Summary
In many organisms, food availability is a proximate cue that synchronizes seasonal development of the reproductive system with optimal environmental conditions. Growth of the gonads and secondary sexual characteristics is orchestrated by the hypothalamic–pituitary–gonadal (HPG) axis. However, our understanding of the physiological mechanisms by which food availability modulates activity of the HPG axis is limited.
It is thought that many factors, including energetic status, modulate seasonal reproductive activation. We tested the hypothesis that food availability modulates the activity of the HPG axis in a songbird. Specifically, we food‐restricted captive adult male Abert's Towhees Melozone aberti for 2 or 4 weeks during photoinduced reproductive development. A third group (control) received ad libitum food throughout. We measured multiple aspects of the reproductive system including endocrine activity of all three levels of the HPG axis [i.e. hypothalamic gonadotropin‐releasing hormone‐I (GnRH‐I), plasma luteinizing hormone (LH) and testosterone (T)], and gonad morphology. Furthermore, because gonadotropin‐inhibitory hormone (GnIH) and neuropeptide Y (NPY; a potent orexigenic peptide) potentially integrate information on food availability into seasonal reproductive development, we also measured the brain levels of these peptides.
At the hypothalamic level, we detected no effect of food restriction on immunoreactive (ir) GnRH‐I, but the duration of food restriction was inversely related to the size of ir‐GnIH perikarya. Furthermore, the number of ir‐NPY cells was higher in food‐restricted than control birds. Food restriction did not influence photoinduced testicular growth, but decreased plasma LH and T, and width of the cloacal protuberance, an androgen‐sensitive secondary sexual characteristic. Returning birds to ad libitum food availability had no effect on plasma LH or T, but caused the cloacal protuberance to rapidly increase in size to that of ad libitum‐fed birds.
Our results support the tenet that food availability modulates photoinduced reproductive activation. However, they also suggest that this modulation is complex and depends upon the level of the HPG axis considered. At the hypothalamic level, our results are consistent with a role for the GnIH and NPY systems in integrating information on energetic status. There also appears to be a role for endocrine function at the anterior pituitary gland and testicular levels in modulating reproductive development in the light of energetic status and independently of testicular growth.
Keywords: gonad development, gonadotropin‐inhibitory hormone, gonadotropin‐releasing hormone, hypothalamic–pituitary–gonadal axis, luteinizing hormone, neuropeptide Y, reproductive physiology, seasonal reproduction, testosterone
Introduction
To maximize reproductive success, organisms often temporally synchronize their breeding period with optimal environmental conditions (Baker 1938; Wingfield & Kenagy 1986; Lindström 1999; Lourdais et al. 2002). This synchronization has been demonstrated in a variety of taxa (Munro, Scott & Lam 1990; Boyd 1991; Nager & van Noordwijk 1995; Olsson & Shine 1998; Olive, Lewis & Beardall 2000). In many adult organisms, the reproductive system is regressed outside the breeding period and must recrudesce before the start of the next breeding period. In these organisms, reproductive recrudescence (i.e. regrowth of the gonads and secondary sexual characteristics) is, therefore, an important determinant of the breeding period. This is the case in most birds, which generally exhibit distinct breeding and non‐breeding life‐history stages that are characterized by dramatic changes in reproductive physiology, morphology and behaviour (Williams 2012; Davies & Deviche 2014).
To correctly time reproductive development, organisms use proximate environmental cues to predict favourable conditions (Ims 1990), and one such cue in birds is the annual change in day length (photoperiod). The annual cycle of photoperiod is constant between years at a given location and can predict favourable conditions. Accordingly, birds use photoperiod as the ‘initial predictive cue’ that stimulates the hypothalamic–pituitary–gonadal (HPG) axis to begin reproductive development (Wingfield 1980; Dawson et al. 2001) and the mechanism by which increasing vernal photoperiod influences the avian HPG axis has been studied extensively. Light is detected by deep brain photoreceptors, and the circadian system is used to measure the length of this light signal (Follett, King & Meddle 1998; Sharp 2005). Information from these photoreceptors is relayed via the pars tuberalis (PT) of the pituitary gland to increase the production and secretion of the neuropeptide gonadotropin‐releasing hormone‐I (GnRH‐I; King & Millar 1982; Sharp & Ciccone 2005). Gonadotropin‐releasing hormone‐I is the primary secretagogue of the gonadotropins, luteinizing hormone (LH) and follicle‐stimulating hormone (FSH), from the anterior pituitary gland (Kuenzel 2000). These hormones stimulate development of the gonads and secretion of the sex steroids testosterone (T) and oestradiol (E2) in males and females, respectively (Murton & Westwood 1977), and these steroids, in turn, stimulate development of secondary sexual organs and promote the expression of reproductive behaviours.
In contrast to photoperiod, many environmental variables often vary from year to year at a given location. Birds may modulate the activity of their reproductive system in the light of supplementary environmental cues, including ambient temperature (Wingfield et al. 2003; Schaper et al. 2012b), rainfall (Hau et al. 2004; Small, Sharp & Deviche 2007), social interactions (Wingfield & Wada 1989; Maney, Goode & Ball 2007; Small et al. 2008a; Stevenson et al. 2008) and food availability (Lack 1968; Hau, Wikelski & Wingfield 2000; Hahn et al. 2005; O'Brien & Hau 2005). Although considerable information is available about the effects of day length on the HPG axis, much less is known about the physiological mechanisms by which these non‐photic environmental factors influence reproductive activity and most studies to date have focused primarily on the pituitary gland. Food availability, in particular, is thought to modulate reproductive activity partly through an individual's energetic status. Accordingly, within the window of opportunity for breeding determined by day length, a bird's energetic status is predicted to constrain the timing of reproductive development (Drent & Daan 1980; Wingfield & Kenagy 1986; Meijer & Drent 1999; Hahn et al. 2005). Thus, birds in good energetic status can begin this development shortly after stimulation by sufficiently long days, whereas birds in poor energetic status delay development until they have acquired sufficient energy stores. Various experimental and correlative approaches, including food supplementation (Schoech 1996, 2009; Scheuerlein & Gwinner 2002; Harrison et al. 2010) and natural variation in the abundance of wild food sources (Ligon 1974; Hahn 1998), found that increased food availability is associated with earlier seasonal breeding in wild birds (reviewed by Davies & Deviche 2014). Captive studies on the effect of food restriction or deprivation on the avian reproductive system have, however, yielded inconsistent results (Dawson 1986; Meijer 1991; Hahn 1995; Perfito et al. 2008).
A candidate neuropeptide that may integrate information on food availability and fine‐tune seasonal reproductive development is gonadotropin‐inhibitory hormone (GnIH; Tsutsui et al. 2000) and its links with hypothalamic cells that produce neuropeptide Y (NPY; McConn et al. 2014). Across vertebrates, GnIH opposes the effect of GnRH on the HPG axis (Greives et al. 2008) and inhibits reproductive function by acting on hypothalamic GnRH neurons, anterior pituitary gland gonadotropes and gonads (Tsutsui et al. 2010, 2012, 2013; Clarke 2011; Clarke & Parkington 2013; Davies & Deviche 2014). In addition, GnIH has an orexigenic effect in birds and mammals (Tachibana et al. 2005, 2008; Clarke et al. 2012; Clarke & Parkington 2013; Tsutsui et al. 2013; McConn et al. 2014). In mammals, GnIH neurons project to hypothalamic regions that regulate appetite and energetic status (Qi, Oldfield & Clarke 2009; Ubuka et al. 2009). These regions contain NPY‐producing neurons, and the orexigenic action of GnIH may, therefore, be mediated by these neurons (Clarke et al. 2009). Neuropeptide Y is among the most potent endogenous orexigenic factors (Boswell 2001; Hill, Elmquist & Elias 2008; Pralong 2010), and, in mammals, NPY‐producing cells integrate information on energetic status via both hormones and metabolites (Marty, Dallaporta & Thorens 2007). Indeed, reciprocal projections from NPY cells to GnIH cells and evidence from mammalian studies suggest that GnIH cells modulate their activity in response to energetic status (Klingerman et al. 2011). Therefore, the GnIH–NPY system appears to simultaneously regulate the activity of the HPG axis and food intake in response to energetic status and may play a role in the modulation of reproductive development by food availability (Davies & Deviche 2014).
Here, we investigated whether food availability modulates reproductive development in a seasonally breeding songbird and examined potential neuroendocrine mechanisms mediating this modulation. Specifically, we hypothesized that energetic status influences reproductive development. To test this hypothesis, we compared hypothalamic levels of GnRH, plasma LH and T, testicular development and cloacal protuberance width of adult male Abert's Towhees Melozone aberti (Baird) subjected to various food availability regimes during photoinduced gonadal development. We predicted that birds with access to ad libitum food and, hence, in good energetic status would undergo reproductive activation at a faster rate than food‐restricted birds in poor energetic status. We also aimed to shed light on the physiological mechanism(s) by which food availability modulates the HPG axis and, for this, quantified hypothalamic levels of GnIH and NPY in response to food availability. To our knowledge, this study is the first in a wild bird species to examine the effects of food restriction on the endocrine regulation of reproductive development at all levels of the HPG axis simultaneously. A better understanding of the physiological mechanisms by which food availability modulates seasonal reproductive activation will improve our understanding of how variation in this environmental cue is transduced into annual variation in the timing of breeding periods.
Materials and methods
Bird Capture and Housing
During January 2011, we caught 24 adult male Abert's Towhees from Robbins Butte Wildlife Area, Maricopa Co., AZ (altitude: 249 m; latitude: 33°19′N; longitude: 112°38′W), using mist nets and conspecific song playback. We determined sex and age using behaviour (singing and aggressive response to conspecific playback in males only), skull pneumatization and wing chord (wing chord ≥92 mm = male; Pyle 1997). We transported birds to Arizona State University and randomly divided them between two environmental chambers, both maintained at 23 °C (±1 °C) with a short light cycle (9 L : 15 D; lights on at 06:00). We individually housed birds in 76 × 46 × 46 cm cages with opaque barriers on three sides of each cage and provided ad libitum food (Mazuri small bird maintenance diet) and water. To permit individual identification, we marked each bird with a numbered aluminium tarsal band. After a 1‐week acclimation period in captivity, we increased the photoperiod to 14 L (lights on at 06:00) to induce reproductive development. To measure the average daily ad libitum food consumption of each individual over 2 weeks, we provided a pre‐weighed amount of food shortly after the lights went on and weighed the remaining food (including spillage on the cage bottom) 24 h later. To minimize spillage, we partially covered food dishes with tape, allowing only a small opening to access food. All procedures were approved by the Arizona State University Institutional Animal Care and Use Committee and conducted under appropriate scientific collecting permits issued by the Arizona Game and Fish Department and the US Fish and Wildlife Service.
