Delivered TT <240 min | 72.1±17.1 | 81.0±14.1 | 73.8±16.3 | 86.3±9.4 | 75.2±16.2 | 83.5±12.3 |
| Average body mass index, kg/m2 | 28.2±1.5 | 27.6±1.5 | 28.1±1.5 | 27.5±1.6 | 28.2±1.6 | 27.5±1.5 |
| Body mass index, kg/m2 | ||||||
| <18.5 | 4.6±3.2 | 5.3±4.0 | 4.6±3.7 | 5.2±3.9 | 4.6±3.7 | 5.2±3.9 |
| 18.5–24.9 | 33.6±7.7 | 36.6±8.3 | 33.5±8.9 | 37.2±8.5 | 33.5±8.9 | 37.1±8.5 |
| 25.0–29.9 | 28.1±6.5 | 27.5±7.2 | 28.6±7.6 | 27.7±7.3 | 28.5±7.5 | 27.9±7.6 |
| ≥30 | 33.6±8.7 | 30.5±8.9 | 33.3±9.2 | 29.9±9.2 | 33.4±9.3 | 29.8±9.0 |
Values are presented as numbers (percentages) of facilities or means±SDs of the percentage of facility patients. Using prescribed TT as an example, a mean of 69.3% of patients at facilities in the ≤75th CMS measure percentile had a prescribed TT <240 minutes compared with a mean of 78.9% of patients at facilities in the >75th CMS measure percentile. CMS, Centers for Medicare and Medicaid Services; KCQA, Kidney Care Quality Alliance; TT, treatment time.
For the CMS measure, the 75th percentile represents 28.3% of facility patients with ultrafiltration rates >13 ml/h per kilogram. Measure 75th percentile and facility data reflect the January of 2012 CMS cohort data.
For the KCQA measure, the 75th percentile represents 20.3% of facility patients with ultrafiltration rates ≥13 ml/h per kilogram and treatment times <240 minutes. Measure 75th percentile reflects the 75th percentile of the KCQA annual measure score, and facility data reflect the January of 2012 KCQA cohort data.
For the KCQA measure without TT restriction, the 75th percentile represents 22.4% of facility patients with ultrafiltration rates ≥13 ml/h per kilogram. Measure 75th percentile reflects the 75th percentile of the KCQA annual measure score, and facility data reflect the January of 2012 KCQA cohort data.
Mean of median household income. Income data were obtained from 2010 US Census on the basis of dialysis facility zip code.
We applied a lower UF rate threshold of 10 ml/h per kilogram to the full cohort and key subgroups (Supplemental Table 4). Among the CMS cohort patients with heart failure (n=38,506), 24.8% had UF rates >13 ml/h per kilogram, and 45.4% had rates >10 ml/h per kilogram. Among patients with histories of intradialytic hypotension (n=30,045), 20.7% had UF rates >13 ml/h per kilogram, and 40.5% had rates >10 ml/h per kilogram. Measure and monthly results were similar.
