Abstract
Nuclear localization signal retinoic acid receptor alpha(NLS-RARα), which forms from the cleavage of promyelocytic leukemia-retinoic acid receptor alpha(PML-RARα) protein by neutrophil elastase(NE), possesses an important role in the occurrence and development of acute promyelocytic leukemia(APL). However, the potential mechanism underlying the effects of NLS-RARα on APL is still not entirely clear. Here, we investigated the effects of NLS-RARα on APL NB4 cells and its mechanism. We found that all-trans retinoic acid(ATRA) could promote differentiation while inhibit proliferation of APL NB4 cells via upregulating the expression of phosphorylated p38α mitogen-activated protein kinase(p-p38α MAPK). We also found that NLS-RARα could inhibit differentiation while accelerate proliferation of NB4 cells via downregulating the expression of p-p38α protein in the presence of ATRA. Furthermore, immunofluorescence and co-immunoprecipitation assays confirmed NLS-RARα interacted with p38α protein directly. Finally, application of PD169316, an inhibitor of p38α protein, suggested that recruitment p38α-combinded NLS-RARα by ATRA eventually caused activation of p38α protein. In summary, our study demonstrated that ATRA cound promote differentiation while inhibit proliferation of APL NB4 cells via activating p38α protein after recruiting p38α-combinded NLS-RARα, while NLS-RARα could inhibit the effects of ATRA in the process.
Keywords: acute promyelocytic leukemia, NB4 cells, all-trans retinoic acid, nuclear localization signal retinoic acid receptor alpha, p38α MAPK.
Introduction
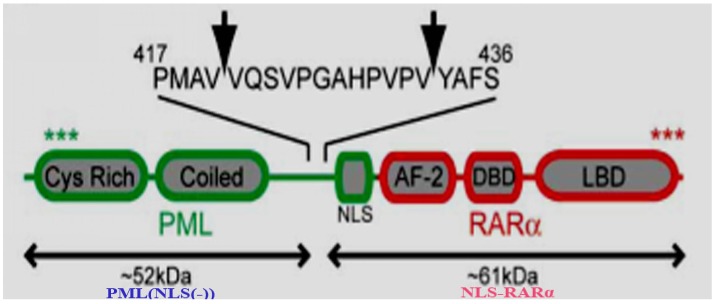
Acute promyelocytic leukemia (APL) is a normal type of acute myeloid leukemia (AML), in which leukemia cells possess the ability to infinitely proliferate. Moreover, cell differentiation in APL is suppressed at immature stages due to the fusion protein, PML-retinoic acid receptor alpha(PML-RARα)1, 2, a strong transcriptional repressor for genes involved in granulocyte differentiation3. PML-RARα is formed by the chromosomal translocation of the RARα gene on chromosome 17 to the PML gene on chromosome 154. It has been found that neutrophil elastase (NE) in early APL cells can cleave PML-RARα into two mutational proteins, PML (NLS(-)) and nuclear localization signal NLS-RARα (Figure 1), which was significant for the development of APL5, 6. Previous researchers have studied the function of the mutational protein, NLS-RARα, and have verified that it can accelerate the proliferation of NB4 cells while inhibit differentiation of HL60 cells 7, 8. Moreover, the function of NLS-RARα proved to be linked with Akt signaling pathway 8.
Figure 1.
Identification of NE cleavage sites in PML-RARα5.Arrows indicate the position of NE cleavage within the PML portion of PML-RARα, after V420 and V432. The approximate expected sizes of the peptide fragments generated by these cleavage events are shown. Several known domains in PML-RARα are labeled: cystine-rich RING/B Box domain(Cys Rich), helical coiled-coil domain(Coiled), nuclear localization signal(NLS), transcriptional activation domain(AF-2),DNA binding domain(DBD), and the ligand binding domain(LBD).
MAPK family members and Akt signaling pathway played crucial roles in the clonal formation of KG1a cells, which mimic a CD34+ cell model 9. In addition to hematological tumors, researchers have found that p38 MAPK and Akt pathways played significant roles in myogenesis and muscle differentiation 10-12. Furthermore, it has been demonstrated that transcription activity of RARα on target genes decreased when it directly interacted with p38α MAPK in the presence of ATRA 13, which is a drug used to treat APL 14.
Thus, we speculated that the activity of p38α MAPK may influence the proliferation and differentiation of APL NB4 cells, and the effects of NLS-RARα on differentiation and proliferation of NB4 cells may be related to the activity of p38α MAPK. Then we explored the potential mechanism underlying the effects of NLS-RARα on NB4 cells.
Materials and Methods
Cell lines
APL cell line NB4 cells, NB4 cells infected with lentivirus only(LV-NC-NB4 ) and NB4 cells infected with NLS-RARα-lentivirus(LV-NLS-RARα-NB4) cells were saved by our own laboratory, and cultured in RPMI-1640 medium supplemented with 10 % fetal bovine serum(FBS; Gibco, Australia) in an environment with 5 % CO2 at 37°C.
