Abstract
OBJECTIVE
Severe traumatic brain injury (TBI) is a dynamic neuropathologic process in which a substantial proportion of patients die within the first 48-hours. The assessment of injury severity and prognosis are of primary concern in the initial management of severe TBI. Supplemental testing that aids in the stratification of patients at high risk for deterioration may significantly improve posttraumatic management in the acute setting.
METHODS
This retrospective study assessed the utility of both single-marker and multimarker models as predictive indicators of acute clinical status after severe TBI. Forty-four patients who sustained severe TBI (admission Glasgow Coma Scale [GCS] score ≤8) were divided into two cohorts according to a dichotomized clinical outcome at 72 hours after admission: Poor status (death or GCS score ≤8) and improved status (GCS score improved to >8). Threshold values for clinical status prediction were calculated for serum S-100B, matrix metalloproteinase-9, and plasma D-dimer, upon admission and at 24 hours after TBI by the use of receiver operating characteristic analysis. Performance characteristics of these single-marker predictors were compared with those derived from a multimarker logistic regression analysis.
RESULTS
Biomarkers with the greatest predictive value for poor status at 72 hours included serum S-100B on admission, as well as plasma D-dimer and serum S-100B at 24 hours, for which, associations were strongly significant. Multimarker analysis indicated no substantial improvement in prediction accuracy over the best single predictors during this time frame.
CONCLUSION
In conjunction with other clinical, physical, and radiologic evidence, blood-derived biochemical markers may serve to enhance prediction of early clinical trends after severe TBI.
Keywords: Biomarkers, D-dimer, MMP-9, S-100B, Trauma, Traumatic Brain Injury
INTRODUCTION
Severe traumatic brain injury (TBI) is a dynamic neuropathologic process in which the primary mechanical injury triggers a heterogeneous complex of vascular, metabolic, cellular, and molecular consequences that promote neurologic deterioration and secondary brain injury (33). The mortality rate associated with severe TBI has been reported between 30% and 50%, with approximately 90% of deaths occurring within 48 hours of insult (28). Therefore, the early assessment of injury severity is of significant importance in the management of patients who have sustained severe TBI (18). Despite the substantial burden of this disease process, however, optimally reliable outcome predictors after head trauma are lacking. Because the early assessment of neurological injury often presents a significant challenge in the intensive are unit setting, the use of biochemical markers may be of value in the identification of patients at greater risk for deterioration and in the guidance of immediate posttraumatic therapeutic strategies (5).
The analysis of disease-specific biomarkers in modern medicine has revolutionized the diagnostic, prognostic, and therapeutic approach of various human pathologies. For example, in myocardial infarction, the evaluation of troponin, creatine kinase MB, D-dimer, and brain natriuretic peptide plays an important diagnostic role (9). In the case of TBI, however, the brain itself introduces multiple unique challenges to the identification of a single reliable marker of injury. Among these include the distribution of heterogeneous cell populations within the central nervous system (CNS) and their respective tolerance or resistance to injury, the overall complexity of ischemic and neuroinflammatory cascades, and the presence of the blood-brain barrier (22). Furthermore, the majority of markers are nonspecific for cerebral injury; rather, they represent various components of the ischemic and neuroinflammatory cascades (22). Thus, despite statistical associations between cerebral injury and individual markers of inflammation, glial activation, and neuronal injury, no single marker has possessed the characteristics required to demonstrate stand-alone diagnostic or prognostic value (22).
The primary aim of this investigation was to evaluate tendencies in select biomarker profiles that could serve as posttraumatic indicators of early clinical trends after severe TBI. Cut-off values for serum S-100B, matrix metalloproteinase-9 (MMP-9), and plasma D-dimer were established, on admission and at 24 hours, to predict clinical status at the 72-hour time point. Performance characteristics of these single-marker predictors were compared with those derived from a multimarker model to assess the predictive value of multimarker monitoring in the early posttraumatic period.
MATERIALS AND METHODS
Patient Population
Ethical and institutional approval for the study was granted by the University of Miami Institutional Review Board prior to study commencement, and informed consent for enrollment was obtained by either the patient’s health care surrogate or closest family member. A retrospective analysis of biochemical data was conducted on patients who were admitted to Ryder Trauma Center at Jackson Memorial Hospital, between the dates of June 2003 and February 2005, with severe TBI (Glasgow Coma Scale (GCS) Score ≤ 8) after initial resuscitation. All patients included in this study were victims of severe TBI—any mechanism of injury—between the ages of 16 and 64 years and were admitted within 3 hours of injury. Patients outside of the stated age range or those who presented after 3 hours after injury, as well as, patients with a history of acute meningitis, cerebral vasculitis, or a history of another recently documented CNS infection were excluded from the analysis. All patients were sedated, intubated, mechanically ventilated, and managed according to a protocol adapted from the guidelines for the management of severe TBI proposed by the Brain Trauma Foundation (4). Corticosteroids were not used in the treatment of these patients.
