Abstract
Parkinson's disease (PD) is a multisystem disorder, involving several monoaminergic neurotransmitter systems resulting in a broad range of motor and non-motor symptoms. Pathological hallmarks of PD are the loss of dopaminergic neurons and the accumulation of alpha-synuclein, however also being present in the serotonergic raphe nuclei early in the disease course. The dysfunction of the serotonergic system projecting to the hippocampus might contribute to early non-motor symptoms such as anxiety and depression. The adult hippocampal dentate gyrus (DG), a unique niche of the forebrain continuously generating new neurons, may particularly present enhanced susceptibility towards accumulating alpha-synuclein levels. The underlying molecular mechanisms in the context of neuronal maturation and survival of new-born neurons are yet not well understood. To characterize the effects of overexpression of human full-length alpha-synuclein on hippocampal cellular and synaptic plasticity, we used a recently generated BAC alpha-synuclein transgenic rat model showing important features of PD such as widespread and progressive alpha-synuclein aggregation pathology, dopamine loss and age-dependent motor decline. At the age of four months, thus prior to the occurrence of the motor phenotype, we observed a profoundly impaired dendritogenesis of neuroblasts in the hippocampal DG resulting in severely reduced survival of adult new-born neurons. Diminished neurogenesis concurred with a serotonergic deficit in the hippocampus as defined by reduced levels of serotonin (5-HT) 1B receptor, decreased 5-HT neurotransmitter levels, and a loss of serotonergic nerve terminals innervating the DG/CA3 subfield, while the number of serotonergic neurons in the raphe nuclei remained unchanged. Moreover, alpha-synuclein overexpression reduced proteins involved in vesicle release, in particular synapsin-1 and Rab3 interacting molecule (RIM3), in conjunction with an altered ultrastructural architecture of hippocampal synapses. Importantly, alterations of the hippocampal serotonergic system were associated with an anxiety-like behavior consisting of reduced exploratory behavior and feeding in transgenic rats. Taken together, these findings imply that accumulating alpha-synuclein severely affects hippocampal neurogenesis paralleled by impaired 5-HT neurotransmission prior to the onset of aggregation pathology and motor deficits in this transgenic rat model of PD.
Keywords: Parkinson's disease, alpha-synuclein, hippocampus, 5-HT, neurogenesis, dendritogenesis, synapse
Introduction
Non-motor symptoms (NMS) like anxiety and depression play an important role in synucleinopathies, such as Parkinson's disease (PD) and often precede motor deficits (Hinnell et al., 2012), severely affecting quality of life (Gallagher et al., 2010). NMS have been linked to monoaminergic deficits within the limbic system including the hippocampus (HC), implicated in affective and cognitive functions (Halliday et al., 2014; van Mierlo et al., 2015). In fact, degeneration within the serotonergic system may occur prior to the progressive loss of nigrostriatal dopaminergic neurons (Braak et al., 2003; Halliday et al., 1990). The raphe nuclei predominantly consisting of serotonergic neurons, show early intracellular accumulation of alpha-synuclein (α-syn) (Braak et al., 2003), accompanied by the loss of serotonergic neurons (Halliday et al., 1990) and reduced expression of tryptophan hydroxylase 2 (TPH2) in the median raphe nucleus (MnR) in PD patients (Kovacs et al., 2003). Both the MnR and the dorsal raphe nuclei (DR) densely innervate the hippocampal formation (McQuade and Sharp, 1997). Furthermore, the interaction of α-syn with the serotonin transporter (SERT) and its consecutive sequestration from the cellular membrane (Wersinger et al., 2006) may connect serotonergic dysfunction with synucleinopathies (Huot and Fox, 2013).
Physiologically, α-syn assembles at presynaptic membranes and participates in vesicle clustering thereby attenuating synaptic transmission (Abeliovich et al., 2000; Vargas et al., 2014). Furthermore, increased α-syn levels were linked to synaptic defects of hippocampal neurons (Boassa et al., 2013; Nemani et al., 2010; Scott and Roy, 2012). The hippocampal dentate gyrus (DG) is a confined area of adult neurogenesis (Cameron et al., 1993). Here, newly generated DG granule neurons integrate functionally into the hippocampal circuit, and connect to pyramidal neurons of the CA3 area via the mossy fibers tract (Bergami et al., 2008; Toni et al., 2008). Undergoing pronounced structural changes during maturation and integration, adult-born hippocampal neurons likely present an enhanced susceptibility in this niche. Serotonergic projections from the raphe nuclei to the HC predominantly terminate at the granular cell layer (GCL) and hilus of the DG (Descarries et al., 2010). Serotonin (5-HT) receptors, in particular 5-HT 1A and 1B are involved in the regulation of adult hippocampal neurogenesis as well as neurite growth and branching (Trakhtenberg and Goldberg, 2012). Consistently, impaired hippocampal neurogenesis contributes to anxiety and depression presumably by dysfunction of serotonergic signaling (Samuels and Hen, 2011). Furthermore, treatment with selective serotonin reuptake inhibitors (SSRI) rescues impaired hippocampal neurogenesis both in models of depression (Sairanen et al., 2005; Santarelli et al., 2003; Surget et al., 2011) and in α-syn transgenic mice (Kohl et al., 2012).
Still, the etiology and treatment of NMS in PD is unsatisfactory most likely since the underlying mechanisms are not yet well understood. We have recently described a transgenic rat model carrying a bacterial artificial chromosome (BAC) construct consisting of human full-length α-syn with pan-neuronal overexpression of α-syn (Nuber et al., 2013). Here, the progressive aggregation of α-syn results in an age-dependent dopaminergic nigro-striatal cell loss with motor deficits starting at the age of 12 months. Using 4-month-old animals, thus prior to the onset of the motor phenotype, we analyzed hippocampal neurogenesis and intra-hippocampal circuitry, previously observed to be directly modulated by overexpression of human α-syn (Marxreiter et al., 2013; Nuber et al., 2008; Winner et al., 2004). Additionally, we addressed the question whether the accumulation of human α-syn results in an early deficit of the serotonergic system, and investigated a possible associated anxiety-like phenotype towards novelty as previously described in models of experimental serotonin depletion (Hohmann et al., 2007; Lipska et al., 1992). Here, we assessed spontaneous exploratory and feeding behavior after transfer into automated home cages.
Material and Methods
Animals
A BAC transgenic rat model over-expressing the full-length human SNCA locus under the control of the endogenous human regulatory elements and wild type littermates were used for this study (Nuber et al., 2013). All animals were housed under a 12h light-dark cycle and had free access to food and water. All animal procedures followed the guidelines by international standards for the care and use of laboratory animals and were approved by the local Animal Welfare and Ethics committee of the Country Commission Tübingen, Germany.
Analysis of hippocampal neurogenesis
To assess proliferating cells in the DG, 3-month-old male BAC alpha-synuclein rats (n=14) and non-transgenic littermates (n=10) received two intraperitoneal (i.p.) injections of bromo-deoxyuridine (BrdU; 100 mg/kg body weight) within a 12 h period. 12 h following the second BrdU injection (24 h pulse paradigm), animals were deeply anaesthetized with a mixture of 10% ketamine (Ketanest; 25 mg/ml), 1% acepromacine (Ventroquil; 0.25 mg/ml), and 2% xylazine (Rompun; 1.25 mg/ml) in 0.9% natriumchloride (NaCl) solution, and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde (PFA) in 0.1 M PBS, pH 7.4 for 10 min. Brains were dissected and postfixed overnight in 4% PFA in PBS solution and stored in 30% sucrose in 0.1 M PBS, pH 7.4 at 4° C until further processing. To determine the survival of newly generated cells in the DG, BrdU was administered daily on five consecutive days by i.p. injections (50 mg/kg body weight; non-tg n=7; α-syn n=8). Four weeks after the first injection, animals were transcardially perfused as described above.