293T cells were saved by our own laboratory and cultured in DMEM medium supplemented with 10 % fetal bovine serum (FBS; Gibco, USA) in an environment with 5 % CO2 at 37°C.
CCK-8 assay
Cell proliferation was quantified by CCK-8 kit (7Sea Cell Counting Kit; Sevenseas Futai Biotechnology Co., Ltd.,Shanghai, China). Cells in each group were seeded in 96-well plates at a density of 5000 cells/well. Then cells were incubated with various of treatments for 3 days. In brief, 10μl of CCK-8 assay was added to each well followed by incubation for 1h at 37°C. The cell proliferation was assessed by detection of absorbance at 450 nm using a spectrophotometer. The optical density value is positive correction with cell proliferation.
Western blot
Cells in each group were washed with ice-cold phosphate-buffered saline (PBS) three times and lysed in RIPA solution containing protease inhibitor phenylmethanesulfonyl fluoride (PMSF), phosphatase inhibitor NaF and Na3VO3. Protein concentration was measured by BCA method. 50 μg total protein was added in 10% sodium dodecyl sulfate-polyacrylamide gel and then transfered to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk for 1 hour and incubated with specific antibodies(polyclonal antibody against p-p38α MAPK; 1:1000; Millipore, USA; polyclonal antidoby against p38α MAPK, HA-Tag; 1:1000; CST, USA; monoclonal antibody against Myc-Tag; 1:1000; CST, USA; polyclonal antibody against RARα; 1:1000; Santa Cruz, USA; polyclonal antibody against C/EBPβ, CD11b; 1:500; Wanleibio; China) overnight at 4 °C and then with secondary antibody(goat anti-rabbit antibody, 1:5000 and goat anti-mouse antibody, 1:2000; Zhongshan Goldenbridge Biotechnology Co. Ltd., Beijing, China) for 1 h at 37 °C. After washing with Tris-Buffered Saline Tween-20 and Tris-Buffered Saline (TBST and TBS), the autoradiograms were scanned and subjected to densitometry. β-actin (monoclonal antibody against β-actin, 1:1000; Zhongshan Goldenbridge Biotechnology Co. Ltd.,Beijing, China) was used as an internal control.
Construction of eukaryotic expression vectors of pCMV-Myc-p38α and LV-NLS-RARα
Primer sequences of p38α(forward: TAA CTCGAG TAA TGT CTC AG G AGA GGC CCA CGT; reverse: TAT TAA GCGGCCGC TCA GGA CTC CAT CTC TTC TTGG) were designed by Primer-Premier 5.0 and synthesized by Sangon Biotech company. Underlined sequences are Restriction Enzyme cutting sequences (Xho1; Not1). cDNAs synthesized from RNA which was extracted from APL NB4 cells were used as PCR(Polymerase Chain Reaction) templates. Reaction system components: PrimeSTARTMHS(Premix)(TaKaRa, Japan), cDNAs, primers of p38α and ddH2O. The PCR conditions were: pre-denaturation at 95°C for 5 min, 29 cycles of denaturation at 98 °C for 10 s, annealing at 68.8 °C for 30 s, and extension at 72 °C for 80s, and a final extension at 72 °C for 5 min. PCR product was CDS of p38α with 1083 bp. Purified products with E.Z.N.A Gel Extraction kit(OMEGA, USA), digested products and pCMV-Myc vector with Restriction Enzyme Xho1 and Not1(Xho1, Xho1; NEB, England), connected products of p38α to vector with T4 DNA ligase (TaKaRa,, Japan). Then transformated connected products into competence DH5α and amplified by bacteria culture. After sequences were proved accurate, Q-PCR (quantitative polymerase chain reaction) and western blot verified pCMV-Myc-p38α MAPK expression plasmid.
Construction of LV-NLS-RARα was as described 8.
Co-immunoprecipitation assay
The binding activity of proteins was determined by co-immunoprecipitation (Co-IP) assay. For this study, total cell lysates were incubated with the desired antibodies for 16 h at 4 °C and the immuno-complex was collected on Protein A/G PLUS-Agarose(Santa Cruz, USA) for 5 h and washed 3 times with lysis buffer prior to boiling in SDS sample buffer. Immunoprecipitated proteins were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes for Western blot analysis.
Indirect immunofluorescence assay
The localization between p38α protein and NLS-RARα was confirmed by indirect immunofluorescence assay. LV-NLS-RARα-NB4 cells suspension were collected, centrifuged and washed by PBS for three times. Cells on glass coverslips were fixed with 4% paraformaldehyde for 20 minutes. Subsequently, cells were permeabilized and blocked respectively with 0.1% Triton X-100(in PBS) and 10% goat serum (in PBS) for 30 minutes at room temperature. For immunofluorescence staining, the rabbit polyclonal antibody against p38α protein, mouse monoclonal antibody against HA-tag(p38α, HA-tag; 1:100; CST, USA) were used to probe p38α protein and NLS-RARα(as NLS-RARα was inserted to eukaryotic expression vector of pCMV-HA) overnight at 4 °C and goat against rabbit-IgG-TRITC, goat against mouse-IgG-FITC(rabbit-IgG-TRITC, mouse-IgG-FITC; 1:200; Zhongshan Goldenbridge Biotechnology Co. Ltd., Beijing, China) was used to detect rabbit and mouse IgG for 1 h at room temperature. Finally, nuclei were stained by DAPI (Beyotime; 1:10) at room temperature for 5 min. All the coverslips were washed with PBS for three times. The coverslips were immobilized on the glass slides by 70% glycerol in PBS and viewed under a fluorescence microscope (Nikon, Tokyo, Japan).