Blood Sampling and Biochemical Measurements
Venous blood samples were obtained from each patient upon admission to Ryder Trauma Center (study entry), as well as at the 24-hour, 48-hour, and 72-hour postadmission time points. Two 5-mL samples were collected, during routine blood drawing from an existing indwelling vascular catheter that had been placed during the patient’s admission. All venous serum samples were centrifuged at 1800 g for 7 minutes at room temperature. Plasma supernatant fractions from peripheral venous blood samples were then separated and stored at −80°C until samples were sent to Alere (formerly Biosite Incorporated, San Diego, California, USA) for batch analysis.
Assessment of Clinical Outcomes
Clinical outcomes after severe TBI in this study were measured at 72 hours after admission and were defined by survival, as well as by assessment using the GCS to evaluate the degree of impaired consciousness in surviving patients. Patients in this study were divided into two main cohorts, for retrospective analysis, based on a dichotomized clinical outcome at 72 hours after admission. The poor clinical status cohort (death or GCS that remained <8) consisted of 30 cases (68%), and the improved clinical status cohort (GCS improved to >8) consisted of 14 cases (32%). As a result of the relatively small sample size, no independent analysis was performed solely on patients who died within the first 72 hours after admission (i.e., seven cases; 16%).
Statistical Analysis
A comparison of median S-100B, MMP-9, and D-dimer values, both on study entry and at 24 hours after study entry, in the different cohorts under investigation was made with the standard Mann-Whitney U-test. The correlation between serum concentrations of S-100B, MMP-9, and D-dimer levels on arrival and poor clinical status (death or GCS ≤8) at 72 hours was assessed with the Spearman rank-order coefficient. In addition, the extent to which each biomarker at a given time point differed between individuals who demonstrated improved (GCS >8 at 72 hours) versus poor (death or GCS <8 at 72 hours) clinical trends was assessed by the use of receiver operating characteristic (ROC) analyses. The ROC plots were estimated by using the algorithm built into the Statistical Package for the Social Sciences (SPSS; SPSS Institute, Cary, North Carolina, USA). Each biomarker was evaluated at its optimal cutoff value on the basis of the standard sensitivity versus 1-specificity and positive predictive value (PPV) and negative predictive value (NPV) measures, both at study entry and 24 hours after study entry, for prediction of unfavorable (poor) clinical status. A cut-off point on the curves was chosen to attain the best compromise between sensitivity and specificity for poor outcome at 72 hours after study entry.
To determine the best combination of biomarkers for early outcome prediction, a multivariate logistic regression model was used. A stepwise procedure was implemented to find the optimal biomarker combination within the first 24 hours after study entry. Various combinations of biomarkers were explored in multiple data analysis iterations, including a wide variety of alternative models and analysis techniques. Measures of predictive performance for the multivariate model were derived from ROC analysis.
RESULTS
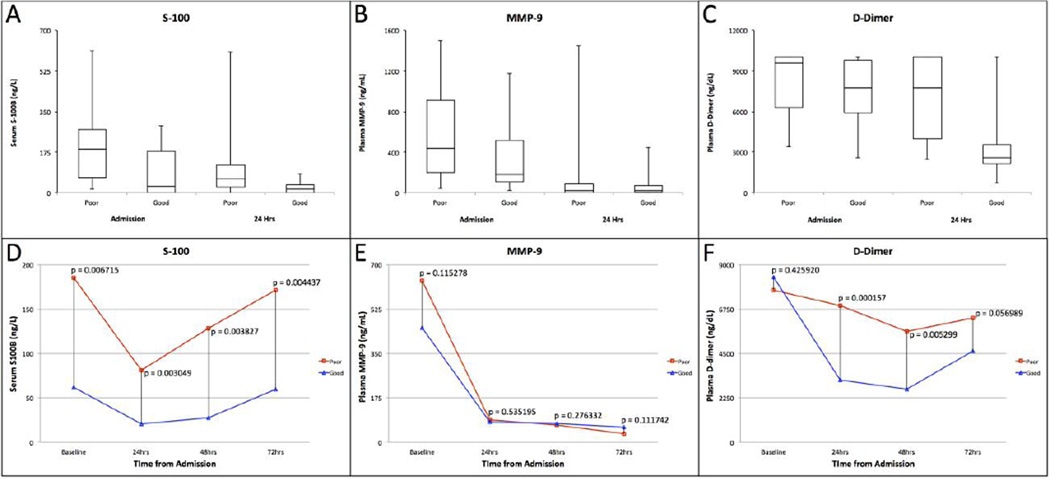
Table 1 depicts the characteristics of the TBI population stratified by outcome measure (poor vs. improved) at 72 hours after severe TBI. Retrospective analysis was performed on 44 patients (31 male and 13 female), ages 16–64 years (median, 28 years), during a period of 20 months. All patients had sustained severe brain trauma (GCS ≤8 on admission) and were subsequently admitted within 3 hours of injury. A total of 30 patients (68%) from this study either died (7 patients) or remained at a GCS ≤8 (23 patients) at 72 hours after admission, whereas 14 patients (32%) demonstrated significant improvement in GCS to >8 during this period. Concentrations of the various biomarkers were determined upon admission with subsequent measurements obtained at 24, 48, and 72 hours after admission in surviving patients. The concentrations of serum S-100B ranged from 0.00 ng/mL to 6.11 ng/mL upon admission after injury and from 0.00 ng/mL to 6.07 ng/mL at 24 hours after the inciting trauma. Median S-100B concentrations were found to be statistically greater, both on admission (P = 0.007) and at 24 hours (P = 0.003), in patients who either died or failed to improve (Figure 1A and D, Table 2). Similarly, D-dimer concentrations ranged from 2587.06 ng/dL to 10,000.00 ng/dL and from 684.38 ng/dL to 10,000.00 ng/dL upon admission and at 24 hours after admission, respectively. Median D-dimer values at 24 hours were significantly greater in patients with poor clinical status (P = 0.00); however, D-dimer values on admission were not significantly different (P = 0.425; Figure 1C and F, Table 2). The concentration of serum MMP-9 on admission ranged from 24.23 ng/mL to 1500.00 ng/mL, whereas the range at 24 hours spanned from 0.00 ng/mL to 1447.71 ng/mL. No significant difference in median MMP-9 concentration was noted between outcome groups on admission (P =0.115) or at the 24-hour time point (P = 0.535) (Figure 1B and E, Table 2).
