Abstract
Pattern recognition receptors (PRRs) are part of the innate immune response and were originally discovered for their role in recognizing pathogens by ligating specific pathogen associated molecular patterns (PAMPs) expressed by microbes. Now the role of PRRs in sterile inflammation is also appreciated, responding to endogenous stimuli referred to as “damage associated molecular patterns” (DAMPs) instead of PAMPs. The main families of PRRs include Toll-like receptors (TLRs), Nod-like receptors (NLRs), RIG-like receptors (RLRs), AIM2-like receptors (ALRs), and C-type lectin receptors. Broad expression of these PRRs in the CNS and the release of DAMPs in and around sites of injury suggest an important role for these receptor families in mediating post-injury inflammation. Considerable data now show that PRRs are among the first responders to CNS injury and activation of these receptors on microglia, neurons, and astrocytes triggers an innate immune response in the brain and spinal cord. Here we discuss how the various PRR families are activated and can influence injury and repair processes following CNS injury.
Keywords: Pattern recognition receptors, Spinal cord injury, Toll-like receptors, NOD-like receptors, Inflammasome, Neuroinflammation
Introduction
Pattern recognition receptors (PRRs)
The innate immune system senses potential pathogens and detects disruptions in tissue homeostasis by several receptor families. Collectively, these receptor families are referred to as pattern recognition receptors (PRRs) (Janeway, 1992). Unlike receptors involved in the adaptive immune response that are customized to recognize a specific proteinor antigen, PRRs detect general “patterns” or sequences/structures commonly present on the surface of potential pathogens called pathogen associated molecular patterns (PAMPs). These receptors are highly conserved across multiple species and can be one of the first lines of defense against a possible infection. In addition to responding to PAMPs, PRRs also respond to “danger” signals or danger-associated molecular patterns (DAMPs). The “danger hypothesis” of immune system function was first proposed by Matzinger (1994, 1998) in direct opposition to the idea that the immune system evolved to recognize self vs. non-self. This theory has grown as more endogenous ligands have been identified that are recognized by PRRs (Table 1). There are several sub-families of PRRs including Toll-like receptors (TLRs), Nod-like receptors (NLRs), Ctype lectin receptors (CLRs), and RIG-like receptors (RLRs); each helps to orchestrate the innate immune response (Fig. 1). Some of these receptors are expressed on the cell surface (i.e. scavenger receptors and some TLRs) and facilitate surveillance of the extracellular environment while others are expressed intracellularly (NLRs, RLRs, some TLRs) and are activated by internalized inflammatory stimuli (e.g., DNA or RNA). Activation of these PRRs leads to production of inflammatory mediators that help remove pathogens or restore tissue homeostasis (Fig. 2). However, chronic activation of these receptors can cause inflammatory disease.
Table 1.
Microbial and endogenous ligands for PRRs.
| Receptor | PAMPs | DAMPs | References |
|---|---|---|---|
| TLR1 | Peptidoglycan (with TLR2), triacylated lipoproteins |
||
| TLR2 | Peptidoglycan, zymosan, lipoteichoic acid |
HSP60, HSP70, HMGB1, versican, necrotic cells |
Asea et al. (2002); Vabulas et al. (2001), Li et al. (2001), Yu et al. (2006); Park et al., 2004 and Kim et al., 2009 |
| TLR3 | Viral dsRNA | mRNA | Kariko et al. (2004) |
| TLR4 | LPS | HMGB1, HSP60, HSP70, hyaluronic acid, fibronectin, fibrinogen |
Ohashi et al. (2000), Asea et al. (2002), Vabulas et al. (2001), Termeer et al. (2002), Okamura et al. (2001), Smiley et al. (2001), Yu et al. (2006), Park et al. (2004) |
| TLR5 | Bacterial flagellin | ||
| TLR6 | Peptidoglycan (with TLR2), diacylated lipoproteins |
||
| TLR7 | ssRNA | miRNA, RNA | Lehmann et al. (2012) |
| TLR8 | ssRNA | ||
| TLR9 | Unmethylated CpG DNA | mtDNA; n-formyl peptides |
Zhang et al. (2010) |
| TLR11 | Uropathogenic E. coli | ||
| NLRs | Bacterial muramyl dipeptide (MDP), DAP-PGN, anthrax toxin, bacterial RNA |
ATP, uric acid crystals, Ca++, K+ efflux, acidosis, amyloid-β |
Halle et al. (2008), Lee et al. (2012), Mariathasan et al. (2006), Martinon et al. (2006); Murakami et al. (2012), Petrilli et al. (2007), Rajamäki et al. (2013), Rossol et al. (2012), Minkiewicz et al. (2013) |
| RLRs | Viral dsRNA, polyA:C | ROS | Tal et al. (2009) |
| ALRs | dsDNA, polyA:T | Cytosolic DNA | Adamczak et al. (2012), Hornung et al. (2009) |
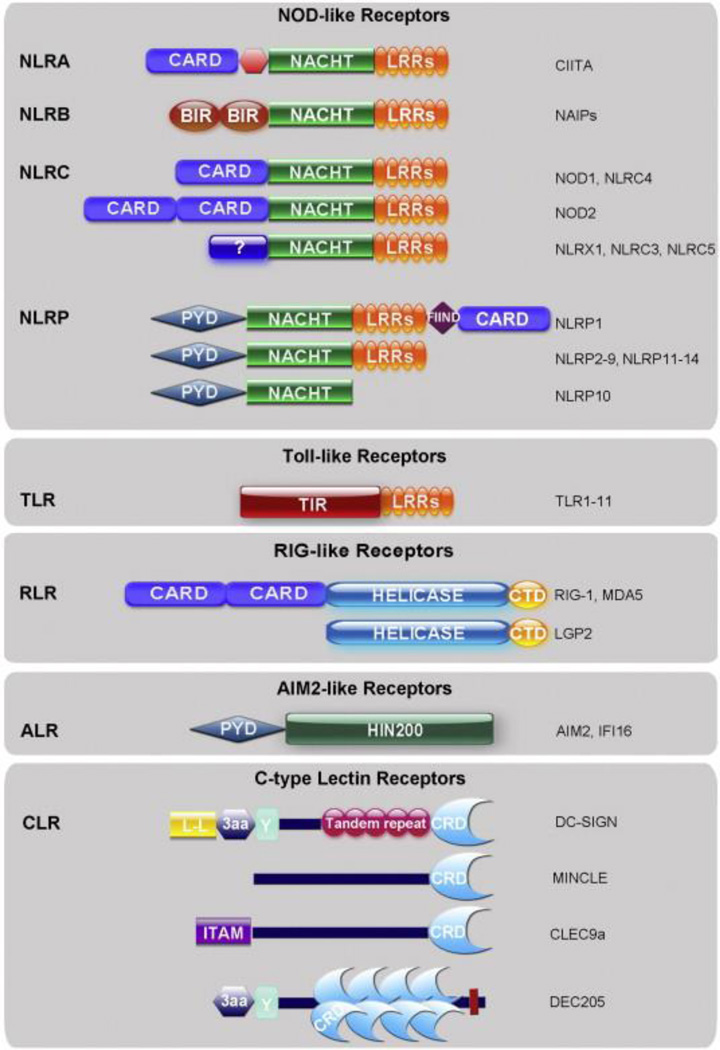
Fig. 1.
Structural domains of pattern recognition families. The major structural domains of TLRs, NLRs, RLRs, ALRs, and CLRs are depicted above. Note that the NLR family is divided into 4 subfamilies: NLRA, NLRB, NLRC and NLRP. CLRs comprise a large family of receptors, thus only those discussed in this review are shown above. Abbreviations: CARD (caspase recruitment domain), LRR (leucine rich repeat), BIR (baculovirus inhibition of apoptosis protein repeat), PYD (pyrin domain), FIIND (function to find domain), TIR (Toll-IL-1 receptor domain), helicase (DExD/H box helicase domain), CTD (carboxy terminal domain), HIN200 (hematopoietic interferon-inducible nuclear antigens with 200 amino acid repeats), L-L (di-leucine motif), 3aa (triad of acidic amino acids), Y (tyrosine-based motif), CRD (carbohydrate recognition domain), ITAM (immunoreceptor tyrosine-based activation motif).