Immunohistochemistry and immunofluorescence
For immunostainings the primary antibodies are listed in supplemental Table S1. To analyze transgenic α-syn expression in the HC the human specific antibody 15G7 (amino acids 91–96; 1:20; Enzo Life Science, Farmingdale, USA) specifically binding to the C-terminus of α-syn was applied. Secondary antibodies used were donkey-derived, species-specific and conjugated with Alexa-488, Alexa-568, Alexa-647, Alexa-660 (all 1:1000, Invitrogen, Carlsbad, USA) or biotin (1:500, Dianova, Hamburg, Germany). DAPI (1:10,000; Sigma, St. Louis, U.S.A.) was used as nuclear counterstain. For further details see supplement.
Microscopy and quantification
The density of BrdU+ and DCX+ cells in the DG was determined as described previously (Kohl et al., 2012), for further details on the analysis of BrdU+ cell see supplement. To analyse the maturation stages of new-born neurons, DCX+ neuroblasts within the DG were categorized corresponding to their morphologies as previously established (Paus et al., 2013; Plumpe et al., 2006). Categorization was based on the presence, size, and shape of apical dendrites (Fig. 3 B). Cells with no or very short processes still bearing proliferative capacity (Lugert et al., 2010) were categorized as “early” neuroblasts. Cells showing dendrites with intermediate length and immature morphology were categorized as “intermediate” DCX+ cells. The processes of intermediate neuroblasts reached the granular layer, but not the molecular layer. “Late” DCX+ neurons were characterized by a more mature appearance. Their dendrites reached the molecular layer and presented dendritic tree branching in the GCL. To count the different DCX stages in the hippocampal DG every 12th coronal section was sampled. Within the ROI all DCX+ cells were classified, divided into the three subgroups and counted at 40× magnification. Absolute cell numbers per DG of one hemisphere were obtained by multiplication with the sections interval (i.e. 12×). The density of DCX+ cell subgroup per total volume of the DG was calculated.
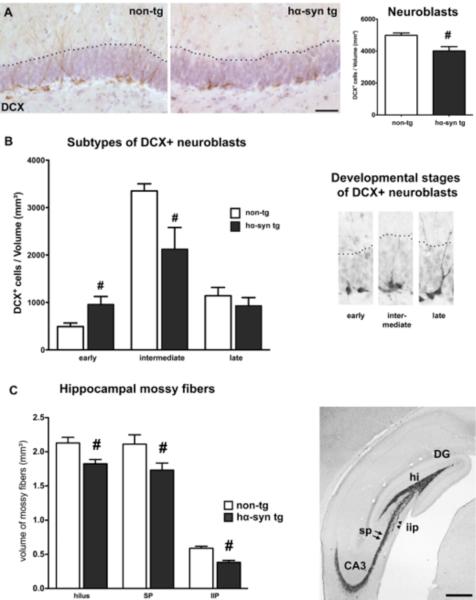
Figure 3.
Altered dendritic and axonal compartment in the DG of hα-syn tg rats. (A) Significantly reduced density of the overall DCX+ neuroblast population in transgenic animals (non-tg n = 7; hα-syn tg n = 8), representative images of DCX+ neuroblasts (brown). (Scale bar: 50μm) (B) Mainly post-mitotic DCX+ neuroblasts classified as intermediate phenotype were reduced, while the number of early DCX+ cells was even higher in transgenic rats. (C) Reduced volume of ZnT3+ mossy fibers representing the axonal projections of DG neurons to the CA3 region in the hilar (hi), suprapyramidal (sp, black arrows), and intra-infrapyramidal region (iip, black arrowheads) (non-tg n = 7; hα-syn tg n = 8; scale bar: 500 μm). # p < 0.05, student's t-test.
The MnR and the DR were identified by cell somata expressing TPH2, the rate limiting enzyme for serotonin synthesis. The raphe nuclei on sagittal sections were outlined in the projected image of every 6th section within Stereo Investigator software and the volume of both regions was calculated. All cells clearly expressing TPH2 were quantified, and the total density of TPH2 serotonergic neurons in the MnR and the DR was determined in BAC alpha-synuclein animals in comparison to age-matched non-transgenic rats.
To assess the size of the sub-portions of the mossy fiber tract every 12th section was stained for the zinc transporter protein ZnT3 (Wenzel et al., 1997), and the hilar mossy fibers, the suprapyramidal mossy fibers, and intra-infra-pyramidal fibers were analyzed. As previously described (Paus et al., 2013) the mossy fiber fields were outlined on the projected images within the StereoInvestigator software using a 10× objective. The area sizes were determined using the area measurement tool. The volumes of the mossy fiber regions were calculated by multiplying the sum of measured areas with the inverse of the sampling fraction (12) and the section thickness (40 μm). Mean values were calculated for the volumes of all three sub-portions of the mossy fiber tract.
Analysis of hippocampal serotonergic and dopaminergic fibers
Fibers of serotonergic neurons expressing SERT in the hilus of the DG including the subgranular zone (SGZ) were determined by the analysis of image stacks (20 μm thickness, 20 slices, size 134.8 × 134.8 μm, 1024 × 1024 pixels) acquired on a ZEISS LSM 780 confocal scanning laser microscope (63× PL APO oil objective, pinhole corresponding to 1 Airy Unit). Maximum intensity projections (MIP) from the SGZ and adjacent hilar region processed by ZEISS ZEN software were further analyzed. On 5 MIPs per animal obtained from randomly chosen areas of the DG the integrated density of fluorescent SERT signal was measured using the measure-tool within ImageJ 1.46r and normalized to the background of the chosen area. Mean background fluorescence was obtained at three different locations without specific SERT signal and subtracted from total fluorescence intensity as previously described (Burgess et al., 2010). To estimate the level of dopaminergic innervation of the DG/CA3 region, we further analyzed confocal overview image stacks of tyrosine-hydroxylase (TH) immunostained sections (15 μm thickness, 6 slices, size 339.7 × 339.7 μm, 1024×1024 pixels) acquired on the identical confocal laser microscope using a 25× APO oil objective (pinhole corresponding to 1 Airy Unit). On 6 MIPs per animal (3 each from the infrapyramidal and the suprapyramidal blade of the DG and adjacent hilar region), the integrated density of fluorescent TH signal was measured as above and calculated accordingly.
Analysis of hippocampal serotonin content
For estimation of hippocampal 5-HT levels by high performance liquid chromatography (HPLC), the CA3 region including the DG and the caudate putamen of 4-month-old rats (n=8 each) were dissected (Fig. 5 A) on a chilled stage (Nuber et al., 2013) and further processed as described previously (Pum et al., 2008). Samples containing 500 pg dihydroxybenzylamine as an internal standard were analyzed by HPLC with electrochemical detection. The column was an ET 125/2, Nucleosil 120-5, C-18 reversed phase column (Macherey-Nagel, Dueren, Germany). For detecting 5-HT, the mobile phase consisted of 75 mM NaH2PO4, 4 mM KCl, 20 mM EDTA, 1.5 mM SDS, 100 ml/l diethylamine, 12% methanol, and 12% acetonitril adjusted to pH 6.0 using phosphoric acid. The electrochemical detector was set at 500 mV versus an ISAAC reference electrode (Antec, Zoeterwoude, Netherlands) at 30° C (Amato et al., 2011).
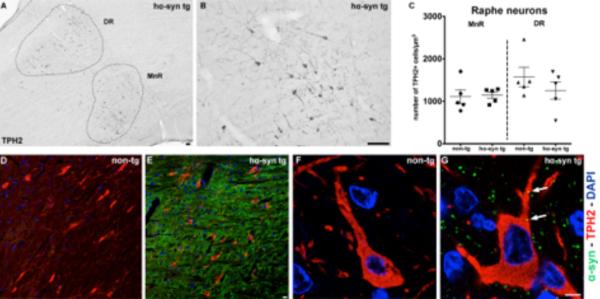
Figure 5.
Quantification of serotonergic cells in rostral raphe nuclei and expression of human α-syn in TPH2+ neurons of hα-syn tg rats. (A) Sagittal overview image of the DR and MnR in hα-syn tg rats. (B) Representative image with higher magnification showing TPH2+ cell bodies and neurites (black) in the DR. (C) Numbers of TPH2+ serotonergic neurons/ mm3 were not altered in MnR or DR of hα-syn tg rats (both n = 5). (D–G) Abundant expression of human α-syn (green) in raphe nuclei (E) and within the soma of TPH2+ neurons (red) of the raphe nuclei (G) in hα-syn tg rats. (D, F) No expression in non-tg animals. DAPI in blue. Scale bars (A, B) 100 μm, (D–G) 10 μm.