Table 1.
Descriptive Characteristics of the Study Population Stratified for Clinical Status (Poor vs Improved) at 72 hours after Severe TBI
| Clinical Data | Severe TBI | Poor Status | Improved Status GCS > 8 |
||
|---|---|---|---|---|---|
| Death | GCS ≤ 8 | Total | |||
| Number of Patients | 44 | 7 | 23 | 30 | 14 |
| Gender | |||||
| Male | 31 | 5 | 14 | 19 | 12 |
| Female | 13 | 2 | 9 | 11 | 2 |
| Age | |||||
| Median | 28 | 20 | 28 | 26 | 30 |
| Range | 16–64 | 18–56 | 17–62 | 17–62 | 16–64 |
| GCS Admission | |||||
| Median | 4 | 4 | 4 | 4 | 4 |
| Range | 3–8 | 3–6 | 3–8 | 3–8 | 3–8 |
| Mechanism of Injury (Cases) | |||||
| MVC (%) | 13 (29.5) | 2 (28.6) | 8 (34.8) | 10 (33.3) | 3 (21.4) |
| MCC w/o helmet (%) | 5 (11.4) | - | 2 (87) | 2 (6.7) | 3 (21.4) |
| MCC w/ helmet (%) | 2 (4.5) | - | 1 (4.3) | 1 (3.3) | 1 (7.1) |
| Fall (%) | 4 (9.1) | - | 1 (4.3) | 1 (3.3) | 3 (21.4) |
| PHBC (%) | 4 (9.1) | - | 3 (13.1) | 3 (10) | 1 (7.1) |
| ATV (%) | 2 (4.5) | - | 2 (8.7) | 2 (6.7) | - |
| Other (%) | 14 (31.8) | 5 (71.4) | 6 (26.1) | 11 (36.7) | 3 (21.4) |
Figure 1.
Box-and-whisker plots for plasma S-100B, matrix metalloproteinase-9 (MMP-9), and plasma D-Dimer levels in the poor versus improved clinical status groups both upon admission and at 24 hours after traumatic brain injury (A, B, C). Temporal profiles of median serum S100-B, MMP-9, and plasma D-Dimer levels in the poor versus improved status cohorts upon admission and at 24, 48, and 72 hours after traumatic brain injury (D, E, F).
Table 2.