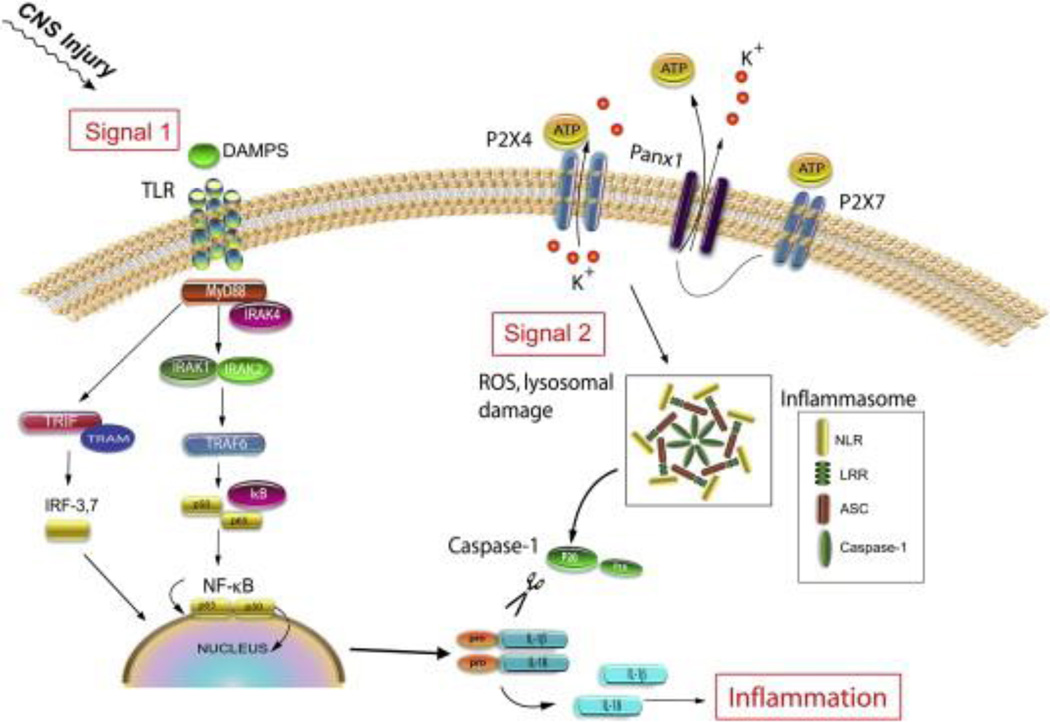
Fig. 2.
Two-signal model of innate immunity. TLRs and NLRs cooperate to orchestrate the innate immune response to injury. Activation of TLRs (via DAMPs released from CNS injury) leads to nuclear translocation of NFκB and transcription of pro-inflammatory cytokines, such as pro-IL-1β. Activation of NLRs (via a second signal) triggers inflammasome formation, caspase-1 activation, and cleavage of pro-IL-1β into its active form.
Pathogen associated molecular patterns and damage associated molecular patterns
Tissue injury, cellular stress, or disease induces the release of molecules that stimulate an innate immune response. Molecules released from pathogens are known as pathogen associated molecular patterns (PAMPs) whereas molecules of endogenous origin that are released from cells or from compartments within the cell into the cytoplasm are termed danger or damage associated molecular patterns (DAMPs) (Tang et al., 2012). DAMPs are released into the cytoplasm after central nervous system (CNS) injury and are recognized by several PRRs. DAMPs are also known as alarmins (Bianchi, 2007) and include heat shock proteins (hsp), hyaluronan, uric acid, galectins, thioredoxin (TRX), adenosine triphosphate (ATP), high mobility group box 1 (HMGB1), IL-1α and IL-33. Alarmins and DAMPs have been recently reviewed (Hirsiger et al., 2012), so only those DAMPs that are known or suspected to activate PRRs following CNS injury are considered in this review.
Pattern recognition receptor families
Toll-Like receptors
Toll-like receptors (TLRs) are homologues of the Toll receptor first identified in Drosophila (Medzhitov et al., 1997; Rock et al., 1998; Taguchi et al., 1996). In Drosophila, Toll plays a role during development in dorsal–ventral patterning and is important for anti-fungal immunity (Anderson et al., 1985a, Anderson et al., 1985b; Hashimoto et al., 1988; Lemaitre et al., 1996). The existence of human TLRs and their pivotal role in innate immune function was first discovered in the 1990’s (Janeway, 1992; Medzhitov et al., 1997; Nomura et al., 1994; Poltorak et al., 1998; Taguchi et al., 1996). To date, 13 murine TLRs and 10 human TLRs have been identified. TLRs are expressed in intracellular endosomal compartments (TLR3, TLR7, TLR8 and TLR9) or as transmembrane (cell-surface) receptors (all other TLRs). The extracellular domains of TLRs contain leucine-rich repeats (LRRs) (Figure 1), which are believed to recognize the molecular structure of PAMPs/DAMPs (Table 1). Bacterial lipopolysaccharide (LPS) was the first identified ligand for TLRs, specifically as a ligand for TLR4 (Poltorak et al., 1998). TLRs belong to the Toll/interleukin-1 receptor (TIR) family and signal via a TIR domain located on the cytosolic end of the receptor (Figure 1). TLR signaling is initiated by dimerization and recruitment of adapter proteins such as MyD88, which is an adapter protein used by all TLRs except TLR3. Recruitment of MyD88 occurs through specific TIR–TIR domain interactions that activate IL-1R-associated kinases (e.g., IRAK4). IRAK activation signals engagement of TRAF6, an E3 ubiquitin ligase. TRAF6 catalyzes formation of a complex that phosphorylates IκBα, leading to its degradation with subsequent nuclear translocation of NFκB and production of inflammatory mediators including TNF, proIL-1β, IL-6 and iNOS (among others; Figure 2). In addition to the MyD88-dependent signaling pathway, TLR4 and TLR3 can signal through a TRIF-dependent pathway. The TRIF signaling pathway recruits TRAF3 resulting in activation of interferon regulatory factor-3 (IRF3) and IRF7. This can trigger production of type I interferons (IFNα or IFNβ) and anti-viral immunity. TLRs also cooperate with a second family of PRRs, the NLR family, to produce active IL-1β and IL-18. For active IL-1β to be produced, two signals are required (Figure 2). The first signal (e.g., DAMP activation of TLRs) leads to production of proIL-1β and the second signal (i.e. activation of NLRs via ATP release) triggers inflammasome assembly followed by cleavage of pro-IL-1β into its active form by the inflammatory caspase, caspase-1. This type of cooperation among families of PRRs helps generate specialized or targeted immune responses to various stimuli. Nod-Like receptors (NLRs) NLRs are primarily involved in sensing and detecting pathogens, but they also have been shown to participate in the inflammatory responses caused by CNS injury or disease. NLRs contain three domains — a carboxy terminal LRR domain, a central NACHT or nucleotide oligomerization domain (NOD) domain, and at the amino terminus a variable interaction domain that can be either a pyrin domain (PYD), a caspase recruitment domain (CARD) or a baculovirus inhibition of apoptosis protein repeat (BIR) (Figure 1) (Di Virgilio, 2013). NLR sequences are conserved among plants and mammals (Jones and Dangl, 2006) and to date, 22 NLRs that have been described in humans and 34 in mice (Lamkanfi and Dixit, 2012; Ting et al., 2008). A thorough review of the different types of NLRs has been recently published (Di Virgilio, 2013). NLRs are best known for their ability to form inflammasomes. Inflammasomes are large multiprotein complexes (~700 kDa) that activate caspase-1, which is essential for enzymatic cleavage and maturation of the precursor cytokines pro-IL-1β and pro-IL-18 (Martinon et al., 2002). In addition to their pivotal role in cytokine production, inflammasomes also influence novel mechanisms of programmed cell death (see pyroptosis discussion later in this review) (Fernandes-Alnemri et al., 2007). NLRP1, 2, 3, 6, 7 and NLRC4 form inflammasomes (Di Virgilio, 2013) that have a common feature of caspase-1 recruitment. Although apoptosis associated speck-like protein containing a CARD (ASC) serves as an adaptor protein that brings together NLR and caspase-1, it can also act as an enhancer of the inflammatory response during inflammasome activation. However, the functional significance of ASC has been debated. Within the CNS, three different NLR inflammasomes have been described: NLRP1 (Abulafia et al., 2009; de Rivero Vaccari et al., 2009; de Rivero Vaccari et al., 2008), NLRP2 (Minkiewicz et al., 2013) and NLRP3 (Halle et al., 2008; Shi et al., 2013). The NLRP1 protein is comprised of a CARD domain at the carboxy terminus, a function to find domain (FIIND), a LRR domain, a NACHT domain and a PYD found at the amino terminus. The FIIND domain is important for inflammasome function (Finger et al., 2012), and through its CARD domain, NLRP1 can form homodimeric interactions with the CARD domain of ASC (Faustin et al., 2007). The NACHT domain has nucleoside triphosphatase activity and is involved in ATP-dependent oligomerization (Di Virgilio, 2013). The LRR domain is believed to be responsible for “sensing” DAMPs or PAMPs, yet there is no compelling data to show that NLRs directly interact with PAMPs or DAMPs. In addition, the LRR domain acts as an autoinhibitor of the inflammasome as it is folded on the NACHT domain, thus preventing oligomerization and activation. The NLRP3 protein has been widely implicated in the pathogenesis of autoimmune diseases and infections (Masters, 2013). It contains a PYD domain, a NACHT domain and a LRR domain (Di Virgilio, 2013). In addition to the LRR, the proteins SGT1 and heat shock protein 90 may bind to NLRP3 to inhibit its activation (Gross et al., 2011). Four isoforms of NLRP2 have been described in humans (Kinoshita et al., 2005) and two in mice (Okamoto et al., 2010). NLRP2 shares structural similarities with NLRP3 in that it is comprised of a PYD, a NACHT domain and a LRR domain. One report suggests that NLRP2 may act as an inhibitor of NFκ-B activation (Bruey et al., 2004), but another report shows that NLRP2 forms a functional inflammasome in human astrocytes that is activated by ATP (Minkiewicz et al., 2013).