Experimental Protocol
Following the 2‐week ad libitum food consumption period, we randomly assigned birds to one of three groups (n = 8 birds per group): (i) ad libitum food, (ii) restricted food availability for 4 weeks (see more below) or (iii) 2 weeks of food restriction followed by 2 weeks of ad libitum food (Fig. 1). We used a stratified random assignment of treatment groups to spatially intersperse the treatments across the two environmental chambers, such that each environmental chamber contained an equal number of birds from each treatment group (i.e. 12 birds per room, with four birds per group). Two birds in the ad libitum food group died during the study due to unknown causes, reducing this group size to six.
Figure 1.

Schematic representation of the food availability regime. During the acclimation period, we quantified the daily food consumption of each bird. Food‐restricted birds received 70% of their individual ad libitum consumption. Photoperiod for the duration of the experiment shown in the schematic was 14 L : 10 D throughout. Arrows indicate the collection of blood samples and morphometric data. Following the third collection period, we also collected the brain and testes from each bird.
To ensure that the food restriction regime caused a marked and consistent reduction in body mass, we food‐restricted birds with the goal of reducing their body mass to 85% of their ad libitum mass. This target body mass is comparable to that used by Lal et al. (1990) in similar studies on cockerels (c. 83% of ad libitum mass) and on European Starlings Sturnus vulgaris (c. 80% of ad libitum mass; Meijer 1991). To reach this target weight and based on a separate pilot study on six Abert's Towhees, experimental birds received 70% of their individual average ad libitum food consumption. We weighed all birds daily immediately prior to dispensing the daily food ration. If a bird's mass decreased below its 85% target mass, this bird was immediately fed the difference between its current mass and its target mass (Morrison et al. 2002). By using this individually customized, flexible restriction regime, we could control food availability and concurrently confirm that the body mass of each bird was reduced and stabilized at the target reduction.
From each bird, we collected a blood sample to quantify plasma LH and T immediately prior to (i) the switch to food restriction (defined as week 0), (ii) the switch back to ad libitum food (week 2) and (iii) the end of the study (week 4; Fig 1). We collected blood (c. 200 μL) from the right jugular vein using a heparinized syringe within 2 min of removing a bird from its cage. The sample was then placed on ice and centrifuged within 1 h. We harvested plasma using a Hamilton glass syringe and froze aliquots at −80 °C until assayed. Immediately following collection of each blood sample, we quantified the furcular fat stores and size of the pectoral muscles of all the birds to estimate their energetic condition. We visually estimated the amount of furcular fat by assigning a score of 0–5 (a score of 0 representing no fat, 5 representing bulging fat deposits; Helms & Drury 1960). Furthermore, because the pectoral muscles in birds are the largest store of protein and muscle protein can be converted into energy via gluconeogenesis, we also estimated the size of the pectoral muscles on a scale ranging from 0 to 3 (adapted from Gosler 1991; 0 representing concave pectoral muscles and a prominent keel, 3 representing convex pectoral muscles that protruded above the keel; Salvante, Walzem & Williams 2007). At this time, we also measured the width of the cloacal protuberance (±0·1 mm; using digital calipers), an androgen‐sensitive secondary sexual characteristic. We staggered the collection of blood samples and morphometrics over two consecutive days by randomly assigning towhees to one of four groups, with six birds in each group. We collected blood samples for any given group always in the same order beginning at 09:00 AM and noted the time that each blood sample was taken.
Perfusion and Tissue Collection
To investigate the effect of food availability on the central control of reproduction and testicular development, at the end of the study, we collected the brain and testes of each bird. Following deep anaesthesia induced by intramuscular injection (250 μL into each pectoral muscle) of a ketamine/xylazine cocktail [ketamine: 8 mg per 0·5 mL (160 mg kg−1); xylazine: 160 μg per 0·5 mL (3·2 mg kg−1) dissolved in 0·9% NaCl solution], we transcardially perfused birds with 35 mL of wash solution (0·9% NaCl and 0·1% NaNO2 in 0·1 m phosphate buffer, PB), followed by 35 mL of fixative (4% paraformaldehyde and 0·1% NaNO2 in 0·1 m PB). We then decapitated birds, exposed the brain and placed heads in the same fixative overnight at 4 °C. We also collected both testes and removed all extra connective tissue before weighing to the nearest 0·1 mg and post‐fixed the tissue as described above for brains. The day after perfusion, we dissected brains out of the skull and post‐fixed them overnight (4% paraformaldehyde and 0·1% NaNO2 in 0·1 m PB). Brains and testes were gelatin‐embedded and cryoprotected according to a modification of a previously published protocol (Saldanha, Deviche & Silver 1994; Small et al. 2008b) and stored at −80 °C until sectioned.
We coronally sectioned brains at a thickness of 30 μm using a cryostat at −21 °C, with the stereotaxic atlas of the canary Serinus canaria brain as a reference (Stokes, Leonard & Nottebohm 1974). Sections were divided into four parallel series by systematically alternating between four separate six‐well Falcon plates containing cryoprotectant solution (Watson et al. 1986) and stored in cryoprotectant at −20 °C until immunolabelled. One series was used for each assay (cGnRH‐I, GnIH or NPY).
GnRH, GnIH and NPY Immunocytochemistry
We immunostained brain sections for GnRH, GnIH and NPY in two assays per peptide, with sections from randomly selected birds in each assay. Previous studies have determined the distribution of these peptides in the avian brain. Gonadotropin‐inhibitory hormone‐I is primarily synthesized in the pre‐optic area (POA; Parry et al. 1997; Dawson & Goldsmith 1997), and GnIH is synthesized solely in the paraventricular nucleus (PVN; Tsutsui et al. 2000; Osugi et al. 2004; Tsutsui et al. 2010). Neuropeptide Y‐producing neurons are widely distributed throughout the avian brain, but the only cell population that responds to energetic status is located in the infundibular nucleus (IN; Kuenzel & McMurtry 1988; Boswell 2001; Boswell, Li & Takeuchi 2002). Therefore, from each bird, we randomly selected sections covering the entire POA, PVN and IN for GnRH, GnIH and NPY, respectively; Fig. 2). For GnIH and NPY, we stained an average of 10 sections per bird and for GnRH, an average of six sections per bird. Since sections were randomly selected from each bird, we assumed that this sampling design gives an unbiased estimate of the number of immunoreactive cells for a given bird and, therefore, calculated the median number of cells per section for each bird.
Figure 2.
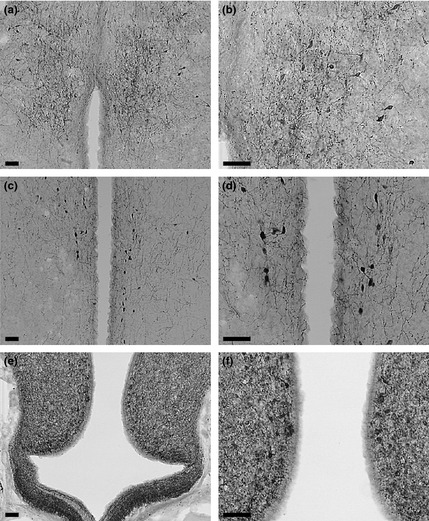
Representative photomicrographs of immunolabelled coronal brain sections of male Abert's Towhees Melozone aberti under lower magnification (left column) and higher magnification (right column). (a and b) immunoreactive gonadotropin‐releasing hormone‐I (GnRH‐I) perikarya in the pre‐optic area. (c and d) immunoreactive gonadotropin‐inhibitory hormone (GnIH) perikarya in the paraventricular nucleus. (e and f) immunoreactive neuropeptide Y (NPY) perikarya in the infundibular nucleus. Scale bars = 50 μm.
The GnRH and GnIH staining protocols have been previously published and validated in our laboratory (Small et al. 2008b). The specificity of the NPY antiserum has been established in the chicken (Kuenzel & McMurtry 1988) and Ring Dove Streptopelia risoria (Strader & Buntin 2001). Pre‐absorption of the NPY antiserum with human/rat NPY (H‐6375; Bachem, Torrance, CA, USA) before application to Abert's Towhee brain sections abolished the staining. Following two washes in 0·1 m PB for 30 min, we serially exposed sections to 0·36% H2O2, washed them 3 × 5 min in 0·1 m PB, blocked background immunoreactivity for 1 h and incubated sections overnight in primary antiserum. We then washed sections three times for 10 min in PB with 0·1% Triton X‐100 (Sigma‐Aldrich Co., St. Louis, MO, USA; 0·1% PBT), incubated for 1 h in secondary antibody, washed three times for 10 min in 0·1% PBT, incubated for 1 h in avidin–biotin complex (ABC Vectastain Elite kit; Vector Laboratories, Burlingame, CA, USA), washed three times for 15 min in 0·1% PBT, incubated in Vector SG peroxidase chromagen for 2 min and washed twice for 5 min in 0·1 m PB. After mounting on glass microscope slides, we allowed immunolabelled sections to dry at room temperature for 24 h before dehydrating through a graded ethanol series, clearing in xylene and coverslipping using Permount mounting medium (Fisher Scientific, Pittsburg, PA, USA).
GnRH
We used anti‐cGnRH‐I rabbit polyclonal antiserum (6DL31/4 prepared by P.J. Sharp) at a dilution of 1 : 10 000 in 0·3% PBT. To block non‐specific sites, we used normal rabbit serum (Vector Laboratories, Inc.; 1 : 200 in 0·3% PBT), and we used biotinylated rabbit anti‐sheep IgG (Vector Laboratories, Inc.; 1 : 200 in 0·3% PBT) as a secondary antibody.
GnIH
We used anti‐Japanese Quail GnIH antiserum (Tsutsui et al. 2000) at a dilution of 1 : 5000 in 0·3% PBT. We used normal horse serum (Vector Laboratories; 1 : 30 in 0·3% PBT) to block non‐specific sites and biotinylated mouse/rabbit IgG (Vector Laboratories; 1 : 100 in 0·3% PBT) as a secondary antibody.