Comparison of Serum S100B, MMP-9, D-Dimer Concentrations on Admission and at 24 hours in Patients who had achieved Poor versus Improved Clinical Satus at 72 hours after Admission
| Biomarkers | Poor Outcome (Death or GCS ≤ 8) |
Good Outcome (GCS > 8) |
p-value | ||
|---|---|---|---|---|---|
| N | Median | N | Median | ||
| Admission | |||||
| S100B (ng/ml) | 30 | 1.88 | 14 | 0.28 | 0.007 |
| MMP-9 (ng/ml) | 30 | 438.26 | 14 | 183.82 | 0.115 |
| D-Dimer (ng/dl) | 30 | 9586.82 | 14 | 7769.50 | 0.425 |
| 24 hours | |||||
| S100B (ng/ml) | 28 | 0.60 | 14 | 0.16 | 0.003 |
| MMP-9 (ng/ml) | 28 | 25.00 | 14 | 22.61 | 0.535 |
| D-Dimer (ng/dl) | 28 | 7753.11 | 14 | 2590.07 | 0.000 |
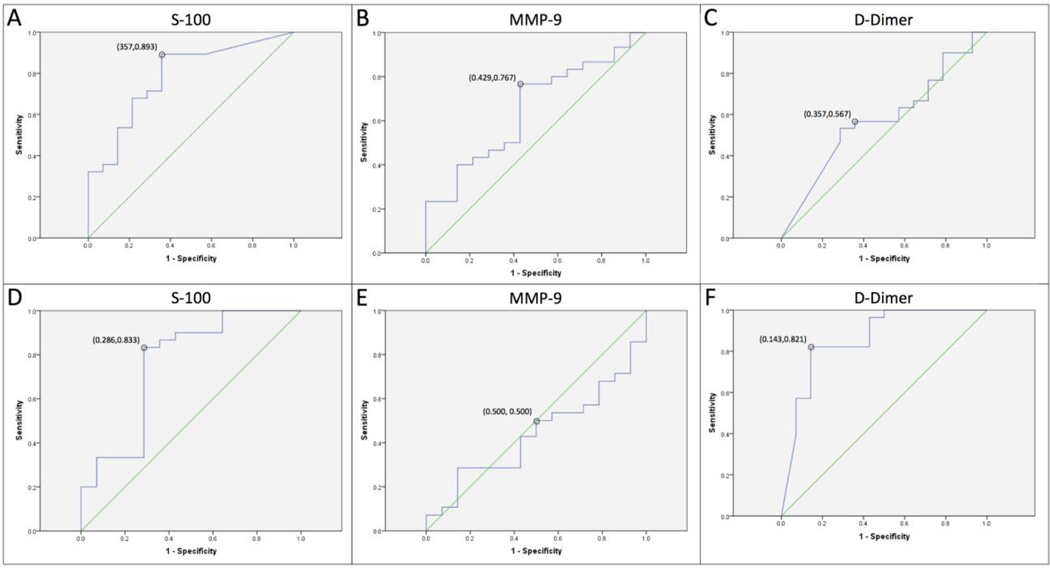
Through the use of ROC analysis, cutoff values on admission and at the 24-hour time point were selected to detect the greatest proportion of patients who achieved poor clinical status at the least compromise to specificity. Performance measures of the threshold values for each individual biomarker are summarized in Table 3. For serum S-100B, threshold values of 0.39 ng/mL at study entry and 0.19 ng/mL at 24 hours were chosen (Figure 2A and D). For D-dimer, plasma concentration values of 8780.11 ng/dL and 3769.55 ng/dL were selected as cut-off points for poor status prediction (Figure 2C and F). However, the plasma D dimer threshold value on admission was not determined to be a statistically significant predictor of poor clinical status at 72 hours (P =0.195). Serum MMP-9 cutoff values were selected to be 193.96 ng/ mL on admission and 23.18 ng/mL at 24 hours. Although the admission cut-off was noted to be a significant predictor of poor clinical status (Figure 2B, Table 3), the 24-hour MMP-9 threshold was not significantly related to early trend prediction (P = 1.000; Figure 2E, Table 3).
Table 3.
Performance Characteristics of Serum Biomarkers on Admission and at 24 Hours as a Test for Poor Clinical Status (Death or GCS ≤ 8) at 72 hours in Patients with Severe Traumatic Brain Injury
| Biomarkers | ROC cut-off | AUC | CI | Sensitivity | Specificity | PPV | NPV | p-value |
|---|---|---|---|---|---|---|---|---|
| Admission | ||||||||
| D-Dimer (ng/dl) | 8780.11 | 0.574 | 0.39–0.75 | 0.567 | 0.643 | 0.773 | 0.409 | 0.195 |
| MMP-9 (ng/ml) | 193.96 | 0.650 | 0.48–0.82 | 0.767 | 0.57 | 0.793 | 0.533 | 0.028 |
| S-100 (ng/ml) | 0.39 | 0.76 | 0.59–0.93 | 0.893 | 0.643 | 0.833 | 0.750 | 0.000 |
| 24 hours | ||||||||
| D-Dimer (ng/dl) | 3769.55 | 0.86 | 0.73–0.99 | 0.821 | 0.857 | 0.920 | 0.706 | 0.000 |
| MMP-9 (ng/ml) | 23.18 | 0.44 | 0.26–0.61 | 0.500 | 0.500 | 0.667 | 0.333 | 1.000 |
| S-100 (ng/ml) | 0.19 | 0.78 | 0.64–0.93 | 0.833 | 0.714 | 0.862 | 0.667 | 0.000 |
Values are in ng/ml
Figure 2.
Receiver operating characteristic (ROC) curve demonstrating plots of sensitivity vs 1-specificity to predict poor clinical status assessed at 72 hours after admission based on cut-off values for serum S-100B, MMP-9, and plasma D-Dimer both upon admission (A, B, C) and at 24 hours after admission (D, E, F), respectively.
Multivariate logistic regression analysis revealed that the combination of serum S-100B on admission, along with plasma D-dimer and serum S-100B at 24-hours, provided the greatest predictive value for poor clinical status at 72 hours. The multivariate model was evaluated at the threshold derived from ROC analysis (Table 4). The threshold selected by this method yielded 29 predicted poor outcomes within our sample population—demonstrating a PPV of 86% and NPV of 77% for death or persistence of low GCS during the acute period after head trauma (P = 0.000).