Dual luciferase reporter assay
293T cells were transiently transfected with 0.4μg pTK-Luc reporter plasmids, 0.01μg pRLtk(Promega), and 0.3μg expression vectors per well of 24 wells plate when cell confluence was about 70%. Cells were cultured in medium containing 10% FBS for 6 hours and then the medium was replaced. After 24 h of transfection (LipoFiterTM Liposomal Transfection Reagent; HANBIO, China), cells were treated for a further 24 h with 10 nM ATRA, then cells were lysed and normalized luciferase activities were determined.
Statistical analysis
All the data were presented as the mean ± SD. Student's t-test was applied for the statistical analysis of three independent groups by GraphPad Prism 5 software. For all tests, *p < 0.05 or **p < 0.01 was considered statistically significant. NS was considered no statistically significant.
Results
Promotion of differentiation while suppression of proliferation of NB4 cells correlated with the activation of p38α protein by ATRA
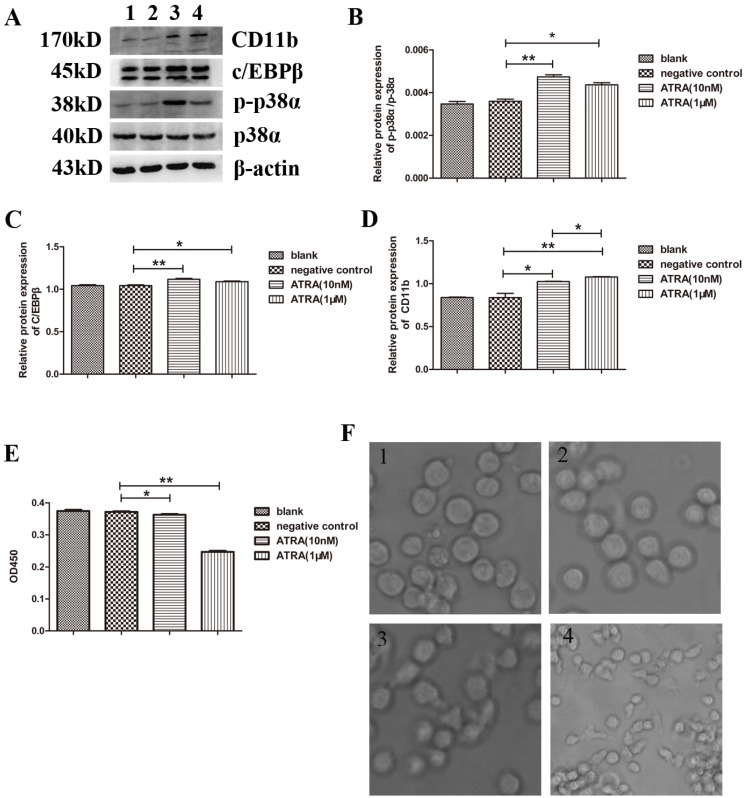
It has been shown that ATRA inhibits differentiation of APL cells while accelerate their proliferation. Moreover, the effects of ATRA have been correlated with activation of p38α MAPK 13, 15, 16. To verify this, and to discover an ATRA concentration that is related to both the biological function(differentiation and proliferation) and the activity of p38α protein in NB4 cells, we first examined the expressions of p-p38α and p38α proteins after treating NB4 cells with ATRA (physiological concentration: 10 nM and pharmacological concentration: 1 µM) for 3 days. We found that the expression of p38α protein was maintained, regardless of ATRA treatment. However, the expression of p-p38α protein increased obviously in the experimental group compared to the negative control (dimethylsulfoxide(DMSO)-treated) group, especially when cells were treated with 10 nM ATRA (Figure 2A and 2B). We further determined the expressions of C/EBPβ, a myeloid differentiation marker protein 17, 18, and CD11b, a surface myeloid differentiation marker protein 13, 19, 20. We found that the expressions of C/EBPβ and CD11b proteins were significantly increased after treating NB4 cells with ATRA (Figure 2A, 2C, and 2D). Moreover, changing trend of C/EBPβ protein paralleled that of p-p38α protein. Based on the above results, we surmised that the differentiation of NB4 cells related to the activity of p38α protein. Next, we determined NB4 cell proliferation with cell counting kit and observed morphological characteristics with an inverted microscope (Figure 2E and 2F). As expected, both concentrations of ATRA inhibited NB4 cell proliferation. Taken together, the above results suggested that not only the differentiation but also the proliferation of NB4 cells correlated with the activation of p38α protein by ATRA.