RIG-like receptors (RLRs)
RLRs are cytoplasmic PRRs that detect RNA viruses resulting in the production of type I interferons (IFNs) such as IFNα and IFNβ (Szabo et al., 2012). The RLR family is comprised of three receptors: retinoic acid-inducible gene-1 (Rig1), melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2). During viral infections, Rig1 is activated by double stranded (ds) RNA or 5′-ppp RNA whereas MDA5 is activated by dsRNA (Wilkins and Gale, 2010). Of the three PRRs, LGP2 remains poorly described and it is believed to regulate Rig1 and MDA5 signaling (Childs et al., 2013). RLRs are located in the cytoplasm and contain a DExD/H-box helicase domain and a carboxy-terminal domain (CTD) that bind RNA (Fig. 1). In addition, Rig1 and MDA5 have a CARD domain at the amino terminus (Kato et al., 2011). After activation of either Rig1 or MDA5, these proteins interact with the mitochondrial associated protein virus induced signaling adaptor (VISA), also known as MAVS, IPS1 or Cardif (Wilkins and Gale, 2010), followed by phosphorylation of interferon regulatory factor 3 or 7 (IRF3/7), and production of IFN stimulated genes (ISG) (Fig. 5). However, it is possible that formation of reactive oxygen species play a prominent role in the activation of these PRRs independent of viral infections (Tal et al., 2009). In addition to their role in mounting an innate immune response to RNA viruses, Rig1 and MDA5 have also been implicated in the inflammatory response of astrocytes after SCI (de Rivero Vaccari et al., 2012b).
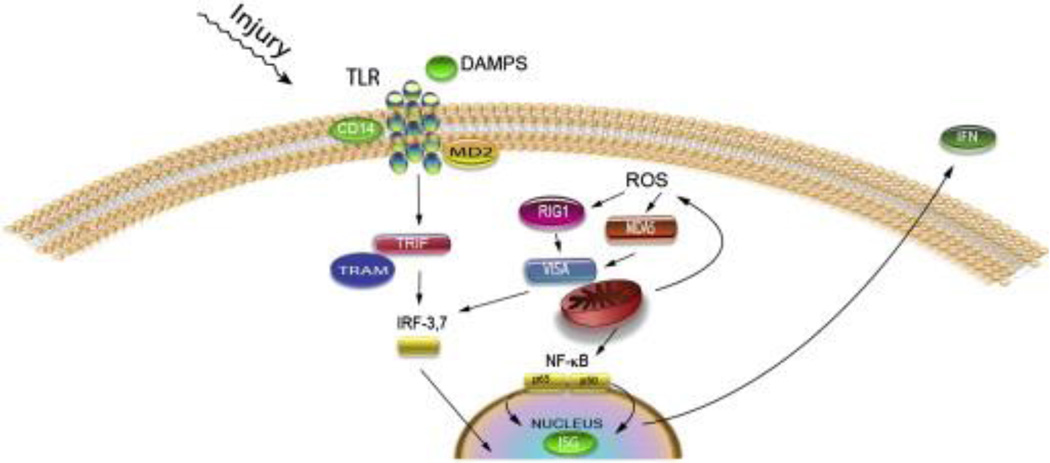
Fig. 5.
Production of type I IFN by TLR and RLR signaling: DAMPS binding to TLR signal through TRIF. TRIF recruits TRAF3 (not shown) resulting in phosphorylation of IRF3 or 7, thus activating interferon stimulated genes and type I IFNs such as IFNα and β. ROS activate RLR signaling either through RIG1 or MDA5. Either protein binds to VISA and is recruited to the mitochondrion, resulting in phosphorylation of IRF3 or 7 or activation of NF-κB, resulting in production of interferon stimulated gene proteins and the type I IFNs (IFNα and β).
Aim-2-like receptors (ALRs)
ALRs are cytoplasmic sensors that are generally activated by DNA viruses (Rathinam et al., 2010), but they have also been implicated in the innate immune response to DNA that is released from dying cells after CNS injury (Adamczak, 2012). Two ALRs have been described: IFI16 and AIM2 (Duan et al., 2011). Both proteins belong to the HIN200 (hematopoietic interferon-inducible nuclear proteins with a 200-aminoacid repeat) family of proteins and through the HIN domain they directly interact with DNA resulting in production of IFNβ (Jin et al., 2012). In addition to the HIN domain, these proteins also have a PYRIN domain and therefore are categorized as members of the PYHIN (PYRIN–HIN) protein family (Goubau et al., 2010; Schattgen and Fitzgerald, 2011) (Fig. 1). The PYRIN domain of AIM2 forms protein–protein interactions with the PYRIN domain of ASC leading to formation of a caspase-1 activating inflammasome (Rathinam et al., 2010). In CNS neurons, AIM2 forms an inflammasome that triggers pyroptosis, a novel but potentially important mode of cell death (Adamczak, 2012).
C-type lectin receptors (CLRs)
The CLR protein family consists of DEC205,Mincle, C-type lectin domain family 9 (CLEC9A) and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN). These proteins sense microbial carbohydrate moieties such as PAMPs. However, in tumor cells CLRs also sense altered glycosylated proteins. Mincle, also known as CLEC4E or Clecsf9, mounts an immune response against the fungus Malassezia (Matsunaga and Moody, 2009). Mincle and CLEC9A also recognize DAMPs released from necrotic cells (but not apoptotic cells), whereas DEC205 can recognize DAMPs released from both necrotic and apoptotic cells (Cambi and Figdor, 2009). In addition, CLRs are involved in the clearing of dying cells by phagocytosis (Ogden et al., 2001). Mincle is also activated upon death of neighboring cells (Yamasaki et al., 2008) by sensing spliceosome-associated protein 130 (SAP130), a noncarbohydrate protein that is released from dying cells. SAP130 is normally involved in spliceosome assembly and is a component of the U2 small nuclear ribonucleoprotein-associated protein complex (Cambi and Figdor, 2009; Menon et al., 2008).
Other PRR families (scavenger receptors, galectins)
Scavenger receptors are PRRs involved in uptake, transport, and metabolism of cholesterol and lipids, and can be divided into type A and type B receptors. Macrophage scavenger receptor A (SR-A) was first identified as a receptor for oxLDL, but since then several other modified self-antigens and microbial components have been identified as ligands (Brown et al., 1980; Goldstein et al., 1979; Platt and Gordon, 2001; Platt et al., 1996). Scavenger receptor B (SR-B) and CD36 are also PRRs that can bind to oxLDL and other modified lipoproteins. These PRRs are involved in binding and phagocytic uptake of PAMPs/DAMPs. In the CNS, these scavenger receptors are expressed on microglia, endothelia and astrocytes (Husemann et al., 2002). In addition tomodified lipoproteins, macrophage SR-A can bind β-amyloid, apoptotic cells, and myelin (da Costa et al., 1997; El Khoury et al., 1996; Paresce et al., 1996; Platt et al., 1996). Advanced glycation end products (AGE), which have been implicated in Alzheimer’s disease pathogenesis, also can bind to SR-A and CD36 on macrophages (Ohgami et al., 2001; Suzuki et al., 1997). CD36 and SR-B can also bind to β-amyloid and can contribute to inflammatory related pathology after stroke (Cho et al., 2005; Coraci et al., 2002; Husemann et al., 2001). Galectins, a family of β-galactoside binding proteins with a conserved carbohydrate recognition domain (CRD) motif, have also been described as PRRs (Vasta, 2012).Galectins bind to glycans on the surface of bacteria and potential pathogens (Sato and Nieminen, 2004), although they were originally thought to bind only to “self” ligands.
Pattern recognition receptors in the CNS
Toll-like receptors in CNS injury
Although TLRs have been traditionally characterized in response to pathogens, they also play an important role in regulating “sterile” inflammation. TLRs can orchestrate the innate immune response to trauma by recognizing DAMPs that are released from injured tissue. Several DAMPs are released after CNS injury and are known ligands for a range of TLRs (Table 1). These include HMGB1, heat-shock proteins (HSP60 and HSP70), degradation products of the ECM (hyaluronic acid, fibronectin) and nucleic acids such as mRNA and miRNAs that are released passively from necrotic cells (Asea et al., 2002; Demarco et al., 2005; Kariko et al., 2004; Li et al., 2001; Ohashi et al., 2000; Okamura et al., 2001; Park et al., 2004; Smiley et al., 2001; Termeer et al., 2002; Vabulas et al., 2001, 2002; Yu et al., 2006). Mitochondrial DNA and proteins also act as DAMPs, particularly mtDNA and N-formyl peptides (Zhang et al., 2010) (Table 1).