Table 4.
Performance Characteristics of the Multivariate Logistic Regression Model as a Test for Poor Clinical Status (Death or GCS ≤ 8) at 72 hours in Patients with Severe Traumatic Brain Injury
| Biomarker | ROC cut-off | AUC | CI | Sensitivity | Specificity | PPV | NPV | p-value |
|---|---|---|---|---|---|---|---|---|
| Model (S100B1+ S100B2 + D-dimer2) |
0.4686 | 0.82 | 0.69–0.95 | 0.893 | 0.714 | 0.862 | 0.769 | 0.000 |
DISCUSSION
Single Biomarker Analysis
In comparing the performance characteristics of the single-marker predictors, serum S-100B levels, both on admission and at 24 hours after injury, were significantly greater in patients with unfavorable outcome compared with those who experienced improvements in GCS to >8 after 72 hours (P =0.000). ROC analysis demonstrates that on admission serum S-100B is the best single predictor of poor clinical status at 72 hours in patients who sustain severe TBI, with an area under the curve (AUC) of 0.76 (confidence interval [CI] 0.59–0.93, P = 0.000) (Figure 2A). The threshold value for S-100B at this time point was associated with a PPV of 83% and a NPV of 75%, meaning that for patients who presented with serum values in excess of this threshold, 83% either died or failed to improve significantly by 72 hours after admission (Table 3). Although greater levels of plasma D-dimer were noted on admission in the poor clinical status group, this difference was not found to be statistically significant (P = 0.195). Conversely, at 24 hours after admission, D-dimer was found to be the strongest predictor of poor status with an AUC of 0.86 (CI 0.73–0.99, P = 0.000; Figure 2F). Furthermore, S-100B at 24 hours provided an AUC of 0.78 (CI 0.64–0.93, P = 0.000; Figure 2D), making it a strong predictor of death or failed improvement at this time point as well.
Multiple Biomarker Analysis
Analysis was limited to measurements obtained during the initial 24 hours postadmission to identify a model for the prediction of early clinical trends after severe TBI. The multivariate model predicts the risk of poor outcome by the use of a combination of biomarker measurements. This model was evaluated at the threshold derived from ROC analysis, which demonstrated the ability of the multimarker model to predict death or no clinical improvement at 72 hours after admission with good predictive performance (Table 4). This approach was preferred over the use of discrete cut-off values to multiple biomarkers to calculate performance measures because such an approach assumes that all biomarkers contribute equally to outcome prediction. Interestingly, the results of this model did not indicate a substantial improvement in performance over the best single predictors obtained on admission or at 24 hours after insult. Thus, the individual biomarkers evaluated in this study provide a more practical, generalizable, and cost effective approach to outcome prediction than the multimarker combination analysis.
S-100B
Astrocytes are the major source of S-100B in the CNS, where it is involved in the regulation of signal transduction pathways, cellular morphology, and astrocytic proliferation via interaction with transcription factors (Table 5) (1, 5, 8, 16, 24). After injury-provoked release into the cerebrospinal fluid, S-100B passes through the arachnoid villi into the bloodstream, where it can be measured (17). Thus, its presence in the serum indicates both neuronal damage as well as increased permeability of the blood-brain barrier, and the measurement of this marker has been suggested in the diagnostic evaluation of various forms of neurotrauma. Numerous studies have focused specifically on the prognostic role of S-100B in TBI, where gross elevations have correlated with poor outcome (5, 7, 10, 12, 13, 18, 19, 25, 32). However, these studies present significant variation in study delineation regarding TBI severity, the presence of associated extracerebral injuries, as well as the temporal profile for S-100B that best reflects the severity of cerebral trauma (5, 25–27, 30–32, 38, 40, 42). Furthermore, several extracerebral sources of S-100B, including adipocytes, chondrocytes, skeletal muscle tissue, bone marrow, and melanoma cells, among others, have been identified (1, 10, 13, 32). Consequently, there has been concern that extracerebral sources of S-100B may confound the interpretation of assays performed on multitrauma patients who present with concomitant head trauma. However, studies involving patients without head injury have demonstrated that the greatest concentrations of serum S-100B were detected acutely after trauma and were significantly decreased thereafter (1), which is likely the result of the overestimation of serum S-100B secondary to extracerebral contributions early after injury (23, 29). Furthermore, several studies have failed to demonstrate a significant correlation between the presence of systemic trauma and the magnitude of neurobiochemical release, thereby supporting the specificity of S-100B for poor outcome regardless of the presence of associated extracerebral injuries (5, 10, 13, 32). The proposed half-life of S-100B ranges between 30 minutes and 113 minutes after its release into the bloodstream (14). On the basis of these data, monitoring changes in serum S-100B for several days after injury would increase the specificity of this marker for poor outcome prediction when noncerebral contributions likely become negligible (1, 5, 37). In this study, we noted an increased specificity of serum S-100B 24 hours after initial injury compared with values on admission, which likely reflects a sustained release of S-100B as a result of evolving secondary posttraumatic insult.