Biochemical study
4-month-old animals were anesthetized and decapitated, and the HC were subdivided from dissected brains on a chilled stage. The DG/CA3 subfield from one hemisphere was separated (depicted in Fig. 5 A) and further processed. To separate proteins belonging to either the soluble membrane or the detergent-resistant membrane (lipid raft) fraction (Rockenstein et al., 2014), sequential extraction was performed as previously described (Nuber et al., 2013; Tofaris et al., 2003). After separating proteins from the soluble and the membrane fraction using TBS+ (50 mM Tris-HCl, pH 7.4, 175 mM NaCl, 5 mM EDTA), protease inhibitor cocktails (Calbiochem, CA), and TBS+ containing 1% of Triton X-100, pellets were further solubilized in TBS+ containing 1 M sucrose, and RIPA buffer (TBS+, 1% NP-40, and 0.5% sodium deoxycholate, 0.1% SDS), each extraction step followed by ultracentrifugation for 20 min at 120,000 × g. The detergent-resistant membrane fraction was finally solubilized in 8M urea/5% SDS.
Western blot analyses using 25 μg of Tris-buffered saline (TBS), and 8 μg of either TX or 8M urea/5% SDS hippocampal protein extracts were run on 4–12% Bis-Tris gels (Invitrogen, Life Technologies, Carlsbad, CA, USA) and electroblotted on nitrocellulose membranes (Millipore, Bedford, MA, USA). After washing in phosphate buffered saline (PBS), membranes were blocked for 30 min in PBST (PBS with 0.2% Tween-20) containing 5% of bovine serum albumine (BSA) at room temperature. Primary antibodies used are listed in supplemental table S1. Antibody incubation was performed in PBST containing 5% of BSA overnight. After washing with PBST, membranes were probed with anti-mouse secondary antibodies (1:5000, American Qualex, CA, USA), visualized with enhanced chemiluminescence (ECL, PerkinElmer, Boston, MA, USA), and analyzed with the VersaDoc gel imaging system (BioRad, Hercules, CA, USA). Proteins were normalized to ß-actin (1:3000). Quantification of signal intensities was performed as previously described (Nuber et al., 2013).
Electronmicroscopy study
For ultrastructural analysis, vibratome sections were post-fixed with 2% of glutaraldehyd/0.1% osmium tetroxide in 0.1M sodium cacodylate buffer, embedded in epoxy and analyzed with a ZEISS EM 10 electron microscope. Image J was used to outline the length of the active zone and the area of the postsynaptic density (PSD) of asymmetric synapses (each group n=3; 82 ± 5 total synapses studied). The average thickness of PSD was calculated by dividing the outlined area by the length of the postsynaptic membrane, as previously described (Dosemeci et al., 2001).
Behavioral analysis
To investigate spontaneous exploratory and feeding behavior we re-evaluated data obtained from an automated cage system (Lab Master home cage apparatus; TSE Systems, Bad Homburg, Germany) (Nuber et al., 2013), specifying on the first minutes after transfer to the new home cage. BAC alpha-synuclein and non-transgenic rats (n=6 each) were individually placed into the Lab Master cage during the light phase with 200g sawdust, and a grid of photocells automatically detected overall and center activity within the first 100 min, consistent with earliest detection of inactivity (linear regression; see result section). Moreover, mean food intake was determined by the home cage system, and body weight of animals was obtained monthly. All possible efforts were made to minimize the number of animals used and distress.
Statistical analysis
Statistical analyses and graphs were performed using Prism v6.0 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± S.E.M.. Differences between means were analyzed by two-tailed t-test, 1-way, and 2-way ANOVA with post-hoc tests as appropriate. Statistical outliers were detected using the `robust regression and outlier removal' method (ROUT; Q = 1%) (Motulsky and Brown, 2006). Statistical significance at p < 0.05 was considered significant.
Results
Expression of full-length human α-syn in maturing DG neurons and hippocampal synapses
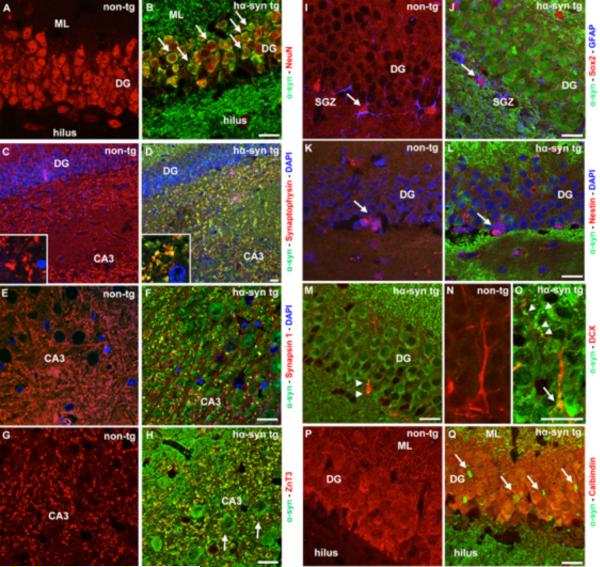
In BAC alpha-synuclein rats, a 2-3-fold overexpression of full-length human α-syn was detected in hippocampal homogenates (Nuber et al., 2013), and is therefore expressed in the range predicted for PD gene multiplication (Kasten and Klein, 2013). Human α-syn was present in neuronal perikarya of the DG labeled either with NeuN or Calbindin (Fig. 1 B and Q). Furthermore, abundant co-localization of human α-syn with the synaptic proteins synaptophysin and synapsin-1 in the hilus of the DG and the adjacent CA3 region was detected (Fig. 1 D and F). α-Syn immunoreactive patches also co-localized with ZnT3, expressed within the mossy fiber tract and its nerve terminals (Fig. 1 H). We further determined the temporal pattern of human α-syn expression during proliferation, migration, and differentiation in newly generated DG cells. Interestingly, early precursor cells in the DG, expressing both Sox2 and GFAP, or Nestin, showed no co-labeling with human α-syn (Fig. 1 J and L). In contrast, human α-syn was present in the cytoplasm as well in developing dendrites of DCX+ neuroblasts (Fig. 1 M and O). Thus, human α-syn was expressed in mature DG neurons and in intermediate and late stage DCX+ neuroblasts as well as at synaptic membranes of hippocampal granule cells projecting to the CA3 region.
Figure 1.
Expression of human alpha-synuclein (α-syn) under its endogenous regulatory sequences in mature and developing neurons and synaptic terminals in the hippocampal dentate gyrus (DG)/CA3 subfield of BAC alpha-synuclein transgenic rats (hα-syn tg). (A, B) Human α-syn (green) in mature DG neurons labelled with NeuN (red, arrows). (C–H) Human α-syn strongly co-localized with synaptophysin (D, red), synapsin-1 (F, red), and the mossy fiber marker ZnT3 (H, red) in the CA3 region of hα-syn tg rats (yellow), while α-syn was also expressed in CA3 neurons (H, arrows). (I–L) Lack of α-syn expression in early neural precursors co-labeling Sox2 (red) and GFAP (blue, arrow), or Nestin (M, red, arrow) in the subgranular layer (SGZ) of the DG of transgenic rats. (M–O) α-Syn is present in neuroblasts expressing doublecortin (DCX), cytoplasmatic (M and O, arrows) and along dendrites of DCX+ cells (O, arrowheads). (P, Q) Young mature Calbindin+ neurons express α-syn (Q, red, arrows). The hippocampus of non-transgenic animals (non-tg) was devoid of transgenic α-syn. Nuclear staining (DAPI) in blue. (Scale bars: 20 μm).