Figure 2.
Promotion of differentiation while suppression of proliferation of NB4 cells correlated with the activation of p38α protein by ATRA. (A) Western blot analysis of the expressions of p38α, p-p38α, differentiation makers, C/EBPβ and CD11b, in NB4 cells cultured with ATRA for 3 days; (B-D) Quantitative analysis of the expression levels of p-p38α/p38α, C/EBPβ, CD11b after normalization with β-actin; (E) NB4 cells were treated with ATRA for 3 days. Then the optical density value at 450nm(OD450) was measured by CCK-8 assay; (F) NB4 cells were treated with ATRA for 3 days. Then Observed morphological characteristics using an inverted microscope. All data are presented as mean±SD. * p < 0.05, ** p < 0.01. (1: blank group(non-manipulated); 2: negative group(dimethylsulfoxide(DMSO)-treated); 3: experimental group treated with 10 nM ATRA; 4: experimental group treated with 1 µM ATRA)
Promotion of cell differentiation while suppression of cell proliferation resulted from activation of p38α protein by ATRA
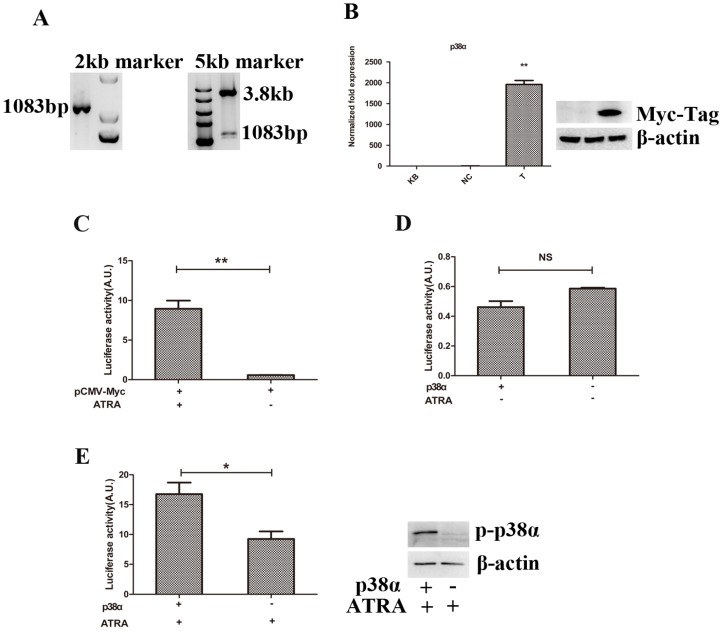
We have known that RARα could regulate the expressions of genes involved in cell differentiation and proliferation after binding to retinoid-responsive elements (RARE) in genes 21, 22, 24-26. However, p38α protein can interact with RARα directly to inhibit the transcriptional activity of RARα in the presence of ATRA 13. Thus, we speculated that p38α protein could regulate the expressions of genes involved in cell differentiation and proliferation similar to RARα. We first constructed a eukaryotic expression plasmid of pCMV-Myc-p38α and tested the availability of the retinoid-responsive reporter plasmid, pTK-Luc, offered by Professor Dmitrii Kamashev and his partners in France 23. The eukaryotic expression plasmid of pCMV-Myc-p38α was successfully constructed (Figure 3A-3B) and pTK-Luc plasmid was proved to be available (Figure 3C).
Figure 3.
Promotion of cell differentiation while suppression of cell proliferation resulted from activation of p38α protein by ATRA. (A) PCR combined with restriction enzyme digestion analysis of p38α gene; (B) RT-qPCR and Western blot analysis of the expression of Myc-tagged p38α gene; (C, D, E) 293T cells were transfected with pCMV-Myc-p38α or pCMV-Myc plasmids(0.3μg), retinoid-responsive reporter plasmid, pTK-Luc(0.4μg), and pRLtk plasmids(0.01μg). 24 hours later, cells were treated with 10 nM ATRA or vehicle for another 24 hours. Then cells were lysed and normalized luciferase activities were determined. All data were presented as mean±SD. * p<0.05, ** p < 0.01, NS: not statistical significant. (KB group: 293T cells were non-manipulated, NC group: 293T cells were transfected with pCMV-Myc plasmids, T group: 293T cells were transfected with pCMV-Myc-p38α plasmid).
Next, We co-transfected 293T cells with plasmids and then treated cells with ATRA or vehicle. As expected, changes of luciferase activity in two groups had no statistical significance when cells treated with vehicle (Figure 3D). However, when cells treated with ATRA, luciferase activity and the expression of p-p38α protein remarkably increased compared to the negative control group (Figure 3E). It suggested that promotion of cell differentiation while suppression of cell proliferation resulted from activation of p38α protein rather than resulted in activation of p38α protein.