Cellular expression in the CNS
Most cells in the CNS express TLRs; however microglia express the full repertoire of TLRs. This likely enhances their ability to survey the CNS and act as a first line of defense against pathogens or traumatic injury (Davalos et al., 2005). In vitro, microglia expressm RNA for TLRs 1–9 and expression of these receptors is upregulated following microglial activation (Bsibsi et al., 2002; Olson and Miller, 2004). TLR4 can detect and respond to peripheral LPS, mediating a paracrine wave of microglial activation throughout the CNS (Laflamme and Rivest, 2001), and TLR2 activation on microglia is critical for an effective immune response against Gram-positive bacteria in the CNS (Esen et al., 2004; Kielian et al., 2005a, 2005b). In addition, intraspinal injection of LPS (TLR4), zymosan (TLR2), polyI:C (TLR3) or R848 (TLR7/8) activates microglia (Fitch et al., 1999; Popovich et al., 2002; Schonberg et al., 2007; Zhang et al., 2005). Astrocytes, neurons and oligodendrocytes also express TLRs in both naïve and pathological CNS. Astrocytes express TLR3 under resting and activated conditions (Bsibsi et al., 2006) and may upregulate TLR2 and TLR4 upon activation (Owens, 2009). Neurons express several TLRs as well. Cortical and DRG neurons express TLR3 and TLR8 (Cameron et al., 2007;Ma et al., 2006). Expression of TLR3 and TLR7 in nociceptors and other primary sensory neurons and TLR8 in axons of spinal cord dorsal horn and dorsal columns implicates TLR signaling in modulating sensations including pain or itching (pruritus) (Liu et al., 2010; Liu et al., 2012). There is limited data describing TLR expression by oligodendrocytes but TLR2 and TLR3 expression have been documented (Bsibsi et al., 2012; Sloane et al., 2010). During the first few weeks after spinal cord injury (SCI), intraspinal expression of TLR1, TLR2, TLR4, TLR6, & TLR7mRNA is increased N2-fold (Kigerl et al., 2007). Expression of several TLR-associated signaling molecules (i.e. MyD88, IRAK-4) also is increased during the first 1–2 weeks post-injury (Kigerl et al., 2007). TLR4 mRNA is strongly induced on activated CNS macrophages in and around the lesion while TLR2 is upregulated on both microglia and astrocytes (Kigerl et al., 2007). These broad and prolonged changes in expression of TLRs and related signaling molecules suggest a prominent role for this receptor family in regulating the complex but interrelated processes of post-injury inflammation, regeneration and cell death/survival.
Influences on axon growth and regeneration
Axon growth and regeneration are limited in the injured mammalian CNS. Neuron intrinsic (e.g., impaired transcriptional control over growth promoting genes) and extrinsic factors (proteins in the microenvironment that impede axon growth) inhibit axon growth or trigger growth cone collapse. The accumulation of DAMPs in the lesion site may also impair axon growth and neuron survival through TLR signaling in neurons (Fig. 3). TLR3, TLR7, and TLR8 — TLRs responsible for recognition of RNA molecules — are expressed on CNS neurons (Cameron et al., 2007; Lehmann et al., 2012; Ma et al., 2006; Ma et al., 2007). TLR3 and TLR8 are expressed in the neuronal endosomes and are also concentrated in growth cones (Cameron et al., 2007; Ma et al., 2006, 2007). TLR7 is expressed in cortical neurons and colocalizes with early endosomal protein 1 (EEA1) in endosomes (Lehmann et al., 2012). Activation of these neuronal RNA-sensing TLRs causes neurodegeneration, growth-cone collapse or inhibition of neurite outgrowth (Cameron et al., 2007; Lehmann et al., 2012; Ma et al., 2006, 2007). These distinct TLR-dependent effects vary as a function of the TLR and activation of downstream signaling pathways. TLR3 is an intracellular TLR activated by viral dsRNA and mRNA released from dead/necrotic cells (Kariko et al., 2004). TLR3 is the only TLR that does not signal through the MyD88-adaptor protein; it signals exclusively through the TRIF pathway (Fig. 2). Axon growth is inhibited in DRG and cortical neurons treated with poly(I:C) (a synthetic TLR3 agonist) or mRNA isolated from mouse brain (Cameron et al., 2007). This effect was shown to be TLR3-dependent, as the growth inhibitory effects of poly(I:C) or mRNA on DRG axon growth was abolished using neurons from TLR3−/− mice. TLR3 colocalizes with f-actin in the growth cone of DRG neurons. After treatment with poly(I:C) or mRNA, TLR3 activation causes rapid growth cone collapse (within 30 min) without inducing neurite retraction or neuron death (Fig. 3). TLR3 activation does not activate NF-κB in neurons, suggesting that its effects on growth cones/axons occur independent of canonical transcription factors (Cameron et al., 2007). Activation of TLR3 in vivo via intrathecal infusion of poly(I:C) also inhibits sensory neuron development (Cameron et al., 2007). TLR7 and TLR8 are intracellular TLRs activated by ssRNA. Recently, specific uridine-rich sequences of RNA and miRNAs with a GU-rich element were identified as endogenous TLR7 ligands (Green et al., 2012; Lehmann et al., 2012). TLR8 expression is developmentally regulated in the mouse brain and is expressed on neurons and axons (Ma et al., 2006, 2007). Although TLR8 levels decrease post-natally, TLR8 expression is detectable in the soma of adult neurons. Treatment of cortical neurons with a synthetic TLR8 agonist (R-848) reduces neurite outgrowth and causes caspase-3 dependent cell death (Fig. 3). Interestingly, neuronal TLR8 activation does not trigger the typical TLR-signaling pathway identified in innate immune cells, i.e., neuronal TLR8 does not activate NF-κB or AP-1, nor do the effects require MAPK activation. Finally, TLR8 effects on neurite outgrowth and neuron survival are independent of MyD88, suggesting that a novel TLR8 signaling pathway exists in neurons (Ma et al., 2007). A recent paper published by Lehmann et al. (2012) documents a previously unreported role for miRNAs in the CNS. Let-7b and other miRNAs with a GU-rich core serve as endogenous ligands for TLR7 found onmicroglia and neurons. In vitro, let-7bmiRNA induces cortical and hippocampal neuron death through a MyD88, and caspase-3 dependent signaling pathway. Neuronal TLR7 signaling also triggers phosphorylation of IRAK4 but does not activate NF-κB. Let-7b also triggers microglia activation in a TLR7-dependent but NF-κB-independent manner. In vivo, intrathecal infusion of let-7b causes axonal injury and neuron death in wild-type but not TLR7−/− mice; however, restoring signaling in TLR7−/− neurons via transfection of TLR7 restores their susceptibility to let-7b-mediated toxicity. This effect was not dependent on activated microglia (Lehmann et al., 2012). To date, all studies documenting direct effects of TLR signaling on neurons and axon growth have involved “RNA-sensing” TLRs, suggesting the presence of a conserved mechanism to recognize and avoid free nucleic acids in the CNS. Collectively these studies define a broader role for TLRs in the CNS beyond recognition of danger signals and innate immune activation. Perhaps this is not surprising given the developmental role for the Toll proteins in dorsoventral patterning in Drosophila (Anderson et al., 1985a, 1985b). Inhibition of DAMP release following SCI and subsequent activation of neuronal TLRs may be an additional therapeutic target to increase regeneration through the injury site. Indeed, TLR signaling can activate RhoA GTPase (Manukyan et al., 2009), an enzyme that is involved in triggering growth cone collapse when myelin inhibitory proteins bind to the Nogo-receptor (Niederost et al., 2002). The concentration of TLRs in neuronal growth cones may indicate that neurons rely on TLRs to activate a general signaling pathway utilized by other “inhibitory” signals in the CNS. Activation of TLRs on glial cells in the CNS can also exert indirect effects on axon growth. For example, zymosan-activated macrophages can stimulate axon growth (Gensel et al., 2009; Yin et al., 2003). Zymosan is a yeast cell-wall protein that is a ligand for TLR2 (and a subset of CLRs). In the eye, zymosan-activated macrophages promote growth of retinal ganglion cell (RGC) axons after optic nerve crush injury; however, these same macrophages also release high molecular weight neurotoxins (Yin et al., 2003). In the spinal cord, zymosan-activated macrophages promote axon growth but cause collateral injury (cell death and demyelination) to neurons and glia (Gensel et al., 2009; Popovich et al., 2002; Schonberg et al., 2007). Intravitreal injection of Pam(3)Cys, a synthetic TLR2 agonist, also promotes RGC axon growth after optic nerve injury (Hauk et al., 2010).