Severely reduced survival of newly generated neurons in the DG of BAC alpha-synuclein rats
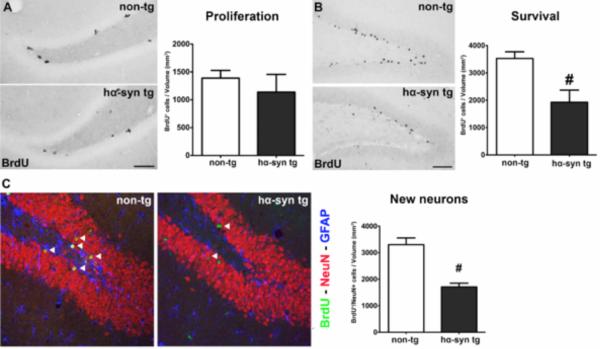
Since α-syn is already strongly expressed during neuronal maturation, we further dissected its impact on hippocampal neurogenesis. In the DG of BAC alpha-synuclein animals we detected a strikingly reduced survival of BrdU+ cells (Fig. 2 B, Tab. 1). We did neither observe significant alterations of cell proliferation in the SGZ of the DG (Fig. 2 A, Tab. 1), nor proportional changes in neuronal differentiation (Tab. 1). This resulted in 48% less newly generated neurons in BAC alpha-synuclein compared to non-transgenic rats (Fig. 2 C, Tab. 1). Together, this suggests that human full-length α-syn strongly affects survival of new neurons in the DG, while proliferation remains unaffected.
Figure 2.
α-Syn accumulation severely decreased hippocampal neurogenesis in hα-syn tg rats. (A) No significant difference of BrdU+ proliferating cells in the DG (density) between hα-syn tg and non-tg animals (non-tg n = 10; hα-syn tg n = 14), representative images of BrdU labelled cells (black). (B) Dramatically reduced density of BrdU+ cells in the DG representing surviving newly generated cells in hα-syn tg rats, representative images (non-tg n = 7; hα-syn tg n = 8; scale bars: 50μm). (C) Strong reduction of newly generated mature neurons in hα-syn tg rats, representative images of triple immunofluorescence showing BrdU+ new cells (green) co-expressing the neuronal marker NeuN (red; arrowheads), glial cells (expressing GFAP) in blue (scale bar: 20μm). # p < 0.05, student's t-test.
Table 1.
Impaired cellular and axonal plasticity in the HC of BAC alpha-synuclem rats (hα-syn tg) compared to controls: Volume of mossy fiber tract projecting from DG granule cells to CA3 pyramidal neurons. Mossy fiber (MF) bundle was visualized using an antibody against zinc-transporter 3 (ZnT3), and the volumes of the three major MF subfields, consisting of hilar MFs (hi), supra-pyramidal (sp) MFs, and intra-infra-pyramidal (iip) MFs, expressed as mm3 ± S.E.M. are presented. The volumes of all three subfields were significantly reduced in hα-syn tg rats compared to non-tg littermates.
Hippocampal neurogenesis in the adult DG of hα-syn tg and non-tg animals for both experimental paradigms. Calculation of densities was performed by division of quantified cells through the corresponding volume of the DG and presented as cells/mm3. While the proliferation of neural stem and precursor cells in hα-syn tg rats showed no significant reduction, the number of surviving BrdU+ cells in the DG of hα-syn tg animals four weeks after BrdU injection was severely decreased, while the proportional differentiation into neuronal (NeuN) and glial (GFAP) phenotypes was unaltered. This resulted in a strongly reduced density of newly generated neurons in the DG of hα-syn tg rats. Moreover, the density of DCX+ neuroblasts in the DG was reduced in hα-syn tg animals. The analysis of developmental stages of DCX+ cells revealed reduced densities of the large group of intermediate stage neuroblasts, while the proportion of late DCX+ cells was unaltered. Additionally, the density of early DCX+ neuroblasts was increased in hα-syn tg animals. All values are given ± S.E.M.. The significance level was set at p< 0.05.
| Non-tg | hα-syn tg | p-value | |
|---|---|---|---|
|
| |||
| Mossy fiber (MF) volumes (mm3) | |||
|
| |||
| Volume of hilar MFs (hi) | 2.13 ± 0.08 | 1.82 ± 0.06 | p = 0.011 |
| Volume of suprapyramidal layer MFs (sp) | 2.11 ± 0.14 | 1.73 ± 0.10 | p = 0.042 |
| Volume of intra-infrapyramidal layer MFs (iip) | 0.59 ± 0.03 | 0.38 ± 0.03 | p = 0.001 |
|
| |||
| Hippocampal neurogenesis | |||
|
| |||
| Proliferation | |||
| Density (BrdU+ cells /mm3) | 1390 ± 138 | 1138 ± 85 | p = 0.115 |
|
| |||
| Survival of newly generated cells | |||
| Density (BrdU+ cells/mm3) | 3528 ± 242 | 1927 ± 157 | p < 0.0001 |
|
| |||
| Differentiation of new cells | |||
| % BrdU+/NeuN+ cells | 93.3 ± 1.0 | 88.7 ± 2.1 | p = 0.084 |
| % BrdU+/GFAP+ cells | 1.9 ± 0.8 | 1.9 ± 0.6 | p = 0.983 |
| % BrdU+ only cells | 4.8 ± 0.9 | 9.3 ± 1.7 | p = 0.042 |
|
| |||
| Newly generated neurons | |||
| Density (BrdU+ neurons/mm3) | 3303 ± 253 | 1707 ± 145 | p < 0.0001 |
|
| |||
| Neuroblasts | |||
| Density (DCX+ cells/mm3) | 4984 ± 147 | 4011 ± 264 | p = 0.009 |
|
| |||
| Subgroups of DCX+ neuroblasts/mm3 | |||
| early | 490 ± 76 | 958 ± 167 | p = 0.030 |
| intermediate | 3352 ± 149 | 2123 ± 457 | p = 0.032 |
| late | 1142 ± 175 | 930 ± 174 | p = 0.407 |
Overexpression of human α-syn reduces dendritic outgrowth of newly generated neurons and impairs the axonal compartment of the DG in BAC alpha-synuclein rats
The decrease in adult hippocampal neurogenesis was also reflected by a significantly reduced number of DCX+ neuroblasts (Fig. 3 A, Tab. 1). The functional integration of new DG neurons in the local circuitry depends on the development of dendritic branching during neuronal maturation (Toni et al., 2008): By categorizing distinct maturation stages of DCX+ cells into early, intermediate, and late neuroblasts via dendritic complexity we detected a significantly reduced density of intermediate neuroblasts in BAC alpha-synuclein rats (Fig. 3 B, Tab. 1). In contrast, early DCX+ cells, still bearing proliferative capacity, were even increased (Fig. 3 B). Thus, an impaired or delayed dendritogenesis of DCX+ neuroblasts may contribute to the overall reduced survival of newly generated neurons in BAC alpha-synuclein rats. We further focussed our analysis on the mossy fiber tract representing the axonal compartment of DG neurons. Here, we observed a reduced volume of all three major subfields of the ZnT3 expressing mossy fiber tract (Paus et al., 2013) projecting to the pyramidal neurons of the CA3 subregion (Fig. 3 C, Tab. 1) indicative for a reduced axonal output from DG neurons. Together, these data suggest that the overexpression of α-syn has detrimental effects on the development of dendrites and axons of newly DG generated neurons.