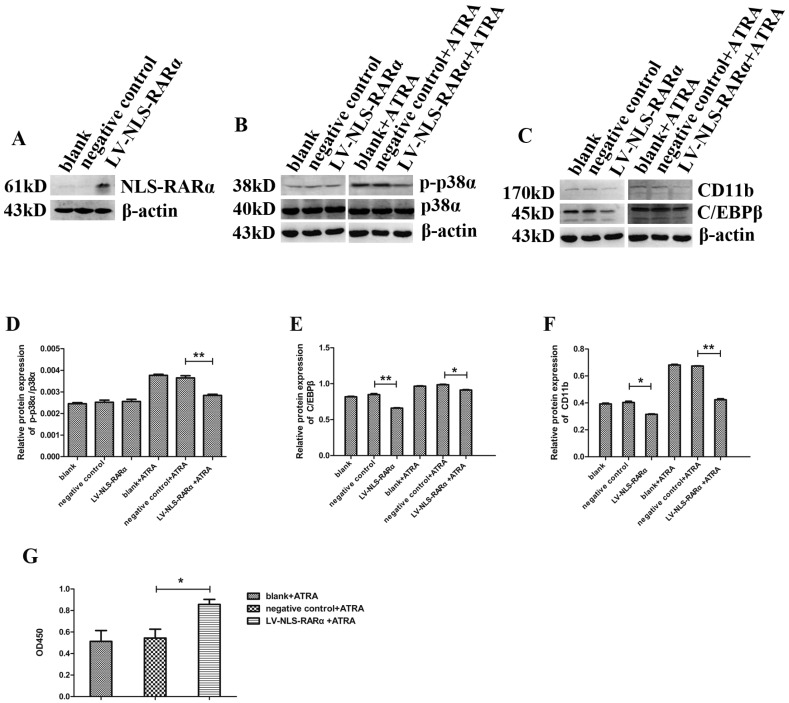
ATRA application led to NLS-RARα-induced differential inhibition while accelerated proliferation of NB4 cells via down regulating p-p38α protein level
We next explored the effects of NLS-RARα on NB4 cells and its underlying mechanism. First, we established an early APL cell model through lentivirus-mediated overexpression of NLS-RARα in NB4 cells (Figure 4A). Then, Western blot detected the expressions of various proteins. Compared to the negative control group, the expressions of p38α and p-p38α proteins maintained (Figure 4B, 4D), while the expressions of differentiation markers, C/EBPβ and CD11b decreased (Figure 4C, 4E-4F). We have observed that the effects of p-p38α protein on differentiation and proliferation of NB4 cells were related to ATRA. Thus, we next detected the expressions of various proteins and cell proliferation after treating cells with 10 nM ATRA for 3 days. Compared to the negative control group, the expressions of p-p38α, C/EBPβ, and CD11b proteins decreased while the expression of p38α protein maintained (Figure 4B). Cell proliferation accelerated when compared to the negative control group (Figure 4G). These data suggested that NLS-RARα could inhibit differentiation while accelerate proliferation of NB4 cells via down regulating p-p38α protein level in the presence of ATRA. These data also implied that NLS-RARα could inhibit differentiation of NB4 cells via an alternative pathway in the absence of ATRA.
Figure 4.
ATRA application led to NLS-RARα-induced differential inhibition while accelerated proliferation of NB4 cells via down regulating p-p38α protein level. (A)Western blot analysis of the overexpression of NLS-RARα in NB4 cells; (B, C) Western blot analysis the expressions of p38α, p-p38α, C/EBPβ and CD11b proteins in NB4 cells after treating cells with/without 10 nM ATRA for 3 days; (D-F) Quantitative analysis of the expression levels of p-p38α/p38α, C/EBPβ, CD11b proteins after normalization with β-actin; (G) NB4 cells were treated with 10 nM ATRA for 3 days. Then the optical density value at 450nm(OD450) was measured by CCK-8 assay. All data are presented as mean±SD. * p < 0.05, p < 0.01.(blank group: non-manipulated, negative control group: lentivirus only).
Downregulation of p-p38α protein level may be induced by a direct interaction between NLS-RARα and p38α protein
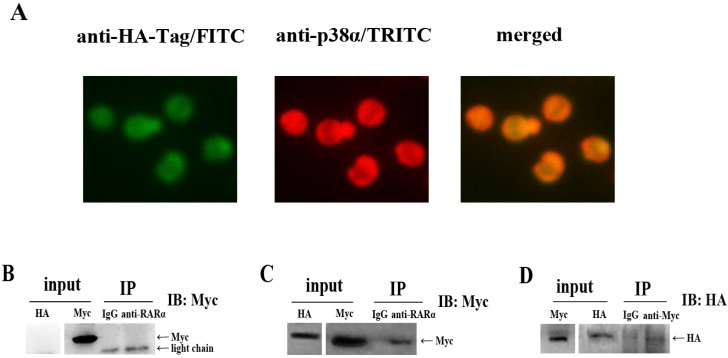
To investigate the underlying mechanism by which NLS-RARα induced downregulation of p-p38α protein level, we carried out an indirect immunofluorescence assay. As shown in Figure 5A, cells appeared yellow, indicating NLS-RARα (green) merged with p38α protein (red). These findings suggested close localization of NLS-RARα and p38α protein in three-dimensional space. However, this assay couldn't distinguish whether it's indirect or direct interaction between them. Therefore, we performed a co-immunoprecipitation experiment in 293T cells. Immunoprecipitation with an anti-RARα antibody resulted in co-precipitation of p38α protein (Myc-tagged) only in cells that overexpressed both proteins (Figure 5C). This interaction was confirmed when NLS-RARα (HA-tagged) instead of p38α protein, was co-immunoprecipitated in complementary experiments (Figure 5D).