Facility TT Burden in the Setting of UF Rate Thresholds
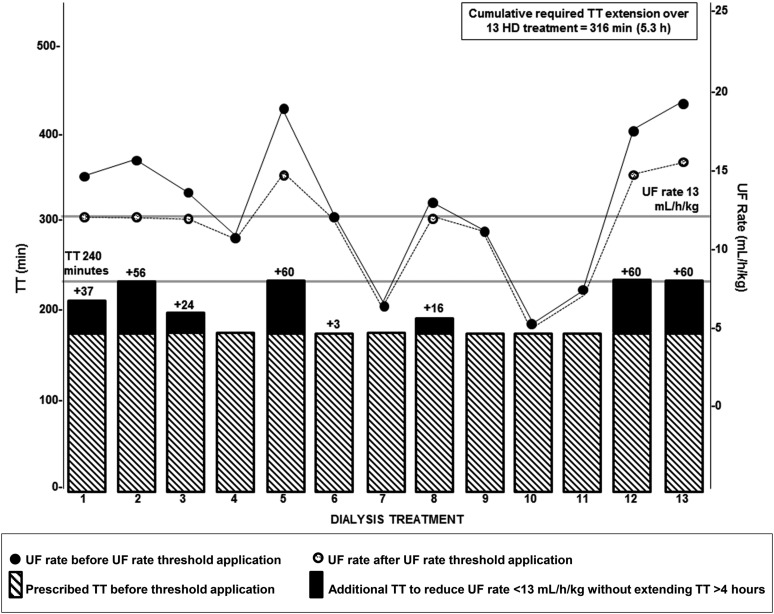
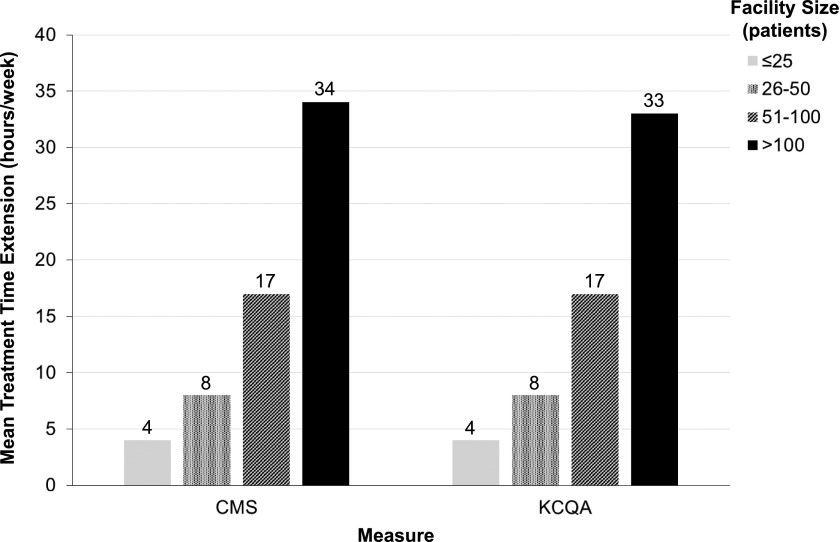
Among 103,850 patients in the January 2012 KCQA cohort, 20,152 (19%) had UF rates ≥13 ml/h per kilogram. Figure 3 depicts a single patient’s TT extension over 1 month in response to application of the KCQA UF rate threshold. To lower UF rates to <13 ml/h per kilogram via TT extension (with total TT remaining at ≥4 hours), facilities would require a mean additional TT of 17±16 h/wk. Larger facilities would require proportionately more TT compared with smaller facilities, with facilities with >100 patients requiring an additional 33 h/wk (Figure 4). Percentages of patients with UF rates remaining ≥13 ml/h per kilogram after application of varied TT extension paradigms are presented in Table 4. Supplemental Figure 2 displays facility TT burden if TTs were extended to bring UF rates to <13 ml/h per kilogram without application of a 4-hour total TT maximum.
Figure 3.
Illustration of treatment time (TT) extension calculation for a single patient with varying prescribed ultrafiltration (UF) rates over 1 month (13 treatments). The black circles represent the prescribed UF rates on the basis of observed interdialytic weight gains and prescribed TTs without application of a UF rate threshold. The white circles represent the updated UF rates after the application of a UF rate threshold of 13 ml/h per kilogram under the constraint of a 4-hour TT maximum. The striped bars represent the prescribed TT (180 minutes) during the month assessed. The black bars represent the calculated amount of additional TT needed to reduce UF rates to <13 ml/h per kilogram without extending total TT beyond 4 hours. For treatments with a prescribed UF rate of <13 ml/h per kilogram, no additional TT was assigned, and no change to the UF rate was made. For treatments with a prescribed UF rate of ≥13 ml/h per kilogram, the additional TT (in minutes) needed to achieve the UF rate <13 ml/h per kilogram was calculated as follows: ([predialysis weight from current treatment − postdialysis weight from previous treatment (milliliters)]/[postdialysis weight from previous treatment (kilograms) ×12.9])×60− prescribed TT (minutes). Total TT was capped at 4 hours per treatment, such that the UF rate remained ≥13 ml/h per kilogram for treatments in which UF rate reduction to rates <13 ml/h per kilogram would result in TT extension beyond 4 hours. Additional patient TTs were then summed for the month. Detailed methods for estimation of TT extension are described in Supplemental Table 2. HD, hemodialysis.