Alpha-synuclein induced early impairment of the hippocampal serotonergic system
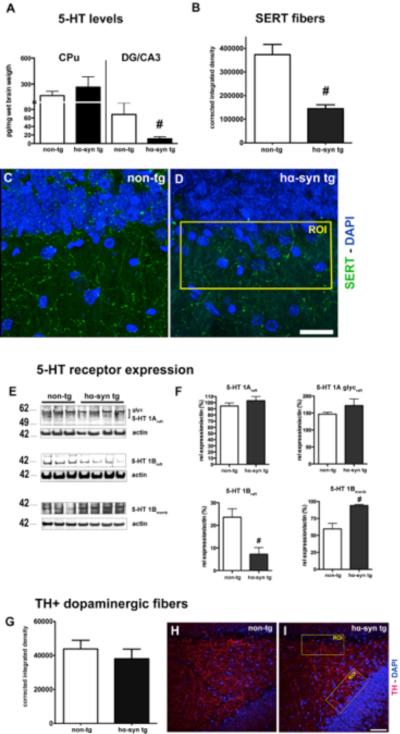
We previously observed a significant effect of SSRI treatment compensating α-syn induced impairments of hippocampal neurogenesis in A53T α-syn transgenic mice (Kohl et al., 2012). Because of the previously described impact of 5-HT on neurogenesis we further estimated the integrity of serotonergic input into the HC of BAC alpha-synuclein rats. We first evaluated 5-HT neurotransmitter levels in both the putamen and the DG/CA3 subfield of the HC in BAC alpha-synuclein rats. α-Syn overexpression induced a decrease of 5-HT levels in the DG/CA3 subfield only (Fig. 4 A), reflecting an early dysfunction of serotonergic terminals within the HC (Vertes et al., 1999). The quantitative analysis of high-resolution image stacks revealed a significant reduction of SERT signal in the HC subregion of BAC alpha-synuclein animals (Fig. 4 B). We next studied the membrane localization of hippocampal 5-HT 1 receptors, known to be implicated in dendrite complexity of new-born neurons (Trakhtenberg and Goldberg, 2012). We detected distinct alterations of their expression pattern: While the levels of 5-HT 1A remained unchanged, 5-HT 1B was strongly reduced in the lipid raft fraction associating with a significantly increased signal in the membrane fraction (Fig. 4 E and F). To address whether there is a rather distinct and early serotonergic hippocampal denervation, we determined TH expressing fibers in the hilus and the adjacent SGZ of the DG. Interestingly, no significant changes of dopaminergic innervation of the DG/CA3 subfield in BAC alpha-synuclein rats were present compared to non-transgenic animals (Fig. 4 G–I). Together, α-syn overexpression induced an early and profound dysfunction of the hippocampal serotonergic input in BAC alpha-synuclein rats.
Figure 4.
α-Syn induced serotonergic deficit in the hippocampus (HC) of hα-syn tg animals. (A) Serotonin (5-HT) levels were significantly reduced in the DG/CA3 subfield of the HC in hα-syn rats tg compared to non-tg littermates, in contrast to the caudate putamen (CPu) (both groups n = 8). (B) A significantly reduced serotonin transporter (SERT) network was present in the DG in transgenic animals, analysis of integrated density of maximum intensity projections (MIP) from confocal image stacks (each group n=6). (C, D) Representative images showing SERT+ network (green), DAPI in blue, example of analyzed region of interest (ROI; yellow). (Scale bar: 20 μm). (E, F) 5-HT receptor 1A levels, both the monomeric (mono) and the glycosylated form (glyc) were unchanged. In contrast, the levels of 5-HT 1B, mainly localized to presynaptic vesicles, were strongly reduced in the lipid raft fraction (raft), its physiological localization, while its content was increased in the membrane bound fraction (memb). (G) No loss of dopaminergic fibers expressing tyrosine-hydroxylase (TH) in the subgranular zone (SGZ) and adjacent hilus of hα-syn tg compared to non-tg rats (each group n = 4) (H, I) Representative images of MIP showing the network of TH expressing fibers (red, DAPI in blue, ROI in yellow), (scale bar: 50 μm). # p<0.05; 1-way ANOVA for 5-HT levels, student's t-test for 5-HT, SERT, and TH analysis.
The number of serotonergic neurons in the raphe nuclei remains unchanged in BAC alpha-synuclein rats
While we detected a reduction of SERT+ nerve terminals in the HC of young BAC alpha-synuclein rats, we further determined the number of serotonergic neurons in the raphe nuclei by quantifying TPH2 expressing perikarya of serotonergic neurons: Interestingly, the density of TPH2+ serotonergic neurons was unaltered in the MnR as well as in the DR in BAC alpha-synuclein rats (Fig. 5 A–C). The number of serotonergic neurons obtained was in the range of previously published studies in rats (Descarries et al., 1982; Vertes and Crane, 1997). However, transgenic α-syn was abundantly present in the raphe nuclei region, as well as in serotonergic neurons expressing TPH2 (Fig. 5 E, G), suggesting that overexpression of α-syn affects serotonergic projections to the HC prior to the perikarya of serotonergic neurons in the raphe nuclei of 4-month-old BAC alpha-synuclein animals.
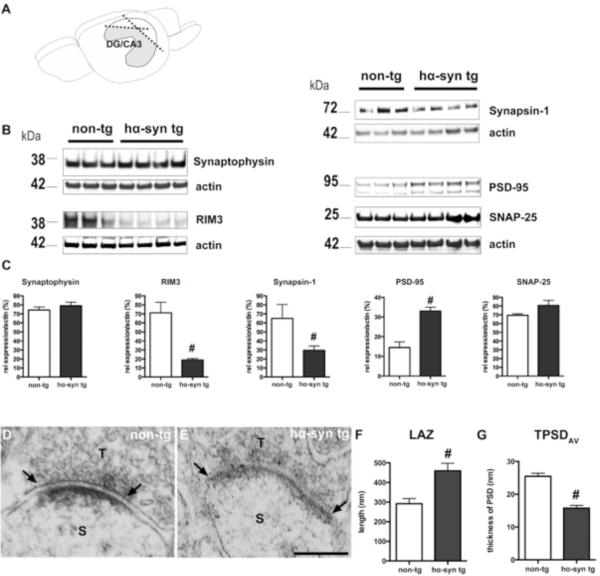
α-Syn affects biochemical composition and ultrastructure of hippocampal synapses in BAC alpha-synuclein rats
Synaptic dysfunction may be an important factor for α-syn mediated pathogenic processes in synucleinopathies. It is hypothesized that α-syn accumulation at synaptic membranes mediates its pathologic role by interfering with synaptic neurotransmission of hippocampal CA1 neurons at the level of vesicular recycling (Nemani et al., 2010). Immunoblot analysis of dissected DG/CA3 tissue (Fig. 6 A) revealed distinct alterations of synaptic proteins in the presence of human α-syn (Fig. 6 B and C): While the levels of synaptophysin and SNAP-25 were unaltered, we detected significantly reduced levels of synapsin-1, physiologically playing an important role in synaptic vesicle trafficking. Moreover, we observed reduced levels of RIM3, a member of the Rab3 Interacting Molecules (RIM) recently described being crucial for dendritic arborization of neurons (Alvarez-Baron et al., 2013). On the contrary, the levels of the postsynaptic protein PSD-95 in the HC of BAC alpha-synuclein rats were increased. This altered expression pattern of distinct synaptic proteins point towards a compromised hippocampal synaptic machinery. Using an ultrastructural approach we observed an increased length of the active zone (LAZ) of CA3 asymmetric synapses in BAC alpha-synuclein compared to non-transgenic rats (291.7 ± 26.2 nm in non-tg vs. 459.3 ± 38.8 nm in tg hα-syn rats; Fig. 6 F). However, the average thickness of the postsynaptic density was reduced in BAC alpha-synuclein animals (25.5 ± 1.0 nm in non-tg vs. 15.7 ± 0.9 nm in tg hα-syn; Fig. 6 G). Thus, the expression of α-syn in conjunction with the reduced serotonergic input resulted in compromised CA3 synapses in BAC alpha-synuclein rats.
Figure 6.
Overexpression of α-syn leads to an altered synaptic structure in the hippocampal DG/CA3 subfield. (A) The DG/CA3 subfield of the HC from one hemisphere was separated from the CA1 area. (B) Note the reduced expression of presynaptic proteins RIM3 and synapsin-1 in hα-syn tg rats, while the postsynaptic protein PSD-95 was increased. (C) Quantification shows significant differences for RIM3, synapsin-1, and PSD-95, but no change for synaptophysin and SNAP-25. (D–G) Representative electron micrographs of hippocampal CA3 synaptic terminals (T) of non-tg (D) and transgenic animals (E). Quantification of the postsynaptic density (PSD) of dendritic spines (S) shows significant changes in the length of the active zone (LAZ; F; line, arrows) and average thickness of the PSD (TPSDAV) of both groups (n= 3 /each). (Scale bar: 200 nm). # p<0.05, student's t-test.