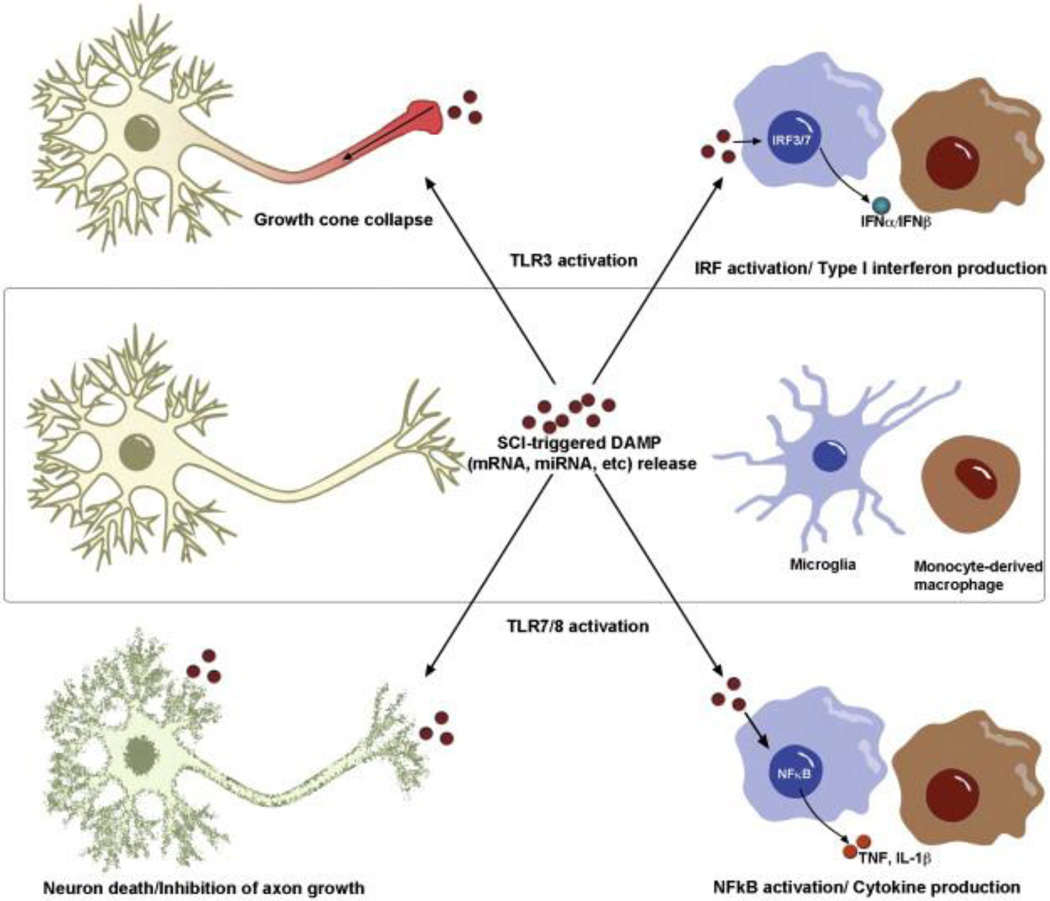
Fig. 3.
Activation of “RNA-sensing” TLRs on neurons and microglia triggers distinct effects. Signaling through TLR3 on neurons triggers rapid collapse of growth cones through a non-canonical signaling pathway, while the same ligand–receptor interaction elicits synthesis/release of type I interferons from activated microglia and monocyte-derived macrophages. TLR7/8 activation leads to neuron death and inhibition of axon outgrowth through undefined mechanisms; however, TLR7/8 activation of CNS macrophages triggers canonical TLR signaling pathways leading to NFκB activation and inflammatory cytokine production.
Influences on neuron and glial survival after SCI
Only three TLRs have been studied in preclinical models of traumatic SCI — TLR2, TLR4 and TLR9 (David et al., 2013; Kigerl et al., 2007). Because the DAMPs that exist in the injured spinal cord (e.g., HMGB1, HSPs, fibronectin) signal through TLR2 and TLR4, these TLRs were the focus of the earliest research paper on TLRs and SCI (Kigerl et al., 2007). Since these TLRs elicit inflammatory signaling in macrophages, we predicted that pathology would be decreased and neurological function improved in SCI mice deficient in either TLR2 or TLR4. However, contrary to our original hypothesis, deficiencies in TLR2 and TLR4 signaling increased myelin pathology and lesion size. This was accompanied by sustained functional impairment and aberrant glial scar formation around the lesion site, particularly in TLR4 deficient mice. To date, the precise mechanisms underlying these unusual pathologic presentations are unknown and have been difficult to reconcile. Indeed, the cellular and molecular data are counterintuitive. Despite a deficiency in signaling via these two highly conserved innate immune receptors, intraspinal inflammation is more pronounced than in SCI wild-type mice and expression of inflammatory cytokine mRNA is reduced (Kigerl et al., 2007). TLR9 is expressed by spinal cord microglia, astrocytes, and neurons (David et al., 2013). TLR9 is activated by bacterial unmethylated CpGDNA and by immune complexes. Intrathecal delivery of a CpG ODN 1826, a TLR9 agonist, into lumbar spinal cord of uninjured wild-type mice robustly increases expression of TNF, IL-1β and CXCL1 mRNA. Cytokine mRNA is not increased by an identical infusion into TLR9−/− mice (David et al., 2013). Following contusive SCI, intrathecal delivery of a TLR9 antagonist, CpG ODN 2088, reduced the number of intraspinal CD45+, CD11b+, and CD3+ cells for the first month post-SCI. Although locomotor function was unaffected, measures of pain (thermal hyperalgesia) and TNF were reduced at 4 weeks postinjury by the TLR9 antagonist (David et al., 2013). A detailed review on the role of neuroinflammation and neuropathic pain is found in this issue (E.T. Walters, 2014). Since SCI increases the expression of many TLRs that ultimately promote inflammatory gene expression, it is logical tomanipulate a point of convergence in the TLR signaling pathway. MyD88 is an adaptor protein shared by many TLRs. Direct intraspinal injection of aMyD88 inhibitory peptide immediately following injury reduced inflammatory cytokine expression, lesion size and improved hind limb function within the first 7–14 days post-injury (Yao et al., 2012). These data suggest that activation of TLRs and subsequent MyD88-dependent signaling is detrimental after SCI; however, the interleukin-1 (IL-1) receptor also uses MyD88. When mice deficient in either MyD88 or IL-1R are subject to SCI, intraspinal neutrophil and monocyte influx is attenuated as is the expression of chemokines involved in leukocyte recruitment (Pineau et al., 2010). Together, these data indicate that MyD88 plays a central role in inflammatory mediated-injury and repair through the regulation of TLR and/or IL-1 receptor pathways.