Figure 5.
Downregulation of p-p38α protein level may be induced by a direct interaction between NLS-RARα and p38α protein. (A) Immunofluorescence microscopy of NLS-RARα staining with FITC(green) and p38α protein staining with TRITC(red) in NLS-RARα-overexpressed NB4 cells; (B, C) NLS-RARα was immunoprecipitated by anti-RARα, p38α protein(Myc-tagged) was detected by Western blot; (D) p38α was immunoprecipitated by anti-Myc, NLS-RARα (HA-tagged) was detected by Western blot. IgG was used as control antibody. The overexpressions of p38α protein and NLS-RARα(input) were determineded with Western blot analysis.
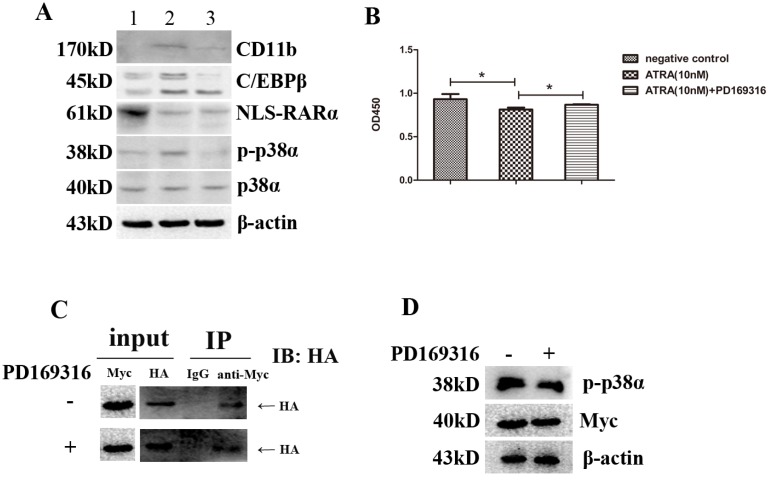
Recruitment p38α-combinded NLS-RARα by ATRA eventually caused activation of p38α protein
We have demonstrated that ATRA could promote differentiation while inhibit proliferation of NB4 cells via activating p38α protein. We also found NLS-RARα could inhibit those effects of ATRA by interacting with p38α protein directly. However, it is faint ATRA directly activates p38α protein and then recruits NLS-RARα or recruitment p38α-combinded NLS-RARα by ATRA eventually causes activation of p38α protein. In order to clarify this question, we investigated the effects of an inhibitor of p38 protein, PD169316 (MCE, USA). We found that PD169316 (10 μM) inhibited the effects of ATRA on NLS-RARα-overexpressed NB4 cells incluing an increase of the expressions of p-p38α, C/EBPβ and CD11b(Figure 6A). It also inhibited the effect of ATRA on NLS-RARα-overexpressed NB4 cell proliferation (Figure 6B). However, it didn't change the effect of ATAR on the expression of NLS-RARα in NLS-RARα-overexpressed NB4 cells (Figure 6A). Moreover, PD169316 (10 μM) couldn't block the direct interaction between NLS-RARα and p38α protein (Figure 6C). Taken together, it suggested that direct interaction between NLS-RARα and p38α protein was naturally existed in cells no matter whether p38α protein was activated or not. Taking this one step further, ATRA recruited p38α-combinded NLS-RARα then caused activation of p38α protein.
Figure 6.
Recruitment p38α-combinded NLS-RARα by ATRA eventually caused activation of p38α protein. (A) Western blot analysis of the expressions of p38α, p-p38α, NLS-RARα, C/EBPβ and CD11b proteins in cultured NLS-RARα-overexpressed NB4 cells after dealing cells with various treatments for 3 days. (B) The proliferation of NLS-RARα-overexpressed NB4 cells in different groups was determined with CCK-8 Kit. (C) NLS-RARα(HA-tagged) was co-immunoprecipitated by anti-Myc in different groups. (D) The expressions of p38α protein(Myc-tagged) and p-p38α protein in different groups. All data are presented as mean±SD. * p < 0.05. (1: negative group(DMSO-treated); 2: experimental group treated with 10 nM ATRA; 3: experimental group treated with 10 nM ATRA+10 μM PD169316)
Discussion
APL is a characteristic type of AML that originates from stem cells in the hematopoietic system of a clonal malignant disease. It is characterized by an unusually aggressive clinical course 27. In our previous study, we have found that NLS-RARα and PML (NLS(-)) resulting from NE-induced PML-RARα cleavage could accelerate APL7, 8, 28. In this study, we further investigated the effects of NLS-RARα on NB4 cells and its potential mechanism.