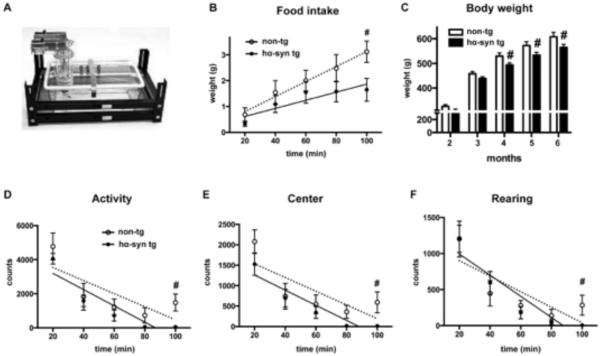
Early decrease in exploratory and feeding behavior in BAC α-synuclein rats
Since we detected a reduced hippocampal neurogenesis in conjunction with deficits of the serotonergic system, we next analyzed its functional consequences using behavioral measurement conducted by an automated cage system (Fig. 7A). This system allows testing for spontaneous exploratory and feeding behavior. A decrease of exploration as well as anxiety-like behavior had been associated with alterations of the serotonergic system, e.g. of the HC (Hohmann et al., 2007; Lipska et al., 1992; Luo et al., 2008). Immediately after transfer, animals face a conflict between avoidance and exploration of the center. BAC alpha-synuclein rats did not show an immediate difference in time spent at the wall or center when compared to non-tg rats, but a more rapid decline in horizontal moving (linear regression: non-tg: slope=−38 ± 18, R2=0.6, p=0.13; α-syn: slope=−48 ± 12, R2 = 0.8, p=0.03) and vertical activity (rearing: linear regression: non-tg: slope=−10.7±4.6, R2=0.6, p=0.1; α-syn: slope=−14.7 ± 3.7, R2=0.8, p=0.02) (Fig. 7 D–F). Moreover, BAC alpha-synuclein rats avoided the center more often as an effect over time (Fig 7 E). In addition, home cage food consumption revealed repressed feeding for the transgenic rats (linear regression: non-tg slope=0.03±0.002, R2=0.9, p=0.006; α-syn slope=0.02±0.005, R2=0.7, Fig. 7 B) that may result in less total body weight by 7% (Fig. 7 C). Together, this is suggestive of altered motivation and conveys the impression of mild anxiety-like behavior in response to a novel environment.
Figure 7.
Time dependent decline of exploratory activity after transfer into the home cage apparatus (example in A) in hα-syn tg rats: Reduced feeding in the novel environment compared to non-tg rats (B), and body weight with significant changes starting as early as 4 months of age (C). After transfer into the automated cage apparatus, hα-syn tg rats showed a more rapid decline in ambulatory (D) and horizontal activity (rearing; F), and a more rapid decline in exploration of the center zone (E). # p<0.05, 2-way ANOVA, post-hoc LSD, linear regression for analysis of slope.
Discussion
Manifold deficits and symptoms, ranging from hyposmia, sleep disturbances, gastrointestinal, genitourinary and cardiovascular dysfunction, to psychopathological symptoms like anxiety and depression in particular, represent NMS frequently occurring in pre-motor stages of PD (Mahlknecht and Poewe, 2013; Weintraub et al., 2015). While from an epidemiological viewpoint it remains ambiguous whether depression is a risk factor for PD or represents an early stage of the disease (Gustafsson et al., 2015), neuropathological studies underline the crucial role of α-syn pathology beyond the nigrostriatal dopaminergic system for pre-motor NMS (Jellinger, 2015). Nevertheless, the molecular mechanisms contributing to NMS in early PD, such as depression and anxiety are still elusive. Moreover, it is not known how overexpression of human α-syn affects the interplay of hippocampal neurogenesis and the serotonergic system, both hypothesized to contribute to the complexity of depression and anxiety disorders (Mahar et al., 2014; Petrik et al., 2012). Here, we demonstrate that accumulating hippocampal α-syn reduced 5-HT levels, impaired the density of serotonergic fibers projecting to the HC, and decreased the expression of 5-HT 1B in the DG/CA3 region of a BAC alpha-synuclein rat model of PD, overexpressing the full-length human gene under its endogenous regulatory sequences. The α-syn mediated alterations of hippocampal synapses were accompanied by an impaired dendritic arborization and a significant mossy fiber loss, leading to a severely reduced survival of new-born neurons. Moreover, the BAC alpha-synuclein rat model showed an early decrease in spontaneous exploratory and feeding behavior towards novelty change.
Impaired serotonergic circuitry in the hippocampus prior to the onset of motor symptoms
Increased levels of α-syn concurred with severe NMS in alpha-synuclein multiplication carriers (Gwinn et al., 2011; Miller et al., 2004) suggesting a gene-dosage effect in PD brains. Interestingly, we observed that the accumulation of α-syn at hippocampal synapses associates with a significant reduction of 5-HT levels in the DG/CA3 subregion, and a profound decrease of SERT+ fibers projecting into the DG. This matches well findings of dystrophic hippocampal SERT+ fibers in PD (Azmitia and Nixon, 2008). In addition, a punctate expression of human α-syn was observed in TPH2+ serotonergic neurons of the raphe nuclei in BAC alpha-synclein animals. This relates to α-syn accumulation detected within serotonergic neurons of the raphe region in PD patients (Halliday et al., 1990; Seidel et al., 2015). Furthermore, decreased hippocampal 5-HT levels as well as reduced SERT binding in the HC were observed in PD reflecting a dysfunctional serotonergic system (Huot and Fox, 2013). Interestingly, 5-HT 1A levels in the HC of BAC alpha-synuclein rats remained unchanged, while the levels of 5-HT 1B were reduced in the lipid raft fraction. The presynaptically localized 5-HT 1B plays an important role in the control of neurotransmitter release, and represents the only 5-HT receptor that is actively transported and released via secretory vesicles into dendritic branches (Liebmann et al., 2012). In conjunction with a recent study detecting reduced 5-HT 1B binding in PD (Varrone et al., 2014), the observed serotonergic dysfunction is likely an underlying pathway contributing to NMS in early PD. The precise onset of NMS is difficult to determine in PD. Models displaying key features of NMS in PD represent a very valuable tool to address this question, as early changes are readily identified and correlated to cellular, biochemical and molecular changes. At 3–6 months of age BAC alpha-synuclein rats display a 2-3 fold overexpression of α-syn, thus leading to a significant accumulation, however without presenting neither α-syn aggregation, nor dopaminergic neurodegeneration (Nuber et al., 2013). Our present study confirms an intact innervation of TH+ fibers to the hippocampal CA3/DG subfield. This is in contrast to early α-syn induced impairments of the serotonergic projections innervating the hippocampal formation being not accompanied by a loss of serotonergic neurons within the MnR and DR of 4-month-old rats. Thus, the loss of serotonergic terminals within the hippocampal target field may reflect an early stage of the α-synucleinopathy, supporting the hypothesis that synaptic loss and axonal degeneration precede neuronal loss in PD (Burke and O'Malley, 2013; Lamberts et al., 2015).
Notably, at this early age, BAC alpha-synuclein rats displayed subtle alterations in emotional behavior, as observed by reduced exploring and food consumption after transfer into a novel environment of home cages. This is in accordance with the chronic unpredicted mild stress rat model of depression, where intra-hippocampal infusion of 5-HT was sufficient to rescue reduced food intake, as well as impaired spontaneous behavior (Luo et al., 2008). Further, experimental 5-HT depletion was shown to associate with increased anxiety in response to novelty (Hohmann et al., 2007); although the impact on anxiety per se is yet not clear, potentially relating to stress as a contributing factor (Andrade and Graeff, 2001; Netto et al., 2002). Consistently, BAC alpha-synuclein rats displayed less visits of the center zone not as an immediate effect but rather a more rapid decline in activity, conveying the impression of less exploratory motivation in response to novelty. Since our findings further imply that these behavioral deficits occur prior to dopaminergic loss and motor deficits, these symptoms are likely related to early α-syn induced impairments of the serotonergic projections that we detected to be decreased in the hippocampal formation. Finally, it remains unclear whether the dysfunctional serotonergic system, impaired neurogenesis associated with a compromised hippocampal circuit or its adverse effects in sequence represent the main underlying mechanism for this behavioral phenotype.