Figure 4.
Weekly required additional facility treatment time (TT) for a maximum run time of 4 hours with the application of a 13-ml/h per kilogram ultrafiltration (UF) rate threshold stratified by facility size. The additional TT required to lower prescribed UF rates to <13 ml/h per kilogram was computed at the facility level and stratified by facility size. Total TT was capped at 4 hours per the Kidney Care Quality Alliance (KCQA) specifications. For these analyses, prescribed UF rate (milliliters per hour per kilogram) was calculated using the formula (predialysis weight from current treatment − postdialysis weight from previous treatment [milliliters])/prescribed TT (hours)/postdialysis weight from previous treatment (kilograms). For treatments with a prescribed UF rate of <13 ml/h per kilogram, no additional TT was assigned. For treatments with a prescribed UF rate of ≥13 ml/h per kilogram, the additional TT (minutes) needed to achieve the UF rate <13 ml/h per kilogram was calculated as follows: ([predialysis weight from current treatment − postdialysis weight from previous treatment (milliliters)]/[postdialysis weight from previous treatment (kilograms) ×12.9])×60− prescribed TT (minutes). Total TT was capped at 4 hours per treatment, such that the UF rate remained at ≥13 ml/h per kilogram for treatments in which UF rate reduction to rates <13 ml/h per kilogram would result in TT extension beyond 4 hours. Additional patient TTs were then summed for the month at the patient and then facility levels. Detailed methods for estimation of TT extension are described in Supplemental Table 2. CMS, Centers for Medicare and Medicaid Services.
Table 4.
Proportion of patients with ultrafiltration rates ≥13 ml/h per kilogram after application of varied hypothetical treatment time extension paradigms
| Facility TT Change | UF Rate ≥13 ml/h per kg before TT Extension, % (no. of patients) | UF Rate ≥13 ml/h per kg after TT Extension, % (no. of patients) |
|---|---|---|
| Extend TT by 15 min if TT is <240 min | 19.4 (20,152) | 15.8. (16,418) |
| Extend TT by 30 min if TT is <240 min | 19.4 (20,152) | 12.8 (13,332) |
| Extend TT to 240 min if TT is <240 min | 19.4 (20,152) | 11.0 (11,395) |
| Extend TT long enough to achieve UF rate <13 ml/h per kg if TT is <240 min | 19.4 (20,152) | 3.1 (3199) |
| Extend TT long enough to achieve UF rate <13 ml/h per kg regardless of TT | 19.4 (20,152) | 0.0 (0) |
Estimation of TT extension was on the basis of the monthly Kidney Care Quality Alliance cohort from January of 2012 (n=103,850). TT, treatment time; UF, ultrafiltration.
Discussion
In this study, we examined UF rate patterns and proposed UF rate quality measures and calculated the facility TT burden with UF rate threshold implementation. Patient UF rates fluctuated over the course of the year, with the highest UF rates occurring in winter and the lowest rates occurring in summer. Patients with higher UF rates were more likely to be women, nonblack, and Hispanic and have lower body weight and heart failure. Facilities with higher percentages of patients with elevated UF rates were more likely to be located in the western Unites States and have fewer black and more Hispanic patients compared with facilities with lower percentages. Extension of TT as a strategy to reduce UF rates resulted in a mean weekly facility burden of 17 TT hours when total TTs were capped at 4 hours.