ATRA is an accepted therapeutic drug for APL because it can promote cell differentiation while inhibit cell proliferation. Moreover, the effects of ATRA have been correlated with activation of p38α MAPK 13, 15, 16. Here, we found that ATRA could promote differentiation while inhibit proliferation of NB4 cells via up-regulating the expression of p-p38α protein rather than the expression of p38α protein (Figure 2, 3). However, it didn't mean the more p-p38α protein expressed, the better for NB4 cells, since we observed a higher expression of CD11b protein and more effective inhibition on cell proliferation while lower expression of p-p38α protein when NB4 cells were treated with1 µM ATRA(Figure 2). Furthermore, dual luciferase reporter assay suggested that p-p38α protein has transcriptional activity (Figure 3). Interestingly, it has been reported that p38α protein could interact with RARα directly then inhibit transcriptional activity of RARα in the presence of ATRA 13. So we thought, p-p38α protein may likely compete with RARα for retinoid-responsive element.
In this study, we also revealed that NLS-RARα could inhibit differentiation while accelerate proliferation of NB4 cells via down regulating the expression of p-p38α protein in the presence of ATRA (Figure 4). Moreover, NLS-RARα could interact with p38α protein directly independent of ATRA (Figure 5). Those suggested that the underlyng mechanism of the effects of NLS-RARα on NB4 cells may be that ATRA recruited p38α-combinded NLS-RARα and then activated p38α protein while p38α-combinded NLS-RARα inhibited the effects of ATRA in the process (Figure 6). Thus, we supposed that patients with APL who are sensitive to ATRA may experience accelerated APL due to an NLS-RARα-induced downregulation of p-p38α protein, while NLS-RARα could promote APL progression by another pathway (e.g., Akt pathway) in patients with APL who are resistant to ATRA. However, it is unclear why there was a consistent variation between the expressions of C/EBPβ and p- p38α protein, even though both myeloid differentiation marker proteins, C/EBPβ and CD11b, increased after dealing NB4 cells with ATRA (Figure 2). Moreover, pathogenesis of APL is still needed to be explored through experiments conducting in ATRA resistant APL cell lines, animal models of APL, and clinical samples.
Acknowledgments
The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81171658) and the Natural Science Foundation Project of CQ CSTC (grant no. 2011BA5037).We are appreciated professor Dmitrii Kamashev and his partners from France for favoring retinoid-responsive reporter construct of pTK-Luc.
Abbreviations
- NLS-RARα
nuclear localization signal retinoic acid receptor alpha
- NE
neutrophil elastase
- PML-RARα
promyelocytic leukemia-retinoic acid receptor alpha
- APL
acute promyelocytic leukemia
- MAPK
mitogen-activated protein kinase
- C/EBPβ
CCAAT/enhancer binding protein beta
- CD11b
integrin subunit alpha M
- ATRA
All-trans Retinoic Acid
- p-p38αMAPK
phosphorylated p38αMAPK
- DMSO
dimethylsulfoxide
- CCK-8
Cell Counting Kit-8
- OD450
Optical density value at 450 nm wavelength
- RARE
retinoic acid responsive element
- LV-NC-NB4
NB4 cells were infected with lentivirusonly
- LV-NLS-RARα-NB4
NB4 cells were infected with NLS-RAR-lentivirus
- PBS
phosphate-buffered-saline
- PMSF
phenylmethanesulfonyl fluo-ride.