NPY
We used anti‐human/rat NPY antiserum (T‐4070; Bachem) at a dilution of 1 : 10 000 in 0·3% PBT. We used normal goat serum (Vector Laboratories; 1 : 30 in 0·3% PBT) to block non‐specific sites and biotinylated rabbit IgG (Vector Laboratories; 1 : 100 in 0·3% PBT) as a secondary antibody.
Immunocytochemistry Data Collection
All data were collected without knowledge of the treatment group. For each bird, we counted the number of cells immunoreactive for GnRH‐I, GnIH and NPY present in each immunostained section. We quantified the area and optical density of GnRH and GnIH immunolabelled perikarya using digital photographs taken at 400× magnification with an Olympus DEI‐750D digital camera mounted on an Olympus BX60 light microscope (Olympus Optical Co., Ltd., Tokyo, Japan). Due to the dense network of NPY‐ir fibres in the IN (Fig. 2), perikaryon area and optical density could not be accurately quantified for this peptide. Light intensity, aperture diameter and camera shutter speed were standardized for all image captures. We photographed five randomly selected perikarya from each section. Only perikarya for which the entire perimeter was unobstructed and clearly visible were used; perikarya with overlapping structures, such as other perikarya, were not analysed. Digitized images were analysed using Image‐Pro Plus (Media Cybernetics, LP, Silver Spring, MD, USA) by manually outlining each perikaryon and then determining the immunolabelled area and optical density (arbitrary units: 0 = no staining, complete light transmission; and 1 = complete staining saturation, no light transmission) of each. All images were standardized for individual variations in background immunolabelling using Image‐Pro's background correction function.
To determine the density of GnRH‐I‐ir and GnIH‐ir fibres in the median eminence (ME), we took pictures from two sections per brain. We corrected for background staining of each image as described above. On the resulting image, we used Image‐Pro Plus to measure the optical density of five areas of interest (AOI, 65 × 65 μm each) per brain section. Areas of interest were evenly spaced from left to right along the ventral edge of the ME. We calculated an average optical density for each section, then an average for each bird.
Testicular Morphology
We sectioned testes at a thickness of 30 μm using a cryostat at −21 °C and stored sections in 0·1 m PB until mounting on glass microscope slides later the same day. After allowing sections to dry at room temperature for 24 h, we rehydrated them through a graded ethanol series before staining with haematoxylin (S212A; Poly Scientific, Bay Shore, NY, USA) for 3 min. We then rinsed the sections for 5 min under running tap water before destaining by dipping them in acid ethanol ten times. Following another 2 min rinse with tap water, we stained sections with eosin (S176, Poly Scientific) for 30 s, dehydrated them through a graded ethanol series, cleared them in xylene and coverslipped using Permount.
Vernal reproductive development in many seasonally breeding birds involves a marked increase in testis size caused by increases in the length and diameter of seminiferous tubules. Seminiferous tubule diameter is, therefore, a sensitive indicator of testicular exocrine function (Amann 1986; Jenkins, Ross & Young 2007). We randomly selected eight sections from each bird (four from each testis) and, using Image‐Pro Plus, measured the shortest diameter of 10 seminiferous tubules per section randomly selected using a grid overlaid on the image. These measurements were used to calculate an average seminiferous tubule diameter for each bird. We also recorded when spermatozoa were present in the seminiferous tubules.
Hormone Assays
To measure plasma LH, we used the radioimmunoassay described previously (Sharp, Dunn & Talbot 1987), with slight modifications. Briefly, the assay reaction volume was 60 μL, comprised of 20 μL of plasma sample or standard, 20 μL of primary rabbit LH antibody and 20 μL of I125‐labelled LH. The primary antibody was precipitated to separate free and bound I125 label using 20 μL of donkey anti‐rabbit precipitating serum and 20 μL of non‐immune rabbit serum. All samples were measured in duplicate in a single assay. The intra‐assay coefficient of variation was 3·6% and the minimum detectable dose was 0·2 ng mL−1. This radioimmunoassay has been used extensively to quantify plasma LH in many avian species (Lal et al. 1990; Lea, Talbot & Sharp 1991; Malecki et al. 1998; Ciccone, Dunn & Sharp 2007; Schaper et al. 2012a; Fraley et al. 2013), including multiple Emberizidae sparrows (Meddle et al. 2002; Deviche, Sabo & Sharp 2008; Deviche et al. 2012a,b; Wingfield et al. 2012).
To quantify plasma T, we used competitive enzyme‐linked immunoassay kits, according to the manufacturer's instructions after 8× dilution in assay buffer containing steroid displacement reagent (Enzo Life Sciences, Ann Arbor, MI, USA). This assay has been validated for Abert's Towhee in our laboratory (Fokidis, Orchinik & Deviche 2009). We assayed samples in duplicate and randomly assigned them to assay plates, except that all samples collected from any given individual were assayed on the same plate. Each plate included a complete standard curve. The average assay sensitivity was 9·4 pg mL−1. The average intra‐ and interassay coefficients of variation were 6·9% and 3·7%, respectively (n = 2 plates; 66 samples). The primary antibody used in this assay has <5% cross‐reactivity with 17β‐oestradiol, 5α‐dihydrotestosterone (DHT), corticosterone and progesterone (manufacturer's specifications).
Statistical Analyses
To analyse the effects of food availability (ad libitum, reinstated ad libitum or restricted) on body mass, furcular fat score, pectoral muscle score, cloacal protuberance width, and plasma LH and T, we used a two‐way analysis of variance with repeated measures (rmanova). The sphericity assumption was tested using Mauchly's test, and if this was violated, we used the Greenhouse‐Geisser correction. To analyse the effect of food availability on the number of GnRH, GnIH and NPY cells, we used Kruskal–Wallis test followed by Dunn's pairwise comparison. Gonadotropin‐releasing hormone‐I and GnIH measures (median number of ir cells, cell body area and optical density, and optical density of fibres in the ME), and seminiferous tubule diameter were compared using one‐way anova. We performed all statistical analyses using pasw version 18.0 (Chicago, Illinois, USA) with an alpha of 0·05 on untransformed data, with the exception of cloacal protuberance width data that were log‐transformed to attain normality. Post hoc comparisons for anovas were performed using Tukey's honestly significant difference (HSD) test. Data analysed using parametric methods are presented as means ± standard error of the mean (SEM), and data analysed using nonparametric methods are presented as medians ± interquartile range (IQR). All graphs depict untransformed data.
Results
Body Mass
Body mass was significantly affected by food availability (F 2,19 = 32·68, P < 0·0001), time (F 2,38 = 93·34, P < 0·0001) and the interaction between these factors (F 4,38 = 39·12, P < 0·0001; Fig. 3). Body mass of ad libitum‐fed birds was similar throughout the experiment (Tukey HSD, P ≥ 0·05). Two weeks of food restriction caused body mass to decrease in both the reinstated ad libitum and the food‐restricted groups (Tukey HSD, P ≤ 0·05). Body mass did not decrease further in food‐restricted birds, but returning birds to ad libitum food availability for 2 weeks resulted in a body mass increase to levels similar to those at the beginning of the study (Tukey HSD, P < 0·05).
Figure 3.

Body mass, furcular fat and pectoral muscles were modulated by food availability in adult male Abert's Towhees Melozone aberti. Body mass (a), furcular fat score (b) and pectoral muscle score (c) were reduced following 2 weeks of food restriction (i.e. 70% of ad libitum consumption), and returning birds to ad libitum availability increased these parameters to levels similar to those of the control group. For details of study design and sample sizes, see Fig. 1 and the Materials and methods section. Data points are means ± SEM, and points with identical letters are not significantly different (P > 0·05; Tukey honestly significant difference test). For visual clarity, points have been separated along the horizontal axis.
Fat Score
Furcular fat scores were significantly affected by food availability (F 2,19 = 10·78, P = 0·001), time (F 2,38 = 74·56, P < 0·0001) and the interaction between these factors (F 4,38 = 15·25, P < 0·0001; Fig. 3). Fat scores of birds exposed to ad libitum food availability decreased over the 4‐week study (Tukey HSD, P < 0·05). Two weeks of food restriction caused fat scores to further decrease in both the reinstated ad libitum and the food‐restricted groups (Tukey HSD, P < 0·05). Fat scores did not decrease further in birds maintained on restricted food availability for another 2 weeks, but returning birds to ad libitum food availability for 2 weeks caused fat score to increase to levels similar to those at the beginning of the study (Tukey HSD, P < 0·05).
Pectoral Muscle Score
Pectoral muscle scores were significantly affected by food availability (F 2,19 = 10·94, P < 0·001), time (F 2,38 = 57·08, P < 0·001) and the interaction between these factors (F 4,38 = 24·72, P < 0·001; Fig. 3). Pectoral muscle scores of birds exposed to ad libitum food availability were similar throughout the experiment (Tukey HSD, P < 0·05). Two weeks of food restriction caused pectoral muscle score to decrease in both the reinstated ad libitum and the food‐restricted groups (Tukey HSD, P < 0·05). Pectoral muscle score did not decrease further in birds maintained on restricted food availability, but returning birds to ad libitum food availability for 2 weeks caused it to increase to levels similar to those at the beginning of the study (Tukey HSD, P < 0·05).
GnRH
We found no effect of food availability treatment on GnRH cells, including the number of GnRH‐I‐ir perikarya per section (H = 0·54, 2 d.f., P = 0·76), the perikaryon area (F 2,21 = 0·24, P = 0·79) or optical density (F 2,21 = 2·07, P = 0·15), or the optical density of ME GnRH‐I‐ir fibres (F 2,21 = 0·40, P = 0·68; Fig. 4).
Figure 4.

The number, area and optical density of perikarya and optical density of fibres in the median eminence (ME) immunolabelled for gonadotropin‐releasing hormone‐I (GnRH‐I), gonadotropin‐inhibitory hormone (GnIH) and neuropeptide Y (NPY) of adult male Abert's Towhees Melozone aberti following food availability treatment. Birds were exposed to ad libitum food availability for 4 weeks (n = 6; ‘ad libitum’), 2 weeks of food restriction (70% of ad libitum consumption) followed by 2 weeks of ad libitum food (n = 8; ‘reinstated ad libitum’) or restricted food availability for 4 weeks (n = 8; ‘food‐restricted’). Superscript letters indicate significant differences between the groups (P < 0·05; Tukey honestly significant difference test). AU, arbitrary units. Data presented are means (±SEM), with the exception of the number of cells, which are presented as medians and interquartile range.