NOD-like receptors (NLRs)
Inflammasomes in the CNS
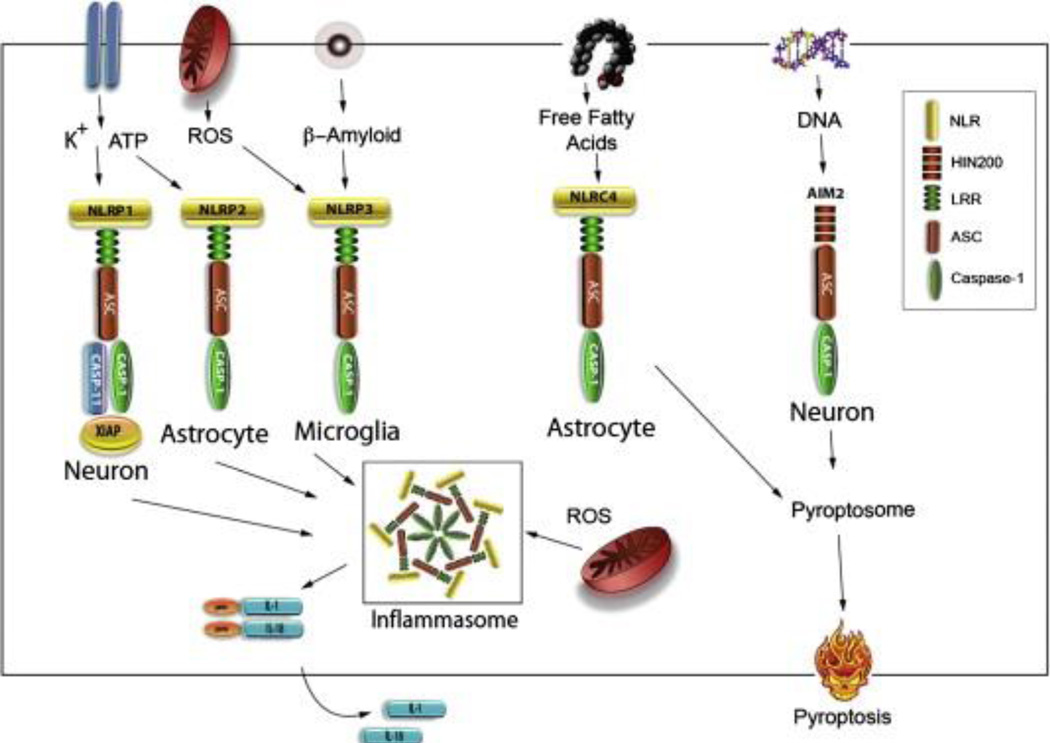
The NLRP1 inflammasome in neurons. The first report of an inflammasome in the CNS was the NLRP1 inflammasome in neurons. The NLRP1 inflammasome was first described after injury to the spinal cord in rodents (de Rivero Vaccari et al., 2008). It is comprised of inflammatory caspases (caspase-1 and caspase-11), the adaptor protein ASC, the NOD-like receptor protein NLRP1 and the inhibitor of apoptosis protein XIAP (Figures 1 & 4) (de Rivero Vaccari et al., 2008). In addition to NRLP1 inflammasome involvement after SCI, this inflammasome has also been described to mediate an innate immune response after traumatic brain injury (de Rivero Vaccari et al., 2009) and stroke (Abulafia et al., 2009). After injury to the brain or spinal cord there is activation of caspase-1 and cleavage of XIAP. The full-length form of XIAP has a greater capacity to inhibit caspase activation than its cleaved fragments (Deveraux et al., 1999). Thus, upon cleavage of XIAP there is decreased inhibition of XIAP on caspase-1 activation, which facilitates inflammasome activation. Due to the role of inflammasome activation on the processing of the inflammatory cytokines IL-1β and IL-18, the inflammasome is a promising therapeutic target to improve outcomes after injury to the CNS. Inhibition of the NLRP1 inflammasome in neurons with a neutralizing antibody against the adaptor protein ASC decreases inflammation and improves histopathological and functional outcomes after SCI (de Rivero Vaccari et al., 2008) and brain injury (de Rivero Vaccari et al., 2009). Also, a neutralizing antibody against NLRP1 inhibits NLRP1 inflammasome activation after stroke leading to decreased inflammation (Abulafia et al., 2009). Taken together, in addition to inflammasomes in astrocytes and microglia, neuronal inflammasomes appear to contribute robustly to the inflammatory response after CNS injury. The NLRP2 inflammasome in astrocytes. The inflammatory role of astrocytes is well documented, but not until recently was it reported that these cells contain inflammasomes. Specifically, astrocytes express an inflammasome that is comprised of the NOD-like receptor NLRP1/NALP2, the adaptor protein ASC and caspase-1 (Figures 1 & 4) (Minkiewicz et al., 2013). The NLRP2 inflammasome in astrocytes forms protein–protein interactions with the purinergic receptor P2X7 and with the channel protein pannexin-1. ATP activates this inflammasome at concentrations of 500 µM–1 mM (Minkiewicz et al., 2013). Moreover, inhibition of pannexin-1 with 1 mM probenecid or with 5 µM brilliant blue G (BBG) attenuates ATP-dependent NLRP2 inflammasome activation. Inhibition of the NLRP2 inflammasome with Probenecid, BBG or knockdown with shRNA against NLRP2 inhibits inflammasome activation in human astrocytes, resulting in decreased processing of IL-1β and IL-18 (Minkiewicz et al., 2013). Thus, the NLRP2 inflammasome in human astrocytes activates caspase-1 and the processing of IL-1 inflammatory cytokines after CNS injury. The NLRP3 inflammasome in microglia. Microglia share many of the phenotypic and functional characteristics of macrophages and are the resident immune cells of the CNS. The NLRP3 inflammasome plays a role in the inflammatory response against Staphylococcus aureus (Hanamsagar et al., 2011) and may also be a pivotal regulator of microglia-mediated pathology in several CNS diseases including Alzheimer’s disease. Stimulation of microglia with amyloid-β activates the NLRP3 inflammasome (Figure 4) (Halle et al., 2008). In the BV2 microglial cell line, the NLRP3 inflammasome has been implicated in regulating the immune response in prion diseases (Shi et al., 2013). A role for the NLRP3 inflammasome in CNS injury has yet to be described. The AIM2 inflammasome in neurons. The AIM2 inflammasome binds DNA and activates caspase-1. The AIM2 inflammasome in cortical neurons is comprised of ASC and caspase-1 (Figure 4) (Adamczak, 2012). Binding of DNA by AIM2 occurs through its HIN domain (Schroder et al., 2009). The AIM2 inflammasome is the only inflammasome in which there is convincing evidence of a direct interaction between the AIM2-like receptor (ALR) protein and the activating ligand. DNA released from damaged cells after traumatic brain injury is recognized by the AIM2 inflammasome, thus activating caspase-1 and processing of IL-1β into its active form. Activation of the AIM2 inflammasome in cortical neurons also can trigger the inflammasome-mediated programmed cell death process of pyroptosis (Adamczak, 2012).
Fig. 4.
Diverse ligands elicit cell-specific inflammasome activation in CNS. High extracellular potassium is required for NLRP1 inflammasome activation in neurons and astrocytes. High concentrations of ATP in the extracellular space stimulate both the NLRP1 and the NLRP2 inflammasome in neurons and astrocytes. The NLRP3 inflammasome is activated by β-amyloid and by ROS. Signals such as ROS appear to be typical activators of all inflammasomes, but thus far has only been shown for NLRP3 inflammasome signaling. NLRC4 inflammasome activation is activated by free fatty acids in astrocytes, and DNA stimulates AIM2 inflammasome activation. Canonical inflammasomes including NLRP1, NLRP3, AIM2 and NLRC4 recruit caspase-1 through interactions with ASC causing pro-IL-1β and pro-IL18 to be cleaved into their active forms IL-1β and IL18. The NRLC4 and AIM2 inflammasome have been involved in the caspase-1 mediated cell death process of pyroptosis by formation of the pyroptosome. NLR: NOD-like receptors, HIN200: hemopoietic IFN-inducible nuclear, LRR: leucine rich repeat, ASC: apoptosis-associated speck-like protein containing a CARD.
Pyroptosis: Inflammasome mediated programmed cell death
Pyroptosis is characterized by the activation of caspase-1 and the formation of the pyroptosome, a complex of oligomerized ASC molecules. Since pyroptosis is caspase-1 dependent, it can be inhibited by the caspase-1 blocker YVAD (Tyr-Val-Ala-Asp) (Fernandes-Alnemri et al., 2007; Fink et al., 2008). In cortical neurons, DNA is responsible for activating the AIM2 inflammasome, resulting in the formation of the pyroptosome and the opening of 2–3 nm membrane pores that are permeable to the fluorescent dye YO-PRO and the cell-impermeant viability indicator ethidium homodimer-2 (Eth-D2). Inhibition with the pannexin-1 channel blockers probenecid or brilliant blue FCF decreases pore formation, indicating that pannexin-1 is the pore that opens during the process of pyroptosis (Adamczak, 2012). Mechanisms of inflammasome activation in the CNS Several PAMPs and DAMPs have been shown to activate inflammasomes outside of the CNS, but there is limited information about the ligands responsible for activation of inflammasomes within the CNS (Figure 4). ATP has recently been shown to activate the NLRP2 inflammasome in human astrocytes (Minkiewicz et al., 2013) whereas amyloid-β activates the NLRP3 inflammasome in microglia (Halle et al., 2008). Palmitate, a saturated free fatty acid, activates the NLRC4 inflammasome in primary rat astrocytes (Liu and Chan, 2012). Reactive oxygen species (ROS) are also capable of activating the inflammasome (Bauer et al., 2010), although the mechanism of activation is controversial. It is possible that mitochondrial dysfunction following injury results in ROS production that leads to activation of inflammasomes. Thioredoxin (TRX)-interacting protein (TXNIP) has been associated with oxidative stress-dependent inflammasome regulation (Lane et al., 2013). TXNIP is initially bound to TRX. Release of ROS from mitochondria causes TXNIP to be released from TRX upon oxidation. TXNIP then binds to inflammasomes leading to their activation (Lane et al., 2013). An important difference between activation of inflammasomes within the CNS and in peripheral tissues is that high extracellular potassium activates CNS inflammasomes (Silverman et al., 2009), whereas potassium efflux is responsible for activating inflammasomes in peripheral tissues (Franchi et al., 2007). In neurons and astrocytes high extracellular potassiumopens the pannexin-1 channel allowing efflux of ATP (Franchi et al., 2007). The NLRP1 inflammasome forms protein–protein interactions with the purinergic receptor P2X7 and with pannexin-1 (Silverman et al., 2009). Thus, the NLRP1 inflammasome is regulated by both ATP and potassium ions. Relatively high concentrations of ATP are required to activate P2X7, but the purinergic receptor P2X4, which is also involved in inflammasome regulation (de Rivero Vaccari et al., 2012a), has a lower threshold for ATP-dependent activation (North and Surprenant, 2000). Taken together, a model of inflammasome activation in the CNS can be explained as follows: ATP is released from dying cells after CNS injury and activates P2X4. Once activated P2X4 releases potassium, which causes the opening of the pannexin-1 channel. This channel releases ATP, which further increases the concentration of ATP available to open the P2X7 receptor and subsequently activates inflammasomes (Bernier, 2012) (Figure 2). The downstream events linking P2X7 receptor activation and cleavage of caspase-1 remain unknown.