Table 5.
Characteristics and Utility of the Biomarkers Monitored in this Study
| Biomarkers | General Features | Ischemic Studies |
|---|---|---|
| MMP-9 | Proteolytic enzyme; Involved in tissue remodeling; Important role in neuroinflammation |
Increased MMP-9 in acute phase; Increased MMP-9 associated with hemorrhagic transformation; Correlated with infarct size and clinical deficit |
| S-100B | Calcium-binding protein; Synthesized in Astroglial Cells; Marker of Brain Damage |
Increased S-100B in acute phase; S-100B associated with clinical deficit, infarct volume, and functional disability |
| D-Dimer | Product of Fibrin Degradation; Marker of Hemostatic Imbalance |
Increased DD in acute, subacute, and chronic phases; Increased DD in cardioembolic stroke; Predictor of new vascular events and prognostic role |
Matrix Metalloproteinase-9
The MMPs are a family of 26 enzymes that are important in normal growth, development, and wound healing (Table 5) (43). These enzymes are synthesized and secreted in the latent form, and their activity is tightly controlled to prevent unwanted tissue damage and ECM degradation (35). A specific form of MMP termed MMP-9, or gelatinase B, has demonstrated specificity for the degradation of type-IV collagen, laminin, and fibronectin, which are major components of the basal membrane and cerebral blood vessels (11). Gelatinases, like MMP-9, specifically target the blood-brain barrier, damaging its structural integrity and altering its permeability, thus facilitating the development of cerebral edema—an integral part of the secondary injury process (2, 34, 39). A study analyzing the temporal profile of MMP-9 in patients with moderate and severe TBI revealed significant increases in MMP-9 in the very acute phase after injury (39). The results of our study are consistent with these findings. MMP-9 in this study yielded marginally significant predictive power in the acute phase upon admission. This was followed by a rapid return of serum values to near normal over the initial 24 hours after injury, with measurements becoming almost undetectable in the ensuing days after admission (Figure 1E). This result is consistent with the trend towards normalization after 24 hours reported previously (39).
D-Dimer
The development of coagulation abnormalities after head trauma has been well documented in the literature (3, 6, 15, 36). In particular, D-dimer, a breakdown product of fibrin, is an end product of both coagulation and fibrinolysis, and increased levels of plasma at the time of admission are thought to reflect the overall up-regulation hemostasis after TBI (6, 15, 20, 36) (Table 5). Elevated levels of D-dimer in the peripheral blood have correlated with poor clinical outcome in patients with CNS disorders such as subarachnoid hemorrhage, ischemic stroke, trauma, and progressive hemorrhagic injury after brain injury (3, 6, 15, 36). High concentrations of D-dimer in the blood have been shown to reflect the severity of TBI (21). In this study we found persistent elevations in D-dimer levels after the initial 24 hours to be predictive of poor clinical status in the acute setting (Figure 1F). As a single predictor, D-dimer represented the strongest marker of poor outcome prediction at the 24-hour time point with a specificity of 86% and a PPV of 93%. The increased predictive power of D-dimer at this time point, compared with values on admission, may reflect the role of D-dimer in the progressive evolution of neurologic deterioration, resulting from activation of inflammatory cascades and development of cerebral edema characteristic of secondary, rather than primary, cerebral injury (41). Monitoring D-dimer levels may be particularly valuable in estimating the prognosis of patients who may be at risk for unpredictable neurological deterioration. Establishing a cut-off value consistent with poor outcome may provide an appropriate triage trigger for further investigation.
STUDY LIMITATIONS
The retrospective nature of this investigation limited our ability to evaluate trends in biomarker profiles beyond the acute setting after severe TBI. A longer followup period would have provided a more longitudinal assessment of prognosis and quality of outcomes. It would also be advantageous to consider these trends in biomarker profiles in other populations, including those suffering from nontraumatic CNS disease, as well as non-CNS trauma. Furthermore, our analysis was limited to poor versus improved clinical status and not death versus survival. This finding was primarily a result of the small number of in-hospital deaths in our sample size, which made it difficult to substantiate this comparison. Nevertheless, it would be advantageous to identify those patients most susceptible to dying early from their injuries to improve treatment strategies in the setting of severe TBI. Consideration of these suggested limitations should be incorporated into the design of future prospective studies.
CONCLUSION
The timely assessment of injury severity is a priority in the initial management of patients suffering from severe TBI. Hence, there is a need for supplementary testing that can aid in the stratification of patients at risk for deterioration after severe head trauma as well as improve initial triage, management strategies, and family counseling in the acute setting. The results of our analysis illustrate the role of serum S-100B and plasma D-dimer as highly accurate predictors of death or failed improvement within the first few days after severe TBI. When taken in conjunction with other parameters of outcome prediction, the simultaneous evaluation of biochemical data may significantly enhance the clinical assessment of patients who have sustained severe TBI.