GnIH
GnIH‐ir perikaryon area was influenced by the experimental treatments (F 2,21 = 3·67, P = 0·045). Ad libitum birds had greater GnIH‐ir perikaryon area than food‐restricted birds (Tukey HSD, P < 0·05). However, there was no effect of food availability on the number of GnIH‐ir cells (H = 0·87, 2 d.f., P = 0·65), the optical density of GnIH‐ir perikarya (F 2,21 = 3·37, P = 0·056) or the density of ME GnIH‐ir fibres (F 2,21 = 0·70, P = 0·51; Fig. 4).
NPY
The number of NPY‐ir cells was a function of food availability treatment (H = 7·56, 2 d.f., P = 0·023; Fig. 4), with food‐restricted birds having more NPY‐ir cells than ad libitum or reinstated ad libitum birds (Dunn's method, P < 0·05).
Plasma Luteinizing Hormone
There was no overall effect of food availability treatment on plasma LH (F 2,19 = 0·29, P = 0·75), nor was there an overall effect of time (F 1·5,28·6 = 0·25, P = 0·72). However, there was an interaction between treatment and time (F 3,28·6 = 3·39, P = 0·031; Fig. 5). Post hoc tests revealed that plasma LH did not change over time in either the ad libitum or the reinstated ad libitum birds (Tukey HSD, P's > 0·05), but plasma LH of food‐restricted birds decreased between weeks 0 and 4 (Tukey HSD, P < 0·05).
Figure 5.

Plasma luteinizing hormone, plasma testosterone and cloacal protuberance width were modulated by food availability in adult male Abert's Towhees Melozone aberti. Plasma luteinizing hormone (a) decreased between weeks 0 and 4 in food‐restricted birds. Plasma testosterone (b) was lower in birds exposed to two (reinstated ad libitum) or four (restricted) weeks of food restriction (70% of ad libitum consumption) compared to control birds with ad libitum food availability. Cloacal protuberance width (c) was reduced by food restriction and returning birds to ad libitum food availability increased it to a size similar to those of the control group. Points with identical letters are not significantly different (P > 0·05; Tukey honestly significant difference test). For details of study design, see Fig. 1 and the Materials and methods section. Data points are means ± SEM. For visual clarity, points have been separated along the horizontal axis.
Testicular Physiology and Morphology
There was an effect of food availability treatment on plasma T (F 2,19 = 10·97, P = 0·001), but there was no effect of time (F 2,38 = 0·042, P = 0·96) or an interaction between these factors (F 4,38 = 1·33, P = 0·28; Fig. 5). Post hoc tests revealed that plasma T of the ad libitum‐fed birds was higher than both reinstated ad libitum and food‐restricted groups (Tukey HSD, P < 0·05), and the latter two groups had similar plasma T (Tukey HSD, P > 0·05). Plasma T in the reinstated ad libitum and food‐restricted groups did not differ at any point (Tukey HSD, P > 0·05). At the time of sacrifice, there was no effect of treatment on paired testis weight (F 2,21 = 2·21, P = 0·14; Table 1) or seminiferous tubule diameter (F 2,21 = 0·61, P = 0·56; Table 1). Furthermore, spermatozoa were present in the seminiferous tubules of all birds.
Table 1.
Photoinduced testicular development of adult male Abert's Towhees Melozone aberti was not affected by food availability. Towhees were exposed to either ad libitum food availability for 4 weeks (‘ad libitum’), 2 weeks of food restriction (70% of ad libitum consumption) followed by 2 weeks of ad libitum food (‘reinstated ad libitum’) or restricted food availability for 4 weeks (‘food‐restricted’) before we collected testes. Data presented are means (± SEM)
| Food availability treatment group | Statistics | |||
|---|---|---|---|---|
| Ad libitum | Reinstated ad libitum | Food‐restricted | P‐value | |
| Paired testis mass (mg) | 348·8 (±18·1) | 357·0 (±30·1) | 294·7 (±17·0) | 0·14 |
| Seminiferous tubule diameter (μm) | 489·1 (±17·1) | 479·1 (±14·2) | 462·9 (±18·4) | 0·56 |
Cloacal Protuberance
Cloacal protuberance width was significantly affected by food availability (F 2,19 = 7·96, P = 0·003), time (F 2,38 = 8·13, P = 0·001) and the interaction between these factors (F 4,38 = 13·68, P < 0·001; Fig. 5). Cloacal protuberance width of birds exposed to ad libitum food availability increased during the first 2 weeks of the experiment (Tukey HSD, P < 0·05), but did not increase further during the last 2 weeks. Two weeks of food restriction caused cloacal protuberance width to decrease in both the reinstated ad libitum and the food‐restricted groups (Tukey HSD, P < 0·05). Cloacal protuberance width did not decrease further in birds maintained on restricted food availability, but returning birds to ad libitum food availability for 2 weeks caused cloacal protuberance width to increase to levels similar to those of the ad libitum birds.
Discussion
It has long been recognized that food availability plays a crucial proximate role in the development of gonads and secondary sexual characteristics of seasonally breeding birds (Lack 1968; Hahn et al. 2005; Williams 2012), but the mechanism mediating this role remains unclear. Food availability is thought to modulate reproductive development partly via energetic status. Limited food availability may constrain this development because birds lack the necessary energy stores for tissue growth and hormone production (Drent & Daan 1980; Wingfield & Kenagy 1986; Meijer & Drent 1999; Hahn et al. 2005). Studies in controlled laboratory conditions aimed at testing whether food availability modulates reproductive development through its effects on energetic status must, therefore, ensure that food availability treatments produce a disparity in the energetic condition. To that end, we measured body mass and energy stores (as estimated by furcular fat stores and pectoral muscle size). All of these parameters were, indeed, reduced by the food restriction regime, indicating that food‐restricted birds were in lower energetic condition than control birds. Furthermore, returning birds to ad libitum food availability caused all of these parameters to increase to levels similar to those at the beginning of the study. Therefore, the food availability treatments resulted in three groups of birds that differed in the duration that they experienced reduced energetic status.
Testicular Growth and Endocrine Activity
Despite differences in energetic status, Abert's Towhees in the three experimental groups had similar testis masses and seminiferous tubule diameters. Furthermore, the seminiferous tubules of all towhees contained spermatozoa. If testis growth and spermatogenesis are energetically expensive processes, we would have expected the energetic constraint imposed by food restriction to reduce photoinduced testis growth. As we detected no such reduction, our observations are consistent with the proposition that testis growth in the Abert's Towhee may not be particularly energetically demanding. This conclusion is consistent with results of a study by Caro & Visser (2009) in which Great Tits Parus major exposed to ambient temperatures of 8 °C or 22 °C during photoperiodically induced reproductive development had different basal metabolic rates, but showed similar testicular growth. We should point out that neither our study nor that of Caro & Visser (2009) can exclude the possibility that testis growth is energetically demanding, but when energy is limited, birds continue to allocate energy to this process at the cost of other processes. The energetic costs of testis growth and maintenance are notoriously difficult to quantify (Vézina & Salvante 2010). Estimates of the metabolic cost of testis growth based solely on tissue energy content are consistent with the proposition that this process is energetically inexpensive (Walsberg 1983). However, as others have pointed out (Vézina & Salvante 2010), these estimates fail to account for the costs of tissue synthesis, maintenance and function. Indeed, the potential costs of testicular function, particularly increased T production and secretion, are likely to be high.
Testosterone plays a key role in regulating life‐history trade‐offs in male vertebrates and promotes investment in sexual traits, which generally comes at a cost to somatic maintenance. Elevated plasma T during breeding promotes expression of male reproductive behaviours, such as singing, courtship, territorial aggression and mate guarding (Foerster et al. 2002; Hau 2007; Kurvers et al. 2008). These behaviours by themselves confer energetic costs (Oberweger & Goller 2001; Ward, Speakman & Slater 2003; Ward & Slater 2005; Hasselquist & Bensch 2008), which are likely compounded by indirect costs through reduction in the time spent at rest and for self‐maintenance behaviours (Lynn et al. 2000) and foraging (Thomas et al. 2003). Behaviours that are stimulated by T also increase predation risk (Schmidt & Belinsky 2013) and decrease survival (Reed et al. 2006). Furthermore, investment in T‐dependent sexual traits generally comes at a cost to somatic maintenance, such as immune performance (Hau 2007). We suggest, therefore, that our finding that plasma T was higher in birds with ad libitum food compared to birds that had experienced a period of food restriction is consistent with T‐mediated reproductive trade‐offs. Specifically, when energy is limited, T production and secretion may be suppressed to avoid the costs of T‐mediated male reproductive behaviours. In support of this suggestion, adult male Zebra Finches Taeniopygia guttata subjected to short‐term (4–10 h) food deprivation decreased plasma T, singing rate and courtship behaviour towards females (Lynn et al. 2010). Furthermore, spring snow storms, which likely reduced food availability and elevated thermoregulatory demands, were associated with reduced plasma T of free‐ranging male Song Sparrows Melospiza melodia (Wingfield 1985). If the hypothesis that T production and secretion are suppressed during periods of energy constraint to avoid the costs associated with T‐mediated male reproductive behaviours is correct, such a system would allow the reproductive morphology of birds to develop at approximately the correct time (as determined by photoperiod), but the characteristic male reproductive behaviours necessary for breeding may be inhibited due to low plasma T. Once food availability, and thus energetic status, is suitable, plasma T might increase, causing the expression of male reproductive behaviours and the commencement of breeding. Our finding that plasma T was still low after the reinstated ad libitum group had 2 weeks of ad libitum food access suggests, however, that there may be lasting (i.e. on the order of weeks) effects of poor energetic status on T production and secretion.