References
- 1.Yoo SJ, Seo EJ, Lee JH. et al. A complex, four-way variant t(15;17) in acute promyelocytic leukemia. Cancer genetics and cytogenetics. 2006;167:168–71. doi: 10.1016/j.cancergencyto.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Kamimura T, Miyamoto T, Harada M. et al. Advances in therapies for acute promyelocytic leukemia. Cancer science. 2011;102:1929–37. doi: 10.1111/j.1349-7006.2011.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitto T, Sawaki K. Molecular Mechanisms of the Antileukemia Activities of Retinoid and Arsenic. Journal of Pharmacological Sciences. 2014;126:179–185. doi: 10.1254/jphs.14r15cp. [DOI] [PubMed] [Google Scholar]
- 4.Kakizuka A, Miller WH Jr, Umesono K. et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991;66:663–74. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 5.Lane AA, Ley TJ. Neutrophil elastase cleaves PML-RARalpha and is important for the development of acute promyelocytic leukemia in mice. Cell. 2003;115:305–18. doi: 10.1016/s0092-8674(03)00852-3. [DOI] [PubMed] [Google Scholar]
- 6.Lane AA, Ley TJ. Neutrophil elastase is important for PML-retinoic acid receptor alpha activities in early myeloid cells. Molecular and cellular biology. 2005;25:23–33. doi: 10.1128/MCB.25.1.23-33.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu XX, Zhong L, Zhang X. et al. NLS-RARalpha promotes proliferation and inhibits differentiation in HL-60 cells. International journal of medical sciences. 2014;11:247–54. doi: 10.7150/ijms.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H, Li L, Zhong L. et al. NLS-RARalpha regulates the proliferation of leukemia cell NB4 by activating Akt pathway. Basic&Clinical Medicine(China) 2016;36:41–46. [Google Scholar]
- 9.Kale VP. Differential activation of MAPK signaling pathways by TGF-beta1 forms the molecular mechanism behind its dose-dependent bidirectional effects on hematopoiesis. Stem cells and development. 2004;13:27–38. doi: 10.1089/154732804773099236. [DOI] [PubMed] [Google Scholar]
- 10.Cabane C, Coldefy AS, Yeow K. et al. The p38 pathway regulates Akt both at the protein and transcriptional activation levels during myogenesis. Cellular signalling. 2004;16:1405–15. doi: 10.1016/j.cellsig.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Alisi A, Spaziani A, Anticoli S. et al. PKR is a novel functional direct player that coordinates skeletal muscle differentiation via p38MAPK/AKT pathways. Cellular signalling. 2008;20:534–42. doi: 10.1016/j.cellsig.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Serra C, Palacios D, Mozzetta C. et al. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Molecular cell. 2007;28:200–13. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianni M, Peviani M, Bruck N. et al. p38alphaMAPK interacts with and inhibits RARalpha: suppression of the kinase enhances the therapeutic activity of retinoids in acute myeloid leukemia cells. Leukemia. 2012;26:1850–61. doi: 10.1038/leu.2012.50. [DOI] [PubMed] [Google Scholar]
- 14.Watts JM, Tallman MS. Acute promyelocytic leukemia: what is the new standard of care? Blood reviews. 2014;28:205–12. doi: 10.1016/j.blre.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Qian X, He J, Zhao Y. et al. Inhibition of p38 MAPK Phosphorylation Is Critical for Bestatin to Enhance ATRA-Induced Cell Differentiation in Acute Promyelocytic Leukemia NB4 Cells. American journal of therapeutics. 2016;23:680–89. doi: 10.1097/01.mjt.0000433950.01406.b3. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Xia L, Gabrilove J. et al. Sorafenib Inhibition of Mcl-1 Accelerates ATRA-Induced Apoptosis in Differentiation-Responsive AML Cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:1211–21. doi: 10.1158/1078-0432.CCR-15-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duprez E, Wagner K, Koch H. et al. C/EBPbeta: a major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells. The EMBO journal. 2003;22:5806–16. doi: 10.1093/emboj/cdg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanaka R, Lekstrom-Himes J, Barlow C. et al. CCAAT/enhancer binding proteins are critical components of the transcriptional regulation of hematopoiesis (Review) International journal of molecular medicine. 1998;1:213–21. doi: 10.3892/ijmm.1.1.213. [DOI] [PubMed] [Google Scholar]
- 19.Zeng C, Xu Y, Xu L. et al. Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC cancer. 2014;14:693. doi: 10.1186/1471-2407-14-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Jin W, Jia X. et al. Transcriptional repression of CDKN2D by PML/RARalpha contributes to the altered proliferation and differentiation block of acute promyelocytic leukemia cells. Cell death & disease. 2014;5:e1431. doi: 10.1038/cddis.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1996;10:940–54. [PubMed] [Google Scholar]
- 22.Minucci S, Ozato K. Retinoid receptors in transcriptional regulation. Current opinion in genetics & development. 1996;6:567–74. doi: 10.1016/s0959-437x(96)80085-2. [DOI] [PubMed] [Google Scholar]
- 23.Kamashev D, Vitoux D, De The H. PML-RARA-RXR oligomers mediate retinoid and rexinoid/cAMP cross-talk in acute promyelocytic leukemia cell differentiation. The Journal of experimental medicine. 2004;199:1163–74. doi: 10.1084/jem.20032226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rachez C, Freedman LP. Mediator complexes and transcription. Current opinion in cell biology. 2001;13:274–80. doi: 10.1016/s0955-0674(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 25.Rastinejad F. Retinoid X receptor and its partners in the nuclear receptor family. Current opinion in structural biology. 2001;11:33–8. doi: 10.1016/s0959-440x(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 26.Kambhampati S, Verma A, Li Y. et al. Signalling pathways activated by all-trans-retinoic acid in acute promyelocytic leukemia cells. Leukemia & lymphoma. 2004;45:2175–85. doi: 10.1080/10428190410001722053. [DOI] [PubMed] [Google Scholar]
- 27.Lo-Coco F, Cicconi L. History of acute promyelocytic leukemia: a tale of endless revolution. Mediterranean journal of hematology and infectious diseases. 2011;3:e2011067. doi: 10.4084/MJHID.2011.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang XQ, Wang H, Jiang KL. et al. The effects of PML ( NLS -) on leukemia cell NB4 mediated by adenovirus-over-expression. Basic & Clinical Medicine(China) 2014;34:1327–32. [Google Scholar]