Existing observational data suggest an association between higher UF rates and adverse outcomes (11,12). Although these associations have not been prospectively tested, they are supported by plausible physiologic mechanisms, including UF–related end organ ischemia and hypervolemia from hemodynamic instability reactive measures (13,14). UF rates are determined by the amount of fluid removed (typically equivalent to IDWG) and HD duration. Dialysis providers prescribe UF volume and TT, rendering UF rates within facility control. Reducing UF volumes to curb UF rates without concurrent IDWG reduction would leave patients disadvantageously volume expanded. Thus, extending TT or adding supplemental treatments would likely be required to lower UF rates. However, patients are generally averse to longer TTs (15), raising concern about patient acceptance and underscoring the need for prospective study. Despite these uncertainties, CMS is considering inclusion of a UF rate quality measure in the 2019 QIP (7).
The ESRD QIP aims to promote patient health by providing financial incentive for dialysis facilities to deliver high-quality care. In this value–based care model, clinical measures are intended to improve outcomes, reduce disparities, and limit unintended consequences (16). We evaluated UF rate patterns and considered different approaches to measure definition. We found that UF rates vary over the calendar year. This month to month variation, plausibly related to seasonal hydration patterns, suggests that a monthly measure, as proposed by CMS, best captures facility UF rate practices. An annual measure, as recommended by KCQA, obscures seasonal variation. Also, we showed that UF rates vary by assessment day, with greater UF rates occurring after the long interdialytic break. Mean–based UF rate measures, as proposed by KCQA, may best capture facility practices and thwart gaming, a practice made easier by reliance on a single value. Although single UF rate values are easier to collect, their fluctuations limit utility, particularly if day of week collection standards are not specified.
Our data highlight patient and facility differences across higher (versus lower) UF rates that may have unintended consequences. We showed that patients with higher UF rates are more likely to be women, nonblack, and Hispanic and have lower body weight. Facilities with higher UF rate measure scores (less favorable) were more likely to be located in the western United States compared with facilities with lower scores. Lower proportions of black patients, higher proportions of Hispanic patients, and body size differences likely account for these geographic differences (Supplemental Table 5). Such discrepancies highlight the importance of additional investigation of the UF rate and outcome association across categories of race, sex, and body size.
Related, both proposed measures apply a universal UF rate threshold of 13 ml/h per kilogram. This one size fits all approach assumes that all patients experience risk at the same UF rate threshold. A post hoc analysis of the Hemodialysis Study showed that UF rate risk rises steeply between 10 and 13 ml/h per kilogram, with the greatest risk at rates >13 ml/h per kilogram. This risk threshold varied by subgroups: patients with heart failure had a higher mortality risk at lower UF rates (10–13 ml/h per kilogram), whereas patients without heart failure did not (12). In this study, we reported that almost 50% of patients with heart failure had UF rates >10 ml/h per kilogram. We found that over one third of other hemodynamically vulnerable subpopulations, such as patients with low pre–HD BP and intradialytic hypotension, had monthly UF rates >10 ml/h per kilogram. The proposed threshold might leave many high–risk patients inadequately protected.