Inflammasome proteins as biomarkers of CNS injury
The inflammasome proteins ASC, NLRP1 and caspase-1 are secreted into the cerebrospinal fluid (CSF) following traumatic brain injury. Interestingly, the levels of these proteins were elevated in brain-injured patients that had poorer Glasgow outcome scale scores (GOS 1–3) when compared to less severely injured patients (GOS scores of 4–5) (Adamczak et al., 2012). GOS scores were obtained five months after injury but inflammasome protein levels were measured in CSF samples collected early after injury. Thus, acute CSF concentrations of inflammasome proteins may be used as biomarkers to predict long-term outcome or to guide therapeutic interventions with the goal of lowering inflammasome protein concentrations in the CSF. A similar relationship may also be present after SCI (unpublished data). The inflammasome as a target for post-traumatic therapeutic hypothermia Post-traumatic therapeutic hypothermia is a promising therapeutic intervention to treat patients with traumatic brain and spinal cord injury (Ahmad et al., 2013; Grulova et al., 2013). Animal studies have shown that the neuroprotective effects associated with hypothermia are vast (Ahmad et al., 2013; Bell et al., 1998; Horiguchi et al., 2002) and include anti-inflammatory effects, mediated in part through inhibition of inflammasomes (Gu et al., 2014). Post-traumatic therapeutic hypothermia in brain-injured rodents inhibits inflammasome activation in the injured cortex and in cultured cortical neurons (Tomura et al., 2012). Thus, hypothermia can inhibit inflammasome activation and confer neuroprotection after trauma.
RLRs after spinal cord injury
After SCI, reactive astrogliosis causes a glial scar to form around the site of injury. This scar is a structural and molecular barrier to successful axon regeneration (Yuan and He, 2013). The PRRs Rig1 and MDA5 contribute to this process of astrocyte activation by increasing glial fibrillary acidic protein (GFAP) and vimentin in astrocytes (de Rivero Vaccari et al., 2012b). Following contusive cervical SCI in rats, Rig1 and MDA5 expression is increased 6 h after injury, leading to increased expression of the type I IFNs IFNα and β (Fig. 5) (de Rivero Vaccari et al., 2012b). Stimulation of Rig1 and MDA5 with synthetic ligands that mimic dsRNA (poly(I:C)) also activates RLRs and IRF-3 phosphorylation, resulting in increased expression of GFAP and vimentin. Stimulation with low molecular weight poly(I:C), which stimulates Rig1 signaling, increased expression of both GFAP and vimentin, whereas stimulation of MDA5 with high molecular weight poly(I:C) increased expression of only GFAP (de Rivero Vaccari et al., 2012b). RLR signaling in astrocytes can be inhibited with mitochondrial E3 ubiquitin protein ligase 1 (MUL1) (de Rivero Vaccari et al., 2012b) suggesting that manipulation of RLRs could be a novel mechanism for overcoming the growth in hibitory effects of glial scarring.
Mincle after traumatic brain injury
TNF is an important cytokine that contributes to the inflammatory innate immune response after CNS injury. TNF singling is in part regulated by the PRR, mincle. Mincle belongs to the C-type lectin (CLR) family of PRRs. Preliminary data suggest that following fluid percussion TBI, cells release SAP130, which binds mincle to activate its signaling as determined by phosphorylation of spleen tyrosine kinase (syk), resulting in production of TNF (de Rivero Vaccari et al., 2013). Mincle contributes to the innate immune response from neurons after TBI in rodents. In addition, preliminary data show SAP130 and mincle are increased in the brain of patients with TBI (de Rivero Vaccari et al., 2013). These data indicate that in addition to NLR and RLR signaling, CLRs may also contribute to the innate immune response in the CNS after injury.
Conclusion
PRRs are a diverse group of receptor families that recognize heterogenous ligands, both self (DAMPs) and non-self (PAMPs), and elicit innate immune activation in response to injury or disease. Broad expression and injury-induced upregulation of these receptor families in the CNS by microglia, astrocytes, and neurons indicates a central role for PRRs in post-injury neurodegeneration and repair. In a complex injury site, such as a traumatic CNS injury, it is likely that these receptor families act synergistically to tailor the inflammatory response. Understanding how these signaling pathways are activated by and respond to CNS injury will lead to identification of novel therapeutic targets designed to modulate the innate immune response.
Acknowledgments
The authors acknowledge support from the following: Craig H. Neilson 164246 (KAK) & 221346 (RWK); NIH NS059836 (RWK); NIH NS043246 (PGP); & the Poppleton Research Designated Chair (PGP).
References
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- Adamczak SE. Molecular Recognition of DNA by the AIM2 Inflammasome Induces Neuronal Pyroptosis: Implications in Infection and Host Tissue Damage (Open Access Dissertations) 2012 [Google Scholar]
- Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J. Neurosurg. 2012;117:1119–1125. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F, Wang MY, Levi AD. Hypothermia for acute spinal cord injury — a review. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal–ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985a;42:1791–798. doi: 10.1016/0092-8674(85)90275-2. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985b;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- Bell TE, Kongable GL, Steinberg GK. Mild hypothermia: an alternative to deep hypothermia for achieving neuroprotection. J. Cardiovasc. Nurs. 1998;13:34–44. doi: 10.1097/00005082-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Bernier LP. Purinergic regulation of inflammasome activation after central nervous system injury. J. Gen. Physiol. 2012;140:571–575. doi: 10.1085/jgp.201210875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Brown MS, Basu SK, Falck JR, Ho YK, Goldstein JL. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J. Supramol. Struct. 1980;13:67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Bruey-Sedano N, Newman R, Chandler S, Stehlik C, Reed JC. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NFkappaB and caspase-1 activation in macrophages. J. Biol. Chem. 2004;279:51897–51907. doi: 10.1074/jbc.M406741200. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. GLIA. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Nomden A, van Noort JM, Baron W. Toll-like receptors 2 and 3 agonists differentially affect oligodendrocyte survival, differentiation, and myelin membrane formation. J. Neurosci. Res. 2012;90:388–398. doi: 10.1002/jnr.22767. [DOI] [PubMed] [Google Scholar]
- Cambi A, Figdor C. Necrosis: C-type lectins sense cell death. Curr. Biol. 2009;19:R375–R378. doi: 10.1016/j.cub.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, Flavell R, Strittmatter SM, Volpe J, Sidman R, Vartanian T. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J. Neurosci. 2007;27:13033–13041. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KS, Randall RE, Goodbourn S. LGP2 plays a critical role in sensitizing mda-5 to activation by double-stranded RNA. PLoS ONE. 2013;8:e64202. doi: 10.1371/journal.pone.0064202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J. Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa CC, van der Laan LJ, Dijkstra CD, Bruck W. The role of the mouse macrophage scavenger receptor in myelin phagocytosis. Eur. J. Neurosci. 1997;9:2650–2657. doi: 10.1111/j.1460-9568.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- David BT, Ratnayake A, Amarante MA, Reddy NP, Dong W, Sampath S, Heary RF, Elkabes S. A Toll-like receptor 9 antagonist reduces pain hypersensitivity and the inflammatory response in spinal cord injury. Neurobiol. Dis. 2013;54:194–205. doi: 10.1016/j.nbd.2012.12.012. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S. P2X4 receptors influence inflammasome activation after spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2012a;32:3058–3066. doi: 10.1523/JNEUROSCI.4930-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Minkiewicz J, Wang X, De Rivero Vaccari JC, German R, Marcillo AE, Dietrich WD, Keane RW. Astrogliosis involves activation of retinoic acid-inducible gene-like signaling in the innate immune response after spinal cord injury. Glia. 2012b;60:414–421. doi: 10.1002/glia.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Brand F, III, Berti A, de Rivero Vaccari JC. Mincle signaling contributes to the inflammatory response after traumatic brain injury. J. Immunol. 2013;190(P1297) [Google Scholar]
- Demarco RA, Fink MP, Lotze MT. Monocytes promote natural killer cell interferon gamma production in response to the endogenous danger signal HMGB1. Mol. Immunol. 2005;42:433–444. doi: 10.1016/j.molimm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol. Rev. 2013;65:872–905. doi: 10.1124/pr.112.006171. [DOI] [PubMed] [Google Scholar]
- Duan X, Ponomareva L, Veeranki S, Panchanathan R, Dickerson E, Choubey D. Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts. Mol. Cancer Res. 2011;9:589–602. doi: 10.1158/1541-7786.MCR-10-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J. Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, Bertin J, Gough PJ. Autolytic proteolysis with in the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J. Neurosci. 2009;29:3956–3968. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. U. S. A. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubau D, Rehwinkel J, Reis e Sousa C. PYHIN proteins: center stage in DNA sensing. Nat. Immunol. 2010;11:984–986. doi: 10.1038/ni1110-984. [DOI] [PubMed] [Google Scholar]