Overexpression of human α-syn leads to impaired intra-hippocampal circuitry and altered synaptic marker expression
The overexpression of human α-syn in BAC alpha-synuclein rats results in widespread aggregation pathology and associates with age-dependent dopamine loss (Nuber et al., 2013). In the HC, human α-syn was present both in DCX+ neuroblasts and in mature neurons, accumulating at synapses and in the cytoplasm of DG neurons and their major target, the CA3 region. Indeed, we observed abundant co-localization of human α-syn with presynaptic proteins of the vesicle cycle. Moreover, accumulation of α-syn in the presynaptic compartment affects spine maintenance in human synucleinopathies (Kramer and Schulz-Schaeffer, 2007). This is in line with the co-localization of α-syn with presynaptic proteins and the mossy fiber marker ZnT3 leading to a loss of mossy fibers in BAC alpha-synuclein rats. In general, these findings match well with imaging data in PD pointing to altered intra-hippocampal connectivity early in the disease course (Carlesimo et al., 2012).
While levels of synaptophysin were unaltered in the DG/CA3 subfield, the presynaptic protein synapsin-1 was significantly reduced in BAC alpha-synuclein rats. This is in line with a previous report showing a similar decrease of synapsins in α-syn transgenic mice (Nemani et al., 2010). Moreover, RIM3, located within the pre- as well as the postsynaptic compartment, was strongly reduced upon α-syn overexpression. RIM3 is expressed in the DG and the CA regions of the HC, and plays an essential role for neuronal arborisation and the development of dendritic spines besides its synaptic function (Alvarez-Baron et al., 2013). Our finding suggests that overexpression of α-syn within the HC impairs synaptic integration and stability by down-regulation of RIM3. In contrast, an increased expression of the postsynaptic protein PSD-95 in the DG/CA3 subfield of BAC alpha-synuclein animals together with an increased LAZ may reflect secondary remodeling of CA3 synapses by extending the neurotransmitter receptive region. An increased PSD length in α-syn overexpressing mice was previously described (Nemani et al., 2010), however a decreased PSD thickness is a novel finding and suggests α-syn induced defects at the level of both the pre- and post-synaptic compartment.
Hippocampal overexpression of human α-syn severely impairs adult neurogenesis
The integration of new-born neurons is critically pending on local and systemic neural circuitries in the neurogenic niche, possibly resulting in an increased susceptibility towards α-syn overexpression or neurotransmitter imbalance. Adult hippocampal neurogenesis was severely reduced to almost half of the level observed in non-transgenic animals without changing cell proliferation. This is in contrast to our previous findings that the olfactory bulb of 4-month-old BAC alpha-synuclein rats contained significantly more newly generated BrdU+ neurons (Nuber et al., 2013). While the number of early DCX+ cells in the HC was even increased, the proportion of intermediate neuroblasts was significantly reduced in BAC alpha-synuclein rats, suggesting a particular impact of α-syn on young neurons during outgrowth of their processes. Interestingly, accumulating and/or oligomerized α-syn may destabilize microtubule cytoskeleton (Cartelli et al., 2012), thereby proportionally damaging intermediate and late-stage neuroblasts. Furthermore, selective activation of 5-HT 1A or 1B is required to promote neurite outgrowth and branching (Persico et al., 2006). So far, the mechanisms underlying compromised DG neurogenesis in PD are far from understood: Overexpressed α-syn impairs dendritogenesis and spine formation of new DG neurons (Lim et al., 2011; Winner et al., 2012), leading to a reduced survival of newly generated hippocampal neurons in murine transgenic α-syn models and human synucleinopathies (Winner et al., 2011). While recent findings underline the significance of adult neurogenesis in humans (Spalding et al., 2013), data on hippocampal neurogenesis in PD patients is yet rather limited. Nevertheless, post-mortem studies in a small number of cases of synucleinopathies show a reduction of immature neurons in the DG (Hoglinger et al., 2004; Johnson et al., 2011; Winner et al., 2012). Various lines of evidence suggest that hippocampal neurogenesis is under serotonergic control: Compounds elevating serotonergic tone like SSRIs enhance neurogenesis in models of depression (Surget et al., 2011). Furthermore, TPH2 is necessary to stimulate exercise induced hippocampal neurogenesis (Klempin et al., 2013), while combined knockout of 5-HT 1A and 1B in mice leads to a reduction, respectively (Xia et al., 2012). Our findings indicate that a deficit in 5-HT neurotransmission is paralleled by severely impaired neurogenesis, the latter previously shown to directly correlate with α-syn overexpression in inducible transgenic mice (Marxreiter et al., 2013; Nuber et al., 2008). In addition, chronic SSRI treatment ameliorates neurogenesis deficits in human α-syn transgenic animals (Deusser et al., 2015; Kohl et al., 2012; Ubhi et al., 2012). Although these findings are associative and require validation via controlled e.g. pharmacological assessment, the present study further establishes a link between 5-HT deficits and the observed hippocampal alterations in α-syn overexpressing animal models of PD.
Thus, early degeneration of the serotonergic input to the HC may severely affect the survival of new-born hippocampal neurons by reduced dendritic and axonal outgrowth via α-syn induced changes in pre-synaptic vesicle release and post-synaptic ultrastructure. The results predict that individuals carrying gene multiplications may show changes from an early age on in the function of hippocampal neural circuits and particularly of susceptible developing neurons. Furthermore, patients with sporadic PD may show, due to an upregulation of α-syn, early dysfunctions of the pontine raphe nuclei prior to the midbrain dopaminergic region (Braak et al., 2003; Seidel et al., 2015). These results further stress a pivotal role of the serotonergic system in the pathogenesis of NMS such as depression and anxiety in pre-motor PD.
Supplementary Material
Acknowledgments
This study was supported by the Bavarian State Ministry of Education, Science, and the Arts (ForNeuroCell II and ForIPS grant), the University Hospital Erlangen (ELAN grants 12-08-06-1, 12-02-22-1, IZKF grants E12, E13, and J32), the Deutsche Forschungsgemeinschaft (DFG grant INST 410/45-1 FUGG), the Albert-Raps-Foundation, and the Eberhard Karls University Tübingen (fortüne program F.15.13141). We are especially thankful to Martina Münch, Stefanie Schmidt, Jeanette Wihan, and Benedikt Quinger for excellent technical assistance, and Margaritha Trejo for ultrastructure data. Klaus-Peter Lesch and Jonas Waider are gratefully acknowledged for the kind gift of the TPH2 antibody.