Acknowledgments
The authors thank Linda Daniels, MD, Enrique Perez, Jonathan Jagid, MD, Philip Villanueva, MD, Matthias Majetschak, MD, Diego Lozano, MD, and Arash Farahvar, MD, PhD, for their assistance in this study. They also thank Dr. Jeff Bishop from Biosite for his assistance in analyzing the samples.
REFERENCES
- 1.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1260. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Hemostatic function and progressing ischemic stroke: D-dimer predicts early clinical progression. Stroke. 2004;35:1421–1425. doi: 10.1161/01.STR.0000126890.63512.41. [DOI] [PubMed] [Google Scholar]
- 4.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007;24(Suppl 1):S55–S58. doi: 10.1089/neu.2007.9988. [Erratum in: J Neurotrauma 25: 276–278, 2008] [DOI] [PubMed] [Google Scholar]
- 5.da Rocha AB, Schneider RF, de Freitas GR, André C, Grivicich I, Zanoni C, Fossá A, Gehrke JT, Pereira Jotz G, Kaufmann M, Simon D, Regner A. Role of serum S100B as a predictive marker of fatal outcome following isolated severe head injury or multitrauma in males. Clin Chem Lab Med. 2006;44:1234–1242. doi: 10.1515/CCLM.2006.218. [DOI] [PubMed] [Google Scholar]
- 6.Delgado P, Alvarez-Sabín J, Abilleira S, Santamarina E, Purroy F, Arenillas JF, Molina CA, Fernández-Cadenas I, Rosell A, Montaner J. Plasma D-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2006;67:94–98. doi: 10.1212/01.wnl.0000223349.97278.e0. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulou I, Korfias S, Dafni U, Anthi A, Psachoulia C, Jullien G, Sakas DE, Roussos C. Protein S-100b serum levels in trauma-induced brain death. Neurology. 2003;60:947–951. doi: 10.1212/01.wnl.0000049931.77887.7f. [DOI] [PubMed] [Google Scholar]
- 8.Dunn KL, Wolf JP, Dorfman DM, Fitzpatrick P, Baker JL, Goldhaber SZ. Normal D-dimer levels in emergency department patients suspected of acute pulmonary embolism. J Am Coll Cardiol. 2002;40:1475–1478. doi: 10.1016/s0735-1097(02)02172-1. [DOI] [PubMed] [Google Scholar]
- 9.Gibler WB, Blomkalns AL, Collins SP. Evaluation of chest pain and heart failure in the emergency department: impact of multimarker strategies and B-type natriuretic peptide. Rev Cardiovasc Med. 2003;4:S47–S55. [PubMed] [Google Scholar]
- 10.Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem. 2008;41:755–763. doi: 10.1016/j.clinbiochem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metallo proteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65:702–708. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann M, Curio N, Jost S, Grubich C, Ebert AD, Fork ML, Synowitz H. Release of biochemical markers of damage to neuronal and glial brain tissue is associated with short and long term neuropsychological outcome after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2001;70:95–100. doi: 10.1136/jnnp.70.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann M, Jost S, Kutz S, Ebert AD, Kratz T, Wunderlich MT, Synowitz H. Temporal profile of release of neurobiochemical markers of brain damage after traumatic brain injury is associated with intracranial pathology as demonstrated in cranial computerized tomography. J Neurotrauma. 2000;17:113–122. doi: 10.1089/neu.2000.17.113. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson H, Johnsson P, Hoglund P, Alling C, Blomquist S. Elimination of S100B and renal function after cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14:698–701. doi: 10.1053/jcan.2000.18444. [DOI] [PubMed] [Google Scholar]
- 15.Juvela S, Siironen J. D-dimer as an independent predictor for poor outcome after aneurysmal subarachnoid hemorrhage volume. Stroke. 2006;7:1451–1456. doi: 10.1161/01.STR.0000221710.55467.33. [DOI] [PubMed] [Google Scholar]
- 16.Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases-2 and-9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998;32:368–372. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 17.Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA, Barnett GH, Janigro D. Serum S100beta: a noninvasive marker of blood brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleindienst A, Hesse F, Bullock MR, Buchfelder M. The neurotrophic protein S100B: value as a marker of brain damage and possible therapeutic implications. Prog Brain Res. 2007;161:317–325. doi: 10.1016/S0079-6123(06)61022-4. [DOI] [PubMed] [Google Scholar]
- 19.Kleindienst A, Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–1200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- 20.Kuo JR, Lin KC, Luc L, Lin HG, Wang CC, Chang CH. Correlation of a high D-dimer level with poor outcome in traumatic intracranial hemorrhage. Eur J Neurol. 2007;14:1073–1078. doi: 10.1111/j.1468-1331.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuo JR, Chou TJ, Chio CC. Coagulopathy as a parameter to predict the outcome in head injury patients—analysis of 61 cases. J Clin Neurosci. 2004;11:710–714. doi: 10.1016/j.jocn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC. Clinical usefulness of a biomarker based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) Study. Stroke. 2009;40:77–85. doi: 10.1161/STROKEAHA.108.516377. [DOI] [PubMed] [Google Scholar]