Selection criteria designation is critical to measure development. The KCQA and the CMS measures differ in numerator specifications. The KCQA measure includes a TT caveat: the numerator includes patients with UF rates ≥13 ml/h per kilogram and TTs<240 minutes. The denominator encompasses all facility patients meeting selection criteria regardless of TT. The CMS measure does not have a numerator TT limitation. This distinction results in notable differences in the percentages of facility patients above UF rate thresholds: 22.3% for the CMS measure versus 17.4% for the KCQA measure (January of 2012). Recent data show an association between higher UF rates and mortality among patients with longer HD times (J. Bragg-Gresham, et al., unpublished data). The KCQA measure does not facilitate UF rate–related harm reduction among patients with TTs>4 hours. The KCQA proponents propose that the TT caveat addresses patient preference for shorter TT. It also gives facilities two ways to comply with the measure: by lowering UF rates to <13 ml/h per kilogram or increasing TT to ≥4 hours. Additionally, the TT restriction minimizes the KCQA measure’s effect on facility operations. In our analyses, facilities treating >100 patients required 33 h/wk of extra treatment to lower UF rates to <13 ml/h per kilogram in the setting of a 4-hour TT maximum. When the 4-hour TT cap was omitted, the additional weekly facility treatment hours tripled. Increased TT is associated with substantial costs related to staffing, supplies, water, and utilities. Furthermore, added TT has implications beyond cost. Extending HD treatments would have consequences for other patients who would be subject to fluctuating start times when earlier patients required longer treatments. In this regard, the 4-hour TT carve out might minimize disruptions to other patients.
Titrated HD treatments pose substantial patient and facility challenges, suggesting that IDWG reduction may represent the most feasible UF rate reduction approach. Intensive dietary salt counseling has been shown to reduce weight gains (17,18). Nutritional programs that teach salt (and fluid) restriction in ways that are relatable to and achievable by patients are needed. However, it is unlikely that dietary restrictions on their own will be enough to lower UF rates for all patients, and facilities will need to adopt a more flexible approach to dialysis provision. Another approach to weight gain reduction is dialysate sodium adjustment, but outcome data are mixed (19,20). Finally, it is plausible that UF rate threshold implementation will incentivize some patients to curb excessive salt and fluid intake to avoid longer treatments, offsetting facility burden.
Overall, our data highlight the challenges, uncertainties, and potential consequences of an ESRD QIP UF rate measure. Incorporation of exceptions and flexibility not previously applied to other QIP measures might optimize the UF rate measure. For example, should facilities be exempted from financial penalties if patients decline longer treatments and the facility documents good faith efforts at IDWG reduction? Would it be feasible to tie reimbursement to dialysis time rather than the current fixed reimbursement per treatment? How can reimbursement policies be altered to incentivize home-based therapies that allow for more frequent treatments with more gradual UF? How should patient preferences be incorporated into measure implementation? The proposed UF rate QIP measure is a critical first step in codifying optimal volume management into high–quality dialysis care, but the challenges of volume management, particularly given the absence of objective measures, introduce complexities that should be recognized.
Our results must be considered in the context of study limitations. First, most importantly, we considered data from a single LDO. The facility–level UF rate patterns that we observed may not be representative of other dialysis organizations’ facility patterns. UF rate patterns may vary under different clinical protocols. Second, our study is descriptive. Conclusions about differences in the UF rate and outcome association across subpopulations cannot be drawn. Third, we estimated the amount of additional facility TT that would be required with UF rate threshold implementation. In these calculations, we used prescribed rather than delivered UF rates and assumed that treatments for all patients with elevated UF rates would be extended. We were unable to account for patient refusal of TT extension or potential effects from more intensive dietary counseling. Fourth, we used UF rate measure definitions and selection criteria according to the CMS and the KCQA specifications. Results cannot be generalized to excluded populations, such as children and patients from facilities with <11 patients.
In conclusion, our findings suggest a need for reconsideration of the proposed UF rate measure definition and additional research regarding the UF rate to outcome association in key subpopulations. Before adoption of UF rate as a dialysis facility quality indicator, prospective studies of patient and facility consequences of UF rate threshold implementation as well as pilot studies of the proposed rule are needed.
Disclosures
J.E.F. has received speaking honoraria from Dialysis Clinic, Incorporated; Renal Ventures; American Renal Associates; the American Society of Nephrology; and Baxter. J.E.F. and M.M.A. have received research funding for studies unrelated to this study from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America.