References
- Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–52. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Alvarez-Baron E, et al. RIM3gamma and RIM4gamma are key regulators of neuronal arborization. J Neurosci. 2013;33:824–39. doi: 10.1523/JNEUROSCI.2229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato D, et al. Dynamic regulation of dopamine and serotonin responses to salient stimuli during chronic haloperidol treatment. Int J Neuropsychopharmacol. 2011;14:1327–39. doi: 10.1017/S1461145711000010. [DOI] [PubMed] [Google Scholar]
- Andrade TG, Graeff FG. Effect of electrolytic and neurotoxic lesions of the median raphe nucleus on anxiety and stress. Pharmacol Biochem Behav. 2001;70:1–14. doi: 10.1016/s0091-3057(01)00512-3. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Nixon R. Dystrophic serotonergic axons in neurodegenerative diseases. Brain Res. 2008;1217:185–94. doi: 10.1016/j.brainres.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D, et al. Mapping the subcellular distribution of alpha-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson's disease pathogenesis. J Neurosci. 2013;33:2605–15. doi: 10.1523/JNEUROSCI.2898-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Burgess A, et al. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A. 2010;107:12564–9. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, O'Malley K. Axon degeneration in Parkinson's disease. Exp Neurol. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, et al. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, et al. Hippocampal abnormalities and memory deficits in Parkinson disease: A multimodal imaging study. Neurology. 2012;78:1939–45. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- Cartelli D, et al. Microtubule destabilization is shared by genetic and idiopathic Parkinson's disease patient fibroblasts. PLoS One. 2012;7:e37467. doi: 10.1371/journal.pone.0037467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, et al. Ultrastructure of the serotonin innervation in the mamalian central nervous system. In: Müller CP, Jacobs BL, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic Press; London: 2010. pp. 65–102. [Google Scholar]
- Descarries L, et al. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–54. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Deusser J, et al. Serotonergic dysfunction in the A53T alpha-synuclein mouse model of Parkinson's disease. J Neurochem. 2015 doi: 10.1111/jnc.13253. 10.1111/jnc.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, et al. Glutamate-induced transient modification of the postsynaptic density. Proc Natl Acad Sci U S A. 2001;98:10428–32. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher DA, et al. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord. 2010;25:2493–500. doi: 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- Gustafsson H, et al. Depression and subsequent risk of Parkinson disease: A nationwide cohort study. Neurology. 2015;84:2422–9. doi: 10.1212/WNL.0000000000001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn K, et al. Clinical features, with video documentation, of the original familial lewy body parkinsonism caused by alpha-synuclein triplication (Iowa kindred) Mov Disord. 2011;26:2134–6. doi: 10.1002/mds.23776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, et al. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord. 2014;29:634–50. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson's disease. Ann Neurol. 1990;27:373–85. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Hinnell C, et al. Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson's disease? Mov Disord. 2012;27:236–41. doi: 10.1002/mds.23961. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–35. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, et al. Neonatal serotonin depletion alters behavioral responses to spatial change and novelty. Brain Res. 2007;1139:163–77. doi: 10.1016/j.brainres.2006.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P, Fox SH. The serotonergic system in motor and non-motor manifestations of Parkinson's disease. Exp Brain Res. 2013;230:463–76. doi: 10.1007/s00221-013-3621-2. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathobiology of non-motor symptoms in Parkinson disease. J Neural Transm. 2015 doi: 10.1007/s00702-015-1405-5. 10.1007/s00702-015-1405-5. [DOI] [PubMed] [Google Scholar]
- Johnson M, et al. Neurogenic marker abnormalities in the hippocampus in dementia with Lewy bodies. Hippocampus. 2011;21:1126–36. doi: 10.1002/hipo.20826. [DOI] [PubMed] [Google Scholar]
- Kasten M, Klein C. The many faces of alpha-synuclein mutations. Mov Disord. 2013;28:697–701. doi: 10.1002/mds.25499. [DOI] [PubMed] [Google Scholar]
- Klempin F, et al. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci. 2013;33:8270–5. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl Z, et al. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci. 2012;35:10–9. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, et al. Nucleus-specific alteration of raphe neurons in human neurodegenerative disorders. Neuroreport. 2003;14:73–6. doi: 10.1097/00001756-200301200-00014. [DOI] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–10. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts JT, et al. Spreading of alpha-synuclein in the face of axonal transport deficits in Parkinson's disease: a speculative synthesis. Neurobiol Dis. 2015;77:276–83. doi: 10.1016/j.nbd.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Liebmann T, et al. A noncanonical postsynaptic transport route for a GPCR belonging to the serotonin receptor family. J Neurosci. 2012;32:17998–8008. doi: 10.1523/JNEUROSCI.1804-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, et al. alpha-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J Neurosci. 2011;31:10076–87. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, et al. Serotonin depletion causes long-term reduction of exploration in the rat. Pharmacol Biochem Behav. 1992;43:1247–52. doi: 10.1016/0091-3057(92)90510-m. [DOI] [PubMed] [Google Scholar]
- Lugert S, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–56. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Luo DD, et al. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull. 2008;77:8–12. doi: 10.1016/j.brainresbull.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Mahar I, et al. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–92. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Mahlknecht P, Poewe W. Is there a need to redefine Parkinson's disease? J Neural Transm. 2013;120(Suppl 1):S9–17. doi: 10.1007/s00702-013-1038-5. [DOI] [PubMed] [Google Scholar]
- Marxreiter F, et al. Glial A30P alpha-synuclein pathology segregates neurogenesis from anxiety-related behavior in conditional transgenic mice. Neurobiol Dis. 2013;59:38–51. doi: 10.1016/j.nbd.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem. 1997;69:791–6. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- Miller DW, et al. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62:1835–8. doi: 10.1212/01.wnl.0000127517.33208.f4. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto SM, et al. Anxiogenic effect of median raphe nucleus lesion in stressed rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1135–41. doi: 10.1016/s0278-5846(02)00248-8. [DOI] [PubMed] [Google Scholar]
- Nuber S, et al. A progressive dopaminergic phenotype associated with neurotoxic conversion of alpha-synuclein in BAC-transgenic rats. Brain. 2013;136:412–32. doi: 10.1093/brain/aws358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber S, et al. Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease. J Neurosci. 2008;28:2471–84. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus M, et al. Enhanced dendritogenesis and axogenesis in hippocampal neuroblasts of LRRK2 knockout mice. Brain Res. 2013;1497:85–100. doi: 10.1016/j.brainres.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Persico AM, et al. Multiple receptors mediate the trophic effects of serotonin on ventroposterior thalamic neurons in vitro. Brain Res. 2006;1095:17–25. doi: 10.1016/j.brainres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Petrik D, et al. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumpe T, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pum ME, et al. Role of medial prefrontal, entorhinal, and occipital 5-HT in cocaine-induced place preference and hyperlocomotion: evidence for multiple dissociations. Psychopharmacology (Berl) 2008;201:391–403. doi: 10.1007/s00213-008-1296-3. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, et al. Accumulation of oligomer-prone alpha-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain. 2014;137:1496–513. doi: 10.1093/brain/awu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, et al. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–94. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–9. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scott D, Roy S. alpha-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci. 2012;32:10129–35. doi: 10.1523/JNEUROSCI.0535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K, et al. The Brainstem Pathologies of Parkinson's Disease and Dementia with Lewy Bodies. Brain Pathology. 2015;25:121–135. doi: 10.1111/bpa.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–27. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–88. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, et al. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278:44405–11. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–7. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakhtenberg EF, Goldberg JL. The role of serotonin in axon and dendrite growth. Int Rev Neurobiol. 2012;106:105–26. doi: 10.1016/B978-0-12-407178-0.00005-3. [DOI] [PubMed] [Google Scholar]
- Ubhi K, et al. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of alpha-synucleinopathy. Exp Neurol. 2012;234:405–16. doi: 10.1016/j.expneurol.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mierlo TJ, et al. Depressive symptoms in Parkinson's disease are related to decreased hippocampus and amygdala volume. Mov Disord. 2015;30:245–52. doi: 10.1002/mds.26112. [DOI] [PubMed] [Google Scholar]
- Vargas KJ, et al. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J Neurosci. 2014;34:9364–76. doi: 10.1523/JNEUROSCI.4787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone A, et al. Positron emission tomography imaging of 5-hydroxytryptamine1B receptors in Parkinson's disease. Neurobiol Aging. 2014;35:867–75. doi: 10.1016/j.neurobiolaging.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol. 1997;378:411–24. doi: 10.1002/(sici)1096-9861(19970217)378:3<411::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, et al. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–82. [PubMed] [Google Scholar]
- Weintraub D, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord. 2015;30:919–27. doi: 10.1002/mds.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, et al. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci U S A. 1997;94:12676–81. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger C, et al. Modulation of the trafficking of the human serotonin transporter by human alpha-synuclein. Eur J Neurosci. 2006;24:55–64. doi: 10.1111/j.1460-9568.2006.04900.x. [DOI] [PubMed] [Google Scholar]
- Winner B, et al. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–51. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- Winner B, et al. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63:1155–66. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- Winner B, et al. Role of alpha-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci. 2012;32:16906–16. doi: 10.1523/JNEUROSCI.2723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, et al. Ventral hippocampal molecular pathways and impaired neurogenesis associated with 5-HT(1)A and 5-HT(1)B receptors disruption in mice. Neurosci Lett. 2012;521:20–5. doi: 10.1016/j.neulet.2012.05.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.