- 23.Missler U, Orlowski N, Notzold A, Dibbelt L, Steinmeier E, Wisemann M. Early elevation of S-100B protein in blood after cardiac surgery is not a predictor of ischemic cerebral injury. Clin Chim Acta. 2002;321:29–33. doi: 10.1016/s0009-8981(02)00061-x. [DOI] [PubMed] [Google Scholar]
- 24.Montaner J, Perea-Gainza M, Delgado P, Ribó M, Chacón P, Rosell A, Quintana M, Palacios ME, Molina CA, Alvarez-Sabín J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280–2287. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 25.Mussack T, Biberthaler P, Kanz KG, Wiedemann E, Gippner-Steppert C, Mutschler W, Jochum M. Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med. 2002;30:2669–2674. doi: 10.1097/00003246-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Nylén K, Ost M, Csajbok LZ, Nilsson I, Hall C, Blennow K, Nellgård B, Rosengren L. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir (Wien) 2008;150:221–227. doi: 10.1007/s00701-007-1489-2. [DOI] [PubMed] [Google Scholar]
- 27.Olivecrona M, Rodling-Wahlström M, Naredi S, Koskinen LO. S-100B and neuron specific enolase are poor outcome predictors in severe traumatic brain injury treated by an intracranial pressure targeted therapy. J Neurol Neurosurg Psychiatry. 2009;80:1241–1247. doi: 10.1136/jnnp.2008.158196. [DOI] [PubMed] [Google Scholar]
- 28.Park E, Bell JD, Baker AJ. Traumatic brain injury: can the consequences be stopped? CMAJ. 2008;178:1163–1170. doi: 10.1503/cmaj.080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raabe A, Kopetsch O, Woszczyk A, Lang J, Gerlach R, Zimmermann M, Seifert V. Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restor Neurol Neurosci. 2003;21:159–169. [PubMed] [Google Scholar]
- 30.Raabe A, Grolms C, Seifert V. Serum markers of brain damage and outcome prediction in patients after severe head injury. Br J Neurosurg. 1999;13:56–59. doi: 10.1080/02688699944195. [DOI] [PubMed] [Google Scholar]
- 31.Raabe A, Grolms C, Keller M, Döhnert J, Sorge O, Seifert V. Correlation of computed tomography findings and serum brain damage markers following severe head injury. Acta Neurochir (Wien) 1998;140:787–791. doi: 10.1007/s007010050180. [DOI] [PubMed] [Google Scholar]
- 32.Rainey T, Lesko M, Sacho R, Lecky F, Childs C. Predicting outcome after severe traumatic brain injury using the serum S100B biomarker: results using a single (24h) time-point. Resuscitation. 2009;80:341–345. doi: 10.1016/j.resuscitation.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Sahuquillo J, Poca MA, Amoros S. Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr Pharm Des. 2001;7:1475–1503. doi: 10.2174/1381612013397311. [DOI] [PubMed] [Google Scholar]
- 34.Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl. 2006;96:130–133. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- 35.Sternlicht MD, Werb Z. How matrix metallo proteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian HL, Chen H, Wu BS, Cao HL, Xu T, Hu J, Wang G, Gao WW, Lin ZK, Chen SW. D-dimer as a predictor of progressive hemorrhagic injury in patients with traumatic brain injury: analysis of 194 cases. Neurosurg Rev. 2010;33:359–365. doi: 10.1007/s10143-010-0251-z. [DOI] [PubMed] [Google Scholar]
- 37.Townend W, Dibble C, Abid K, Vail A, Sherwood R, Lecky F. Rapid elimination of protein S-100B from serum after minor head trauma. J Neurotrauma. 2006;23:149–155. doi: 10.1089/neu.2006.23.149. [DOI] [PubMed] [Google Scholar]
- 38.Townend WJ, Guy MJ, Pani MA, Martin B, Yates DW. Head injury outcome prediction in the emergency department: a role for protein S-100B? J Neurol Neurosurg Psychiatry. 2002;73:542–546. doi: 10.1136/jnnp.73.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilalta A, Sahuquillo J, Rosell A, Poca MA, Riveiro M, Montaner J. Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med. 2008;34:1384–1392. doi: 10.1007/s00134-008-1056-1. [DOI] [PubMed] [Google Scholar]
- 40.Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, van Geel W, de Reus H, Biert J, Verbeek MM. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–1310. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 41.Williams MT, Aravindan N, Wallace MJ, Riedel BJ, Shaw AD. Venous thromboembolism in the in the intensive care unit. Crit Care Clin. 2003;19:185–207. doi: 10.1016/s0749-0704(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 42.Woertgen C, Rothoerl RD, Metz C, Brawanski A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J Trauma. 1999;47:1126–1130. doi: 10.1097/00005373-199912000-00026. [DOI] [PubMed] [Google Scholar]
- 43.Young VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]