Hypothalamic and Anterior Pituitary Gland Endocrine Activity
The mechanism(s) by which energetic status affects T production is/are unclear, but potentially involves modulation at multiple levels of the HPG axis. This mechanism may involve reduced gonadotropin production and/or secretion. We found that plasma LH did not change over the study in either the ad libitum‐fed birds or the birds subjected to 2 weeks of food restriction; by contrast, food restriction for 4 weeks caused plasma LH to decrease. This finding is in line with other food availability manipulation studies of wild and domesticated avian and mammalian species, which generally show that food restriction decreases plasma LH and FSH (Tanabe, Ogawa & Nakamura 1981; Hoshino et al. 1988; Lal et al. 1990; Hahn 1995; Kobayashi, Cockrem & Ishii 2002; Hill, Elmquist & Elias 2008; but see Perfito et al. 2008). Declines in plasma gonadotropins in response to negative energetic status appear to be due to decreases in the anterior pituitary gland content of their corresponding subunit mRNAs (Kobayashi, Cockrem & Ishii 2002; Kobayashi & Ishii 2002; Ciccone, Dunn & Sharp 2007), but it remains unclear whether energetic status directly modulates levels of these mRNA subunits. Indeed, food restriction also reduces the sensitivity of the pituitary gland gonadotrope to GnRH (Tanabe, Ogawa & Nakamura 1981).
Decreased production and secretion of LH may also result from impairment of the GnRH system. In laying hens, food restriction appears to reduce the releasable store of GnRH‐I in the ME (Bruggeman et al. 1998). Furthermore, providing laying hens with ad libitum food after a period of food restriction increased hypothalamic GnRH‐I mRNA (Ciccone, Dunn & Sharp 2007). In cockerels, food restriction decreased the in vitro baseline and depolarization‐induced release of GnRH‐I from isolated mediobasal hypothalami (Lal et al. 1990). Taken together, these studies in chickens suggest that food restriction inhibits production of GnRH‐I mRNA and release of the peptide in this species. However, we found no effect of food restriction on the hypothalamic GnRH system of Abert's Towhees or on the amount of their ME GnRH‐I‐ir. It is unclear whether results in domesticated species apply to free‐ranging birds. It is possible that changes in GnRH‐I expression similar to those observed in chickens occurred in food‐restricted towhees, but these changes are not reflected in hypothalamic levels of the peptide.
The GnIH–NPY System
Alternatively, food availability may modulate the production and secretion of gonadotropins through effects on the GnIH–NPY system. There is evidence in seasonally breeding birds that GnIH controls reproductive activation through inhibitory actions on gonadotropes (Bentley et al. 2003; Calisi, Rizzo & Bentley 2008; Small et al. 2008b; Perfito et al. 2011). Furthermore, via their interactions with NPY cells in the IN, GnIH cells may modulate this inhibitory influence in response to energetic status (Clarke et al. 2009; Klingerman et al. 2011). In the present study, the area of GnIH‐ir perikarya was inversely related to the amount of time that birds experienced negative energetic status. In addition, food‐restricted birds had more IN NPY‐ir perikarya than ad libitum‐fed birds. These observations are consistent with the idea that negative energetic status was associated with upregulation of the NPY system that, in turn, promoted the release of GnIH. Enhanced GnIH secretion in food‐restricted birds may account for the reduced LH secretion and consequently plasma T in these birds.
Another neuropeptide whose activity is related to energetic status is GnRH‐II, a second form of GnRH that is highly conserved across almost all vertebrates (Schneider & Rissman 2008). In the Musk Shrew Suncus murinus midbrain levels of GnRH‐II show the opposite response to negative energetic status as compared to that of NPY: GnRH‐II content declined in response to food restriction and increased after refeeding (Kauffman et al. 2006). Crucially, these energetically aware GnRH‐II cells were associated with NPY‐ir fibres and, therefore, appear to work with NPY cells to coordinate the reproductive system response to energetic status (Bojkowska et al. 2008). Although, to our knowledge, experimental studies of the response of GnRH‐II to negative energetic status in birds are lacking, the available evidence in birds is consistent with a role similar to that in mammals (Maney, Richardson & Wingfield 1997; Stevenson & MacDougall‐Shackleton 2005; Stevenson et al. 2008; Perfito et al. 2011). In particular, GnRH‐II cells in the White‐crowned Sparrow Zonotrichia leucophrys possess putative GnIH receptors (Bentley et al. 2006). Thus, GnRH‐II may contribute to the reproductive system response to negative energetic status of birds. We suggest, therefore, that improving our understanding of the interactions between GnIH, NPY, GnRH‐II and their role in integrating information on energetic status into the reproductive system may shed light on the physiological mechanisms by which food availability influences the HPG axis.
Gonadal GnIH
Finally, food availability may affect plasma T through gonadally produced GnIH. Avian testes express this peptide and its receptors, and experimental evidence supports an inhibitory role for GnIH in the control of T secretion (Bentley et al. 2009; McGuire & Bentley 2010; Tsutsui et al. 2012), including in response to signals of metabolic stress (McGuire, Koh & Bentley 2013). Overall, the effects of food availability on T production are potentially mediated at multiple levels of the HPG axis. Further research is needed, however, to shed light on the specific mechanism(s) involved.
Secondary Sexual Characteristics
In contrast to the long‐term effects on T secretion, cloacal protuberance width appears to be highly sensitive to changes in energetic status. Indeed, 2 weeks of food restriction caused this organ to decrease in size. Furthermore, returning food‐restricted birds to ad libitum food availability for 2 weeks caused cloacal protuberance width to rapidly increase and reach a size similar to that of ad libitum‐fed birds. This increase, despite no associated change in plasma T, suggests that factors other than plasma T regulate development of the cloacal protuberance and that these factors are responsive to energetic status. The T metabolite DHT stimulates growth of the cloacal protuberance (Tramontin, Wingfield & Brenowitz 2003; Owen‐Ashley, Hasselquist & Wingfield 2004). We are aware of just one study that has examined plasma DHT in birds experiencing food restriction. In male Garden Warblers Sylvia borin caught during their spring migration, during which they also develop their gonads, food restriction reduced body mass but had no effect on plasma DHT (Bauchinger, Van't Hof & Biebach 2008). Therefore, further research is needed to examine whether DHT is also responsive to food availability.
Conclusion
Many birds use food availability as a proximate environmental cue to optimally time seasonal reproductive development. However, despite appreciating the importance of this cue for decades, the physiological mechanisms by which information on food availability is integrated into the HPG axis remain unclear. We hypothesized that seasonal reproductive activation is constrained by energetic status. Indeed, we found that energetic status modulates activity of the HPG axis in male Abert's Towhees; however, the response is complex and appears to vary with the level of the HPG axis considered. Our results are consistent with a role for the GnIH–NPY system in integrating information on energetic status at the level of the hypothalamus. However, this does not appear to involve a modulation of the amount of hypothalamic GnRH‐I. There also appears to be a role for anterior pituitary gland and/or testicular endocrine function in modulating reproductive development in the light of energetic status. Despite no evidence that energetic status modulated testicular growth, plasma LH and T were reduced in response to poor energetic status. A further illustration of the complexity by which energetic status affects the reproductive system is that the cloacal protuberance – but not LH or T secretion – was responsive to returning food‐restricted birds to ad libitum food availability. Future research is warranted to elucidate the relative importance of the hypothalamus and the gonads in integrating information on energetic status.
Supporting information
Lay Summary
Acknowledgements
This research was funded by a research grant to SD from Arizona State University's Central Arizona – Phoenix Long‐Term Ecological Research programme (CAP LTER; under National Science Foundation grant no. DEB‐0423704) and Roslin Institute Strategic Grant funding from the BBSRC (SLM). We thank Kyle Waites and Kirsten Heller for help with bird husbandry, and two anonymous reviewers who's insightful comments greatly improved the manuscript. We have no conflict of interest to declare.
Data accessibility
Data are permanently deposited and available in the NSF LTER Network data base (link: https://caplter.asu.edu/data/data-catalog/?id=613).