- Green NM, Moody KS, Debatis M, Marshak-Rothstein A. Activation of autoreactive B cells by endogenous TLR7 and TLR3 RNA ligands. J. Biol. Chem. 2012;287:39789–39799. doi: 10.1074/jbc.M112.383000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol. Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Grulova I, Slovinska L, Nagyova M, Cizek M, Cizkova D. The effect of hypothermia on sensory–motor function and tissue sparing after spinal cord injury. Spine J. 2013 Dec 1;13(12):1881–1891. doi: 10.1016/j.spinee.2013.06.073. [DOI] [PubMed] [Google Scholar]
- Gu LJ, Xiong XX, Ito T, Lee J, Xu BH, Krams S, Steinberg GK, Zhao H. Moderate hypothermia inhibits brain inflammation and attenuates stroke induced immunodepression in rats. CNS Neurosci. Ther. 2014 Jan;20(1):67–75. doi: 10.1111/cns.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1beta/IL-18 processing are influenced by distinct pathways in microglia. J. Neurochem. 2011;119:736–748. doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hauk TG, Leibinger M, Muller A, Andreadaki A, Knippschild U, Fischer D. Stimulation of axon regeneration in the mature optic nerve by intravitreal application of the Toll-like receptor 2 agonist Pam3Cys. Invest. Ophthalmol. Vis. Sci. 2010;51:459–464. doi: 10.1167/iovs.09-4203. [DOI] [PubMed] [Google Scholar]
- Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediat. Inflamm. 2012;2012:315941. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi T, Shimizu K, Ogino M, Yamaguchi N, Suga S, Inamasu J, Kawase T. Neuroprotection role of adenosine under hypothermia in the rat global ischemia involves inhibition of not dopamine release but delayed postischemic hypoperfusion. Brain Res. 2002;952:222–231. doi: 10.1016/s0006-8993(02)03242-0. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Kodama T, Silverstein SC. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrillar betaamyloid. J. Neuroimmunol. 2001;114:142–150. doi: 10.1016/s0165-5728(01)00239-9. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. GLIA. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr The immune system evolved to discriminate infectious non self from noninfectious self. Immunol. Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA, Xiao TS. Structures of the HIN domain: DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36:561–571. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kato H, Takahasi K, Fujita T. RIG-I-like receptors: cytoplasmic sensors for nonself RNA. Immunol. Rev. 2011;243:91–98. doi: 10.1111/j.1600-065X.2011.01052.x. [DOI] [PubMed] [Google Scholar]
- Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. GLIA. 2005a;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect. Immun. 2005b;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009 Jan 1;457(7225) doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Wang Y, Hasegawa M, Imamura R, Suda T. PYPAF3, a PYRIN containing APAF-1-like protein, is a feedback regulator of caspase-1-dependent interleukin-1beta secretion. J. Biol. Chem. 2005;280:21720–21725. doi: 10.1074/jbc.M410057200. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- Lane T, Flam B, Lockey R, Kolliputi N. TXNIP shuttling: missing link between oxidative stress and inflammasome activation. Front. Physiol. 2013;4:50. doi: 10.3389/fphys.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kalin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S. An unconventional role for miRNA: let- 7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- Liu L, Chan C. Activation of NLC4 inflammasome in primary rat astrocytes by palmitate enhances Alzheimer’s disease-like changes in primary neurons. Alzheimers Dement. 2012:8. [Google Scholar]
- Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7mediates pruritus. Nat. Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J. Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Haynes RL, Sidman RL, Vartanian T. TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle. 2007;6:2859–2868. doi: 10.4161/cc.6.23.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J. Immunol. 2009;182:3522–3529. doi: 10.4049/jimmunol.0802280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Masters SL. Specific inflammasomes in complex diseases. Clin. Immunol. 2013;147:223–228. doi: 10.1016/j.clim.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J. Exp. Med. 2009;206:2865–2868. doi: 10.1084/jem.20092533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Matzinger P. An innate sense of danger. Semin. Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Menon S, Tsuge T, Dohmae N, Takio K, Wei N. Association of SAP130/SF3b-3 with Cullin-RING ubiquitin ligase complexes and its regulation by the COP9 signalosome. BMC Biochem. 2008;9:1. doi: 10.1186/1471-2091-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkiewicz J, de Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013 Jul;61(7):1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N, Nagase T, Miyajima N, Sazuka T, Tanaka A, Sato S, Seki N, Kawarabayasi Y, Ishikawa K, Tabata S. Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041-KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1 (supplement) DNA Res. 1994;1:251–262. doi: 10.1093/dnares/1.5.251. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Ohgami N, Nagai R, Ikemoto M, Arai H, Kuniyasu A, Horiuchi S, Nakayama H. Cd36, a member of the class b scavenger receptor family, as a receptor for advanced glycation end products. J. Biol. Chem. 2001;276:3195–3202. doi: 10.1074/jbc.M006545200. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, Dinarello CA, Fujita M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J. Biol. Chem. 2010;285:6477–6488. doi: 10.1074/jbc.M109.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous systeminnate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Owens T. Toll-like receptors in neurodegeneration. Curr. Top. Microbiol. Immunol. 2009;336:105–120. doi: 10.1007/978-3-642-00549-7_6. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav. Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Platt N, Gordon S. Is the class Amacrophage scavenger receptor (SR-A)multifunctional? — The mouse’s tale. J. Clin. Invest. 2001;108:649–654. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Rajamäki K, Nordström T, Nurmi K, Åkerman KE, Kovanen PT, Öörni K, Eklund KK. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 2013;288:13410–13419. doi: 10.1074/jbc.M112.426254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. U. S. A. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schöneberg T, Schaefer M, Krügel U, Smajilovic S, Bräuner-Osborne H, Baerwald C, Wagner U. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Nieminen J. Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj. J. 2004;19:583–591. doi: 10.1023/B:GLYC.0000014089.17121.cc. [DOI] [PubMed] [Google Scholar]
- Schattgen SA, Fitzgerald KA. The PYHIN protein family as mediators of host defenses. Immunol. Rev. 2011;243:109–118. doi: 10.1111/j.1600-065X.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- Schonberg DL, Popovich PG, McTigue DM. Oligodendrocyte generation is differentially influenced by Toll-Like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J. Neuropathol. Exp. Neurol. 2007:66. doi: 10.1097/nen.0b013e31815c2530. [DOI] [PubMed] [Google Scholar]
- Schroder K, Muruve DA, Tschopp J. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr. Biol. 2009;19:R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Shi F, Yang Y, Kouadir M, Fu Y, Yang L, Zhou X, Yin X, Zhao D. Inhibition of phagocytosis and lysosomal acidification suppresses neurotoxic prion peptideinduced NALP3 inflammasome activation in BV2 microglia. J. Neuroimmunol. 2013;260:121–125. doi: 10.1016/j.jneuroim.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J. Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Szabo A, Bene K, Gogolak P, Rethi B, Lanyi A, Jankovich I, Dezso B, Rajnavolgyi E. RLR-mediated production of interferon-beta by a human dendritic cell subset and its role in virus-specific immunity. J. Leukoc. Biol. 2012;92:159–169. doi: 10.1189/jlb.0711360. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Mitcham JL, Dower SK, Sims JE, Testa JR. Chromosomal localization of TIL, a gene encoding a protein related to the Drosophila transmembrane receptor Toll, to human chromosome 4p14. Genomics. 1996;32:486–488. doi: 10.1006/geno.1996.0150. [DOI] [PubMed] [Google Scholar]
- Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal OS that spur autophagy and immunity. Immunol. Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J. Exp. Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J. Cereb. Blood Flow Metab. 2012;32:1939–1947. doi: 10.1038/jcbfm.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Vasta GR. Galectins as pattern recognition receptors: structure, function, and evolution. Adv. Exp. Med. Biol. 2012;946:21–36. doi: 10.1007/978-1-4614-0106-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET. Neuroinflammatory contributions to pain after SCI: Roles for central glial mechanisms and nociceptor-mediated host defense. Exp. Neurol. 2014;258:48–61. doi: 10.1016/j.expneurol.2014.02.001. (in this issue) [DOI] [PubMed] [Google Scholar]
- Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr. Opin. Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- Yao AH, Jia LY, Zhang YK, Ma QR, Cheng P, Liu L, Ju G, Kuang F. Early blockade of TLRs MyD88-dependent pathway may reduce secondary spinal cord injury in the rats. Evid. Based Complement. Alternat. Med. 2012;2012:591298. doi: 10.1155/2012/591298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013;29:421–435. doi: 10.1007/s12264-013-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Trautmann K, Schluesener HJ. Microglia activation in rat spinal cord by systemic injection of TLR3 and TLR7/8 agonists. J. Neuroimmunol. 2005;164:154–160. doi: 10.1016/j.jneuroim.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]