Supplementary Material
Acknowledgments
The authors thank Alan Brookhart for data access and his insightful comments on analyses and Diane Reams for her data and contract management assistance. The authors also thank DaVita Clinical Research for providing data for this study.
M.M.A. is supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health grant T32 DK007750.
DaVita Clinical Research had no role in the design or implementation of this study or the decision to publish.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Fluid First or Not So Fast: Ultrafiltration Rate and the ESRD Quality Incentive Program,” on pages 1330–1332.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13441215/-/DCSupplemental.
References
- 1.Arneson TJ, Liu J, Qiu Y, Gilbertson DT, Foley RN, Collins AJ: Hospital treatment for fluid overload in the Medicare hemodialysis population. Clin J Am Soc Nephrol 5: 1054–1063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stålhammar NO: Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 9: 2124–2132, 2014 [DOI] [PMC free article] [PubMed]
- 3.Weiner DE, Brunelli SM, Hunt A, Schiller B, Glassock R, Maddux FW, Johnson D, Parker T, Nissenson A: Improving clinical outcomes among hemodialysis patients: A proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis 64: 685–695, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Agar JW: Personal viewpoint: Limiting maximum ultrafiltration rate as a potential new measure of dialysis adequacy. Hemodial Int 20: 15–21, 2016 [DOI] [PubMed] [Google Scholar]
- 5.National Quality Forum: About us. Available at: http://www.qualityforum.org/story/About_Us.aspx. Accessed November 30, 2015
- 6.National Quality Forum: Renal: Draft report. Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwja5cqlzr7JAhXDPiYKHTQUCHUQFggcMAA&url=http%3A%2F%2Fwww.qualityforum.org%2FProjects%2Fn-r%2FRenal_Measures%2FDraft_Report_for_Comment.aspx&usg=AFQjCNFGMoefydMrs7WvIqT7_l1_3wncjw&sig2=f0jP__24ngTVnc9pohVNBA. Accessed November 30, 2015
- 7.National Archives and Records Administration: Federal Register. Available at: http://www.gpo.gov/fdsys/pkg/FR-2015-11-06/pdf/2015-27928.pdf. Accessed November 30, 2015
- 8.Cabrera C, Brunelli SM, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stålhammar NO, Stefánsson BV: A retrospective, longitudinal study estimating the association between interdialytic weight gain and cardiovascular events and death in hemodialysis patients. BMC Nephrol 16: 113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Care Partners: Available at: http://kidneycarepartners.com/. Accessed February 22, 2016
- 10.US Renal Data System: USRDS 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, USA: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013
- 11.Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G: Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 22: 3547–3552, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Flythe JE, Kimmel SE, Brunelli SM: Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntyre CW, Odudu A: Hemodialysis-associated cardiomyopathy: A newly defined disease entity. Semin Dial 27: 87–97, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Flythe JE, Mangione TW, Brunelli SM, Curhan GC: Patient-stated preferences regarding volume-related risk mitigation strategies for hemodialysis. Clin J Am Soc Nephrol 9: 1418–1425, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services: End-Stage renal disease quality incentive program. Available at: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/esrdqip/index.html. Accessed November 30, 2015
- 17.Chazot C: Can chronic volume overload be recognized and prevented in hemodialysis patients? Use of a restricted-salt diet. Semin Dial 22: 482–486, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, Toz H, Can LH, Basci A, Ok E: The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant 24: 956–962, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Davenport A, Cox C, Thuraisingham R; PanThames Renal Audit Group : The importance of dialysate sodium concentration in determining interdialytic weight gains in chronic hemodialysis patients: The PanThames Renal Audit. Int J Artif Organs 31: 411–417, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Mc Causland FR, Waikar SS: Optimal dialysate sodium-what is the evidence? Semin Dial 27: 128–134, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KCQA: KCQA Measure Development. Available at: http://kidneycarepartners.com/kidney-care-quality-alliance-kcqa/measure-development-process/. Accessed February 22, 2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.