References
- Amann, R.P. (1986) Detection of alterations in testicular and epididymal function in laboratory animals. Environmental Health Perspectives, 70, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, J.R. (1938) The evolution of breeding seasons Evolution: Essays on Aspects of Evolutionary Biology (ed DeBeer G.B.), pp. 161–177. Clarendon Press, Oxford, UK. [Google Scholar]
- Bauchinger, U. , Van't Hof, T. & Biebach, H. (2008) Migratory stopover conditions affect the developmental state of male gonads in garden warblers (Sylvia borin). Hormones and Behavior, 54, 312–318. [DOI] [PubMed] [Google Scholar]
- Bentley, G.E. , Perfito, N. , Ukena, K. , Tsutsui, K. & Wingfield, J.C. (2003) Gonadotropin‐inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken‐gonadotropin‐releasing hormone. Journal of Neuroendocrinology, 15, 794–802. [DOI] [PubMed] [Google Scholar]
- Bentley, G.E. , Jensen, J.P. , Kaur, G.J. , Wacker, D.W. , Tsutsui, K. & Wingfield, J.C. (2006) Rapid inhibition of female sexual behavior by gonadotropin‐inhibitory hormone (GnIH). Hormones and Behavior, 49, 550–555. [DOI] [PubMed] [Google Scholar]
- Bentley, G.E. , Ubuka, T. , McGuire, N.L. , Calisi, R. , Perfito, N. , Kriegsfeld, L.J. et al (2009) Gonadotrophin‐inhibitory hormone: a multifunctional neuropeptide. Journal of Neuroendocrinology, 21, 276–281. [DOI] [PubMed] [Google Scholar]
- Bojkowska, K. , Hamczyk, M.M. , Tsai, H.‐W. , Riggan, A. & Rissman, E.F. (2008) Neuropeptide Y influences acute food intake and energy status affects NPY immunoreactivity in the female musk shrew (Suncus murinus). Hormones and Behavior, 53, 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell, T. (2001) Regulation of feeding by neuropeptide Y Avian Endocrinology (eds Dawson A. & Chaturvedi C.M.), pp. 349–360. Alpha Science, Pangbourne, UK. [Google Scholar]
- Boswell, T. , Li, Q. & Takeuchi, S. (2002) Neurons expressing neuropeptide Y mRNA in the infundibular hypothalamus of japanese quail are activated by fasting and co‐express agouti‐related protein mRNA. Molecular Brain Research, 100, 31–42. [DOI] [PubMed] [Google Scholar]
- Boyd, I.L. (1991) Environmental and physiological factors controlling the reproductive cycles of pinnipeds. Canadian Journal of Zoology, 69, 1135–1148. [Google Scholar]
- Bruggeman, V. , D'hondt, E. , Berghman, L. , Onagbesan, O. , Vanmontfort, D. , Vandesande, F. et al (1998) The effect of food intake from 2 to 24 weeks of age on LHRH‐I content in the median eminence and gonadotrophin levels in pituitary and plasma in female broiler breeder chickens. General and Comparative Endocrinology, 112, 200–209. [DOI] [PubMed] [Google Scholar]
- Calisi, R.M. , Rizzo, N.O. & Bentley, G.E. (2008) Seasonal differences in hypothalamic EGR‐1 and GnIH expression following capture‐handling stress in house sparrows (Passer domesticus). General and Comparative Endocrinology, 157, 283–287. [DOI] [PubMed] [Google Scholar]
- Caro, S.P. & Visser, M.E. (2009) Temperature‐induced elevation of basal metabolic rate does not affect testis growth in great tits. Journal of Experimental Biology, 212, 1995–1999. [DOI] [PubMed] [Google Scholar]
- Ciccone, N.A. , Dunn, I.C. & Sharp, P.J. (2007) Increased food intake stimulates GnRH‐I, glycoprotein hormone [alpha]‐subunit and follistatin mRNAs, and ovarian follicular numbers in laying broiler breeder hens. Domestic Animal Endocrinology, 33, 62–76. [DOI] [PubMed] [Google Scholar]
- Clarke, I.J. (2011) Control of GnRH secretion: one step back. Frontiers in Neuroendocrinology, 32, 367–375. [DOI] [PubMed] [Google Scholar]
- Clarke, I.J. & Parkington, H.C. (2013) Gonadotropin inhibitory hormone (GnIH) as a regulator of gonadotropes. Molecular and Cellular Endocrinology, 385, 36–44. [DOI] [PubMed] [Google Scholar]
- Clarke, I.J. , Qi, Y. , Puspita Sari, I. & Smith, J.T. (2009) Evidence that RF‐amide related peptides are inhibitors of reproduction in mammals. Frontiers in Neuroendocrinology, 30, 371–378. [DOI] [PubMed] [Google Scholar]
- Clarke, I.J. , Smith, J.T. , Henry, B.A. , Oldfield, B.J. , Stefanidis, A. , Millar, R.P. et al (2012) Gonadotropin‐inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology, 95, 305–316. [DOI] [PubMed] [Google Scholar]
- Davies, S. & Deviche, P. (2014) At the crossroads of physiology and ecology: food supply and the timing of avian reproduction. Hormones and Behavior, 66, 41–55. [DOI] [PubMed] [Google Scholar]
- Dawson, A. (1986) The effect of restricting the daily period of food availability on testicular growth of starlings Sturnus vulgaris . Ibis, 128, 572–575. [Google Scholar]
- Dawson, A. & Goldsmith, A.R. (1997) Changes in gonadotrophin‐releasing hormone (GnRH‐I) in the pre‐optic area and median eminence of starlings (Sturnus vulgaris) during the recovery of photosensitivity and during photostimulation. Journal of Reproduction and Fertility, 111, 1–6. [DOI] [PubMed] [Google Scholar]
- Dawson, A. , King, V.M. , Bentley, G.E. & Ball, G.F. (2001) Photoperiodic control of seasonality in birds. Journal of Biological Rhythms, 16, 365–380. [DOI] [PubMed] [Google Scholar]
- Deviche, P. , Sabo, J. & Sharp, P.J. (2008) Glutamatergic stimulation of luteinising hormone secretion in relatively refractory male songbirds. Journal of Neuroendocrinology, 20, 1191–1202. [DOI] [PubMed] [Google Scholar]
- Deviche, P. , Gao, S. , Davies, S. , Sharp, P.J. & Dawson, A. (2012a) Rapid stress‐induced inhibition of plasma testosterone in free‐ranging male rufous‐winged sparrows, Peucaea carpalis: characterization, time course, and recovery. General and Comparative Endocrinology, 177, 1–8. [DOI] [PubMed] [Google Scholar]
- Deviche, P. , Dawson, A. , Sabo, J. , Fokidis, B. , Davies, S. & Hurley, L. (2012b) Up to the challenge? Hormonal and behavioral responses of free‐ranging male cassin's sparrows, Peucaea cassinii, to conspecific song playback. Hormones and Behavior, 61, 741–749. [DOI] [PubMed] [Google Scholar]
- Drent, R.H. & Daan, S. (1980) The prudent parent: energetics in avian breeding. Ardea, 68, 225–252. [Google Scholar]
- Foerster, K. , Poesel, A. , Kunc, H. & Kempenaers, B. (2002) The natural plasma testosterone profile of male blue tits during the breeding season and its relation to song output. Journal of Avian Biology, 33, 269–275. [Google Scholar]
- Fokidis, H.B. , Orchinik, M. & Deviche, P. (2009) Corticosterone and corticosteroid binding globulin in birds: relation to urbanization in a desert city. General and Comparative Endocrinology, 160, 259–270. [DOI] [PubMed] [Google Scholar]
- Follett, B.K. , King, V.M. & Meddle, S.L. (1998) Rhythms and photoperiodism in birds Biological Rhythms and Photoperiodism in Plants (eds Lumsden P.J. & Millar A.J.), pp. 231–242. BIOS Scientific Publishers Ltd, Oxford, UK. [Google Scholar]
- Fraley, G.S. , Coombs, E. , Gerometta, E. , Colton, S. , Sharp, P.J. , Li, Q. et al (2013) Distribution and sequence of gonadotropin‐inhibitory hormone and its potential role as a molecular link between feeding and reproductive systems in the pekin duck (Anas platyrhynchos domestica) . General and Comparative Endocrinology, 184, 103–110. [DOI] [PubMed] [Google Scholar]
- Gosler, A.G. (1991) On the use of greater covert moult and pectoral muscle as measures of condition in passerines with data for the great tit Parus major . Bird Study, 38, 1–9. [Google Scholar]
- Greives, T.J. , Kriegsfeld, L.J. , Bentley, G.E. , Tsutsui, K. & Demas, G.E. (2008) Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction? Proceedings of the Royal Society of London. Series B: Biological Sciences, 275, 1943–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, T.P. (1995) Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the red crossbill, Loxia curvirostra (Aves: Carduelinae). Journal of Experimental Zoology, 272, 213–226. [Google Scholar]
- Hahn, T.P. (1998) Reproductive seasonality in an opportunistic breeder, the red crossbill, Loxia curvirostra . Ecology, 79, 2365–2375. [Google Scholar]
- Hahn, T.P. , Pereyra, M.E. , Katti, M. , Ward, G.M. & MacDougall‐Shackleton, S.A. (2005) Effects of food availability on the reproductive system Functional Avian Endocrinology (eds Dawson A. & Sharp P.J.), pp. 167–180. Narosa, New Delhi. [Google Scholar]
- Harrison, T.J.E. , Smith, J.A. , Martin, G.R. , Chamberlain, D.E. , Bearhop, S. , Robb, G.N. et al (2010) Does food supplementation really enhance productivity of breeding birds? Oecologia, 164, 311–320. [DOI] [PubMed] [Google Scholar]
- Hasselquist, D. & Bensch, S. (2008) Daily energy expenditure of singing great reed warblers Acrocephalus arundinaceus . Journal of Avian Biology, 39, 384–388. [Google Scholar]
- Hau, M. (2007) Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays, 29, 133–144. [DOI] [PubMed] [Google Scholar]
- Hau, M. , Wikelski, M. & Wingfield, J.C. (2000) Visual and nutritional food cues fine‐tune timing of reproduction in a neotropical rainforest bird. Journal of Experimental Zoology, 286, 494–504. [PubMed] [Google Scholar]
- Hau, M. , Wikelski, M. , Gwinner, H. & Gwinner, E. (2004) Timing of reproduction in a Darwin's finch: temporal opportunism under spatial constraints. Oikos, 106, 489–500. [Google Scholar]
- Helms, C.W. & Drury, W.H. (1960) Winter and migratory weight and fat field studies on some North American buntings. Bird Banding, 31, 1–40. [Google Scholar]
- Hill, J.W. , Elmquist, J.K. & Elias, C.F. (2008) Hypothalamic pathways linking energy balance and reproduction. American Journal of Physiology. Endocrinology and Metabolism, 294, E827–E832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, S. , Suzuki, M. , Kakegawa, T. , Imai, K. , Wakita, M. , Kobayashi, Y. et al (1988) Changes in plasma thyroid hormones, luteinizing hormone (LH), estradiol, progesterone and corticosterone of laying hens during a forced molt. Comparative Biochemistry and Physiology. Part A, Physiology, 90, 355–359. [DOI] [PubMed] [Google Scholar]
- Ims, R.A. (1990) The ecology and evolution of reproductive synchrony. Trends in Ecology & Evolution, 5, 135–140. [DOI] [PubMed] [Google Scholar]
- Jenkins, L.K. , Ross, W.L. & Young, K.A. (2007) Increases in apoptosis and declines in bcl‐XL protein characterise testicular regression in American crows (Corvus brachyrhynchos). Reproduction, Fertility and Development, 19, 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman, A.S. , Bojkowska, K. , Wills, A. & Rissman, E.F. (2006) Gonadotropin‐releasing hormone‐II messenger ribonucleic acid and protein content in the mammalian brain are modulated by food intake. Endocrinology, 147, 5069–5077. [DOI] [PubMed] [Google Scholar]
- King, J.A. & Millar, R.P. (1982) Structure of chicken hypothalamic luteinizing hormone‐releasing hormone. I. Structural determination on partially purified material. Journal of Biological Chemistry, 257, 10722–10728. [PubMed] [Google Scholar]
- Klingerman, C.M. , Williams, W.P. III , Simberlund, J. , Brahme, N. , Prasad, A. , Schneider, J.E. et al (2011) Food restriction‐induced changes in gonadotropin‐inhibiting hormone cells are associated with changes in sexual motivation and food hoarding, but not sexual performance and food intake. Frontiers in Endocrinology, 2, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, M. , Cockrem, J.F. & Ishii, S. (2002) Effects of starvation and refeeding on gonadotropin and thyrotropin subunit mRNAs in male Japanese quail. Zoological Science, 19, 449–461. [DOI] [PubMed] [Google Scholar]
- Kobayashi, M. & Ishii, S. (2002) Effects of starvation on gonadotropin and thyrotropin subunit mRNA levels and plasma hormone levels in the male japanese quail (Coturnix coturnix japonica). Zoological Science, 19, 331–342. [DOI] [PubMed] [Google Scholar]
- Kuenzel, W.J. (2000) Central nervous system regulation of gonadal development in the avian male. Poultry Science, 79, 1679. [DOI] [PubMed] [Google Scholar]
- Kuenzel, W.J. & McMurtry, J. (1988) Neuropeptide Y: brain localization and central effects on plasma insulin levels in chicks. Physiology & Behavior, 44, 669–678. [DOI] [PubMed] [Google Scholar]
- Kurvers, R.H.J.M. , Roberts, M.L. , McWilliams, S.R. & Peters, A. (2008) Experimental manipulation of testosterone and condition during molt affects activity and vocalizations of male blue tits. Hormones and Behavior, 54, 263–269. [DOI] [PubMed] [Google Scholar]
- Lack, D.L. (1968) Ecological Adaptations for Breeding in Birds. Methuen, London, UK. [Google Scholar]
- Lal, P. , Sharp, P.J. , Dunn, I.C. & Talbot, R.T. (1990) Absence of an effect of naloxone, an opioid antagonist, on luteinizing hormone release in vivo and luteinizing hormone‐releasing hormone I release in vitro in intact, castrated, and food restricted cockerels. General and Comparative Endocrinology, 77, 239–245. [DOI] [PubMed] [Google Scholar]
- Lea, R.W. , Talbot, R.T. & Sharp, P.J. (1991) Passive immunization against chicken vasoactive intestinal polypeptide suppresses plasma prolactin and crop sac development in incubating ring doves. Hormones and Behavior, 25, 283–294. [DOI] [PubMed] [Google Scholar]
- Ligon, J.D. (1974) Green cones of the pinon pine stimulate late summer breeding in the pinon jay. Nature, 250, 80–82. [DOI] [PubMed] [Google Scholar]
- Lindström, J. (1999) Early development and fitness in birds and mammals. Trends in Ecology & Evolution, 14, 343–348. [DOI] [PubMed] [Google Scholar]
- Lourdais, O. , Bonnet, X. , Shine, R. , DeNardo, D. , Naulleau, G. & Guillon, M. (2002) Capital‐breeding and reproductive effort in a variable environment: a longitudinal study of a viviparous snake. Journal of Animal Ecology, 71, 470–479. [Google Scholar]
- Lynn, S.E. , Houtman, A.M. , Weathers, W.W. , Ketterson, E.D. & Nolan, V. Jr (2000) Testosterone increases activity but not daily energy expenditure in captive male dark‐eyed juncos, Junco hyemalis . Animal Behaviour, 60, 581–587. [DOI] [PubMed] [Google Scholar]
- Lynn, S.E. , Stamplis, T.B. , Barrington, W.T. , Weida, N. & Hudak, C.A. (2010) Food, stress, and reproduction: short‐term fasting alters endocrine physiology and reproductive behavior in the zebra finch. Hormones and Behavior, 58, 214–222. [DOI] [PubMed] [Google Scholar]
- Malecki, I.A. , Martin, G.B. , O'Malley, P.J. , Meyer, G.T. , Talbot, R.T. & Sharp, P.J. (1998) Endocrine and testicular changes in a short‐day seasonally breeding bird, the emu (Dromaius novaehollandiae), in Southwestern Australia. Animal Reproduction Science, 53, 143–155. [DOI] [PubMed] [Google Scholar]
- Maney, D.L. , Goode, C.T. & Ball, G.F. (2007) Transduction of a non‐photic cue: from the auditory system to a neuroendocrine response? Journal of Ornithology, 148, 527–538. [Google Scholar]
- Maney, D.L. , Richardson, R.D. & Wingfield, J.C. (1997) Central administration of chicken gonadotropin‐releasing hormone‐II enhances courtship behavior in a female sparrow. Hormones and Behavior, 32, 11–18. [DOI] [PubMed] [Google Scholar]
- Marty, N. , Dallaporta, M. & Thorens, B. (2007) Brain glucose sensing, counterregulation, and energy homeostasis. Physiology, 22, 241–251. [DOI] [PubMed] [Google Scholar]
- McConn, B. , Wang, G. , Yi, J. , Gilbert, E.R. , Osugi, T. , Ubuka, T. et al (2014) Gonadotropin‐inhibitory hormone‐stimulation of food intake is mediated by hypothalamic effects in chicks. Neuropeptides, 48, 327–334. [DOI] [PubMed] [Google Scholar]
- McGuire, N.L. & Bentley, G.E. (2010) A functional neuropeptide system in vertebrate gonads: gonadotropin‐inhibitory hormone and its receptor in testes of field‐caught house sparrow (Passer domesticus). General and Comparative Endocrinology, 166, 565–572. [DOI] [PubMed] [Google Scholar]
- McGuire, N.L. , Koh, A. & Bentley, G.E. (2013) The direct response of the gonads to cues of stress in a temperate songbird species is season‐dependent. PeerJ, 1, e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle, S.L. , Romero, L.M. , Astheimer, L.B. , Buttemer, W.A. , Moore, I.T. & Wingfield, J.C. (2002) Steroid hormone interrelationships with territorial aggression in an arctic‐breeding songbird, gambel's white‐crowned sparrow, Zonotrichia leucophrys gambelii . Hormones and Behavior, 42, 212–221. [DOI] [PubMed] [Google Scholar]
- Meijer, T. (1991) The effect of a period of food restriction on gonad size and moult of male and female starlings Sturnus vulgaris under constant photoperiod. Ibis, 133, 80–84. [Google Scholar]
- Meijer, T. & Drent, R. (1999) Re‐examination of the capital and income dichotomy in breeding birds. Ibis, 141, 399–414. [Google Scholar]
- Morrison, A.R. , Evans, H.L. , Ator, N.A. & Nakamura, R.K. eds. (2002) NIMH (national Institute of Mental Health). Methods and Welfare Considerations in Behavioral Research with Animals: Report of a National Institutes of Health Workshop. Anonymous, U.S., Government Printing Office, Washington, DC. [Google Scholar]
- Munro, A.D. , Scott, A.P. & Lam, T.J. (1990) Reproductive Seasonality in Teleosts: Environmental Influences. CRC Press, Boca Raton, FL. [Google Scholar]
- Murton, R.K. & Westwood, N.J. (1977) Avian Breeding Cycles. Clarendon Press, Oxford. [Google Scholar]
- Nager, R.G. & van Noordwijk, A.J. (1995) Proximate and ultimate aspects of phenotypic plasticity in timing of great tit breeding in a heterogeneous environment. The American Naturalist, 146, 454–474. [Google Scholar]
- Oberweger, K. & Goller, F. (2001) The metabolic cost of birdsong production. Journal of Experimental Biology, 204, 3379–3388. [DOI] [PubMed] [Google Scholar]
- O'Brien, S. & Hau, M. (2005) Food cues and gonadal development in neotropical spotted antbirds (Hylophylax naevioides). Journal of Ornithology, 146, 332–337. [Google Scholar]
- Olive, P.J.W. , Lewis, C. & Beardall, V. (2000) Fitness components of seasonal reproduction: an analysis using Nereis virens as a life history model. Oceanologica Acta, 23, 377–389. [Google Scholar]
- Olsson, M. & Shine, R. (1998) Timing of parturition as a maternal care tactic in an alpine lizard species. Evolution, 52, 1861–1864. [DOI] [PubMed] [Google Scholar]
- Osugi, T. , Ukena, K. , Bentley, G.E. , O'Brien, S. , Moore, I.T. , Wingfield, J.C. et al (2004) Gonadotropin‐inhibitory hormone in Gambel's white‐crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. Journal of Endocrinology, 182, 33–42. [DOI] [PubMed] [Google Scholar]
- Owen‐Ashley, N.T. , Hasselquist, D. & Wingfield, J.C. (2004) Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. The American Naturalist, 164, 490–505. [DOI] [PubMed] [Google Scholar]
- Parry, D.M. , Goldsmith, A.R. , Millar, R.P. & Glennie, L.M. (1997) Immunocytochemical localization of GnRH precursor in the hypothalamus of European starlings during sexual maturation and photo refractoriness. Journal of Neuroendocrinology, 9, 235–243. [DOI] [PubMed] [Google Scholar]
- Perfito, N. , Kwong, J.M.Y. , Bentley, G.E. & Hau, M. (2008) Cue hierarchies and testicular development: is food a more potent stimulus than day length in an opportunistic breeder (Taeniopygia g. guttata)? Hormones and Behavior, 53, 567–572. [DOI] [PubMed] [Google Scholar]
- Perfito, N. , Zann, R. , Ubuka, T. , Bentley, G. & Hau, M. (2011) Potential roles for GNIH and GNRH‐II in reproductive axis regulation of an opportunistically breeding songbird. General and Comparative Endocrinology, 173, 20–26. [DOI] [PubMed] [Google Scholar]
- Pralong, F.P. (2010) Insulin and NPY pathways and the control of GnRH function and puberty onset. Molecular and Cellular Endocrinology, 324, 82–86. [DOI] [PubMed] [Google Scholar]
- Pyle, P. (1997) Identification Guide to North American Birds, Part 1: Columbidae to Ploceidae. Braun‐Brumfield Inc, Bolinas, CA. [Google Scholar]
- Qi, Y. , Oldfield, B.J. & Clarke, I.J. (2009) Projections of RFamide‐related peptide‐3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. Journal of Neuroendocrinology, 21, 690–697. [DOI] [PubMed] [Google Scholar]
- Reed, W.L. , Clark, M.E. , Parker, P.G. , Raouf, S.A. , Arguedas, N. , Monk, D.S. et al (2006) Physiological effects on demography: a long‐term experimental study of testosterone's effects on fitness. The American Naturalist, 167, 667–683. [DOI] [PubMed] [Google Scholar]
- Saldanha, C.J. , Deviche, P.J. & Silver, R. (1994) Increased VIP and decreased GnRH expression in photorefractory dark‐eyed juncos (Junco hyemalis). General and Comparative Endocrinology, 93, 128–136. [DOI] [PubMed] [Google Scholar]
- Salvante, K.G. , Walzem, R.L. & Williams, T.D. (2007) What comes first, the zebra finch or the egg: temperature‐dependent reproductive, physiological and behavioural plasticity in egg‐laying zebra finches. The Journal of Experimental Biology, 210, 1325–1334. [DOI] [PubMed] [Google Scholar]
- Schaper, S.V. , Dawson, A. , Sharp, P.J. , Caro, S.P. & Visser, M.E. (2012a) Individual variation in avian reproductive physiology does not reliably predict variation in laying date. General and Comparative Endocrinology, 179, 53–62. [DOI] [PubMed] [Google Scholar]
- Schaper, S.V. , Dawson, A. , Sharp, P.J. , Gienapp, P. , Caro, S.P. & Visser, M.E. (2012b) Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. The American Naturalist, 179, E55–E69. [DOI] [PubMed] [Google Scholar]
- Scheuerlein, A. & Gwinner, E. (2002) Is food availability a circannual zeitgeber in tropical birds? A field experiment on stonechats in tropical Africa. Journal of Biological Rhythms, 17, 171–180. [DOI] [PubMed] [Google Scholar]
- Schmidt, K.A. & Belinsky, K.L. (2013) Voices in the dark: predation risk by owls influences dusk singing in a diurnal passerine. Behavioral Ecology and Sociobiology, 67, 1837–1843. [Google Scholar]
- Schneider, J.S. & Rissman, E.F. (2008) Gonadotropin‐releasing hormone II: a multi‐purpose neuropeptide. Integrative and Comparative Biology, 48, 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoech, S.J. (1996) The effect of supplemental food on body condition and the timing of reproduction in a cooperative breeder, the Florida scrub‐jay. Condor, 98, 234–244. [Google Scholar]
- Schoech, S.J. (2009) Food supplementation experiments: a tool to reveal mechanisms that mediate timing of reproduction. Integrative and Comparative Biology, 49, 480–492. [DOI] [PubMed] [Google Scholar]
- Sharp, P.J. (2005) Photoperiodic regulation of seasonal breeding in birds. Annals of the New York Academy of Sciences, 1040, 189–199. [DOI] [PubMed] [Google Scholar]
- Sharp, P.J. & Ciccone, N. (2005) The gonadotrophin releasing hormone neurone: key to avian reproductive function Functional Avian Endocrinology (eds Dawson A. & Sharp P.J.), pp. 59–72. Narosa, New Delhi. [Google Scholar]
- Sharp, P.J. , Dunn, I.C. & Talbot, R.T. (1987) Sex differences in the LH responses to chicken LHRH‐I and ‐II in the domestic fowl. Journal of Endocrinology, 115, 323–331. [DOI] [PubMed] [Google Scholar]
- Small, T.W. , Sharp, P.J. & Deviche, P. (2007) Environmental regulation of the reproductive system in a flexibly breeding Sonoran desert bird, the rufous‐winged sparrow, Aimophila carpalis . Hormones and Behavior, 51, 483–495. [DOI] [PubMed] [Google Scholar]
- Small, T.W. , Sharp, P.J. , Bentley, G.E. , Millar, R.P. , Tsutsui, K. , Strand, C. et al (2008a) Auditory stimulation of reproductive function in male rufous‐winged sparrows, Aimophila carpalis . Hormones and Behavior, 53, 28–39. [DOI] [PubMed] [Google Scholar]
- Small, T.W. , Sharp, P.J. , Bentley, G.E. , Millar, R.P. , Tsutsui, K. , Mura, E. et al (2008b) Photoperiod‐independent hypothalamic regulation of luteinizing hormone secretion in a free‐living Sonoran desert bird, the Rufous‐winged sparrow (Aimophila carpalis). Brain, Behavior and Evolution, 71, 127–142. [DOI] [PubMed] [Google Scholar]
- Stevenson, T.J. & MacDougall‐Shackleton, S.A. (2005) Season‐and age‐related variation in neural cGnRH‐I and cGnRH‐II immunoreactivity in house sparrows (Passer domesticus). General and Comparative Endocrinology, 143, 33–39. [DOI] [PubMed] [Google Scholar]
- Stevenson, T.J. , Bentley, G.E. , Ubuka, T. , Arckens, L. , Hampson, E. & MacDougall‐Shackleton, S.A. (2008) Effects of social cues on GnRH‐I, GnRH‐II, and reproductive physiology in female house sparrows (Passer domesticus). General and Comparative Endocrinology, 156, 385–394. [DOI] [PubMed] [Google Scholar]
- Stokes, T.M. , Leonard, C.M. & Nottebohm, F. (1974) The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. The Journal of Comparative Neurology, 156, 337–374. [DOI] [PubMed] [Google Scholar]
- Strader, A. & Buntin, J. (2001) Neuropeptide‐Y: a possible mediator of prolactin‐induced feeding and regulator of energy balance in the ring dove (Streptopelia risoria). Journal of Neuroendocrinology, 13, 386–392. [DOI] [PubMed] [Google Scholar]
- Tachibana, T. , Sato, M. , Takahashi, H. , Ukena, K. , Tsutsui, K. & Furuse, M. (2005) Gonadotropin‐inhibiting hormone stimulates feeding behavior in chicks. Brain Research, 1050, 94–100. [DOI] [PubMed] [Google Scholar]
- Tachibana, T. , Masuda, N. , Tsutsui, K. , Ukena, K. & Ueda, H. (2008) The orexigenic effect of GnIH is mediated by central opioid receptors in chicks. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 150, 21–25. [DOI] [PubMed] [Google Scholar]
- Tanabe, Y. , Ogawa, T. & Nakamura, T. (1981) The effect of short‐term starvation on pituitary and plasma LH, plasma estradiol and progesterone, and on pituitary response to LH‐RH in the laying hen (Gallus domesticus). General and Comparative Endocrinology, 43, 392–398. [DOI] [PubMed] [Google Scholar]
- Thomas, R.J. , Cuthill, I.C. , Goldsmith, A.R. , Cosgrove, D.F. , Lidgate, H.C. & Burdett Proctor, S.L. (2003) The trade‐off between singing and mass gain in a daytime‐singing bird, the European robin. Behaviour, 140, 387–404. [Google Scholar]
- Tramontin, A.D. , Wingfield, J.C. & Brenowitz, E.A. (2003) Androgens and estrogens induce seasonal‐like growth of song nuclei in the adult songbird brain. Journal of Neurobiology, 57, 130–140. [DOI] [PubMed] [Google Scholar]
- Tsutsui, K. , Saigoh, E. , Ukena, K. , Teranishi, H. , Fujisawa, Y. , Kikuchi, M. et al (2000) A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochemical and Biophysical Research Communications, 275, 661–667. [DOI] [PubMed] [Google Scholar]
- Tsutsui, K. , Bentley, G.E. , Bedecarrats, G. , Osugi, T. , Ubuka, T. & Kriegsfeld, L.J. (2010) Gonadotropin‐inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Frontiers in Neuroendocrinology, 31, 284–295. [DOI] [PubMed] [Google Scholar]
- Tsutsui, K. , Ubuka, T. , Bentley, G.E. & Kriegsfeld, L.J. (2012) Gonadotropin‐inhibitory hormone (GnIH): discovery, progress and prospect. General and Comparative Endocrinology, 177, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui, K. , Ubuka, T. , Bentley, G.E. & Kriegsfeld, L.J. (2013) Review: regulatory mechanisms of gonadotropin‐inhibitory hormone (GnIH) synthesis and release in photoperiodic animals. Frontiers in Neuroscience, 7, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka, T. , Lai, H. , Kitani, M. , Suzuuchi, A. , Pham, V. , Cadigan, P.A. et al (2009) Gonadotropin‐inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. The Journal of Comparative Neurology, 517, 841–855. [DOI] [PubMed] [Google Scholar]
- Vézina, F. & Salvante, K.G. (2010) Behavioral and physiological flexibility are used by birds to manage energy and support investment in the early stages of reproduction. Current Zoology, 56, 767–792. [Google Scholar]
- Walsberg, G.E. (1983) Avian ecological energetics Avian Biology (eds Farner D.S., King J.R. & Parkes K.C.), pp. 161–220. Academic Press, New York, NY, USA. [Google Scholar]
- Ward, S. & Slater, P.J.B. (2005) Raised thermoregulatory costs at exposed song posts increase the energetic cost of singing for willow warblers Phylloscopus trochilus . Journal of Avian Biology, 36, 280–286. [Google Scholar]
- Ward, S. , Speakman, J.R. & Slater, P.J.B. (2003) The energy cost of song in the canary, Serinus canaria . Animal Behaviour, 66, 893–902. [Google Scholar]
- Watson, R.E. , Wiegand, S.J. , Clough, R.W. & Hoffman, G.E. (1986) Use of cryoprotectant to maintain long‐term peptide immunoreactivity and tissue morphology. Peptides, 7, 155–159. [DOI] [PubMed] [Google Scholar]
- Williams, T.D. (2012) Physiological Adaptations for Breeding in Birds, Princeton University Press, Princeton, NJ, USA. [Google Scholar]
- Wingfield, J.C. (1980) Fine temporal adjustment of reproductive functions Avian Endocrinology (eds Epple A. & Stetson M.H.), pp. 367–389. Academic Press, New York, NY. [Google Scholar]
- Wingfield, J.C. (1985) Influences of weather on reproductive function in male song sparrows, Melospiza melodia . Journal of Zoology, 205, 525–544. [Google Scholar]
- Wingfield, J.C. & Kenagy, G.J. (1986) Natural regulation of reproductive cycles Vertebrate Endocrinology: Fundamentals and Biomedical Implications (eds Pang P.K.T. & Schreibman M.P.), pp. 181–241. Academic Press, San Diego, CA. [Google Scholar]
- Wingfield, J.C. & Wada, M. (1989) Changes in plasma levels of testosterone during male‐male interactions in the song sparrow, Melospiza melodia: time course and specificity of response. Journal of Comparative Physiology A, 166, 189–194. [Google Scholar]
- Wingfield, J.C. , Hahn, T.P. , Maney, D.L. , Schoech, S.J. , Wada, M. & Morton, M.L. (2003) Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white‐crowned sparrows, Zonotrichia leucophrys oriantha . General and Comparative Endocrinology, 131, 143–158. [DOI] [PubMed] [Google Scholar]
- Wingfield, J.C. , Sullivan, K. , Jaxion‐Harm, J. & Meddle, S.L. (2012) The presence of water influences reproductive function in the song sparrow (Melospiza melodia morphna). General and Comparative Endocrinology, 178, 485–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lay Summary


