Abstract
Context:
The increase in the detection of unruptured cerebral aneurysms has led to management dilemma. Prediction of risk based on the size of the aneurysm is not always accurate. There is no objective way of predicting rupture of aneurysm so far. Computational fluid dynamics (CFDs) was proposed as a tool to identify the rupture risk.
Aims:
To know the correlation of CFD findings with intraoperative microscopic findings and to know the relevance of CFD in the prediction of rupture risk and in the management of unruptured intracranial aneurysms.
Settings and Design:
A prospective study involving nine cases over a period of 6 months as an initial analysis.
Subjects and Methods:
Both males and females were included in the study. Preoperative analysis was performed using computed tomography angiogram, magnetic resonance imaging in all cases and digital substraction angiogram in some cases. Intraoperatively microscopic examination of the aneurysm wall was carried out and images recorded. The correlation was done between microscopic and CFD images.
Results:
Seven cases were found intraoperatively to have a higher risk of rupture based on the thinning of the wall. Two cases had an atherosclerotic wall. All cases had low wall shear stress (WSS).Only two cases with atherosclerotic wall had a correlation with low WSS.
Conclusions:
While the pressure measured with CFD technique is a good predictor of rupture risk, the WSS component is controversial. Multicentric trials involving a larger subset of population are needed before drawing any definite conclusions. On-going development in the CFD analysis may help to predict the rupture chances accurately in future.
Key words: Computational flow dynamics, intraoperative microscopic findings, unruptured intracranial aneurysms, wall changes
Introduction
Intracranial aneurysms are focal dilatations of vessel walls with a risk of rupture. Aneurysms are present in approximately 1% of the adult population, varying between <1% in young adults to 4% in the elderly.[1] The mortality from a ruptured aneurysm is quite high and in patients surviving it, the morbidity is high.[2,3] It is difficult to know which aneurysm will rupture in future. Classically rupture risk was based on the morphological characteristics of an aneurysm, i.e., size, lobulation or daughter sacs and aspect ratio.[4,5] In practice even small aneurysms rupture.[6] The management of unruptured aneurysms is not without complications. The morbidity and mortality can be as high as 10% and 2.5%, respectively.[7]
In recent years, there have been some studies regarding using computational fluid dynamics (CFD) to predict the rupture chances of an unruptured aneurysm. CFD, a branch of fluid mechanics in mechanical engineering, has been in use in industry in aerodynamics, hydrodynamics of vehicles and a lot of other fields.[8] It uses numerical analysis and algorithms to analyze and solve problems that involve fluid flows. Its use in biomedical engineering has progressed only in the last decade. Many simulations have been used to study carotid and intra-cranial cerebrovascular diseases. We were interested in is its utilization in cerebral aneurysms in specific.
CFD is being evaluated as a potential tool in knowing aneurysm hemodynamics and predicting aneurysm rupture risk. There are many controversies regarding the interpretation of CFD. While some advocate that lower wall shear stress (WSS) increases rupture risk others propose that higher WSS increase the rupture risk. What we aim to do is compare the preoperative CFD images and the intraoperative microscopic images of the aneurysm and know whether there is correlation and if there is any predictive value for CFD.
Subjects And Methods
A prospective cohort study was conducted during a 6-month period in 2015, from March to August, in nine patients with saccular intracranial aneurysms using computed tomography (CT) angiogram, CFD and digital substraction angiogram. Permission was taken from the institutional ethics committee for the study. Patient consent was taken for the study. The cases selected for surgery were the ones which had increased rupture risk as per CFD findings. The CFD software used was Hemoscope 2 (Ziosoft corporation, Minato ward, Tokyo, Japan). CFD codes contain three main components (1) a preprocessor, (2) a solver, and (3) a postprocessor.
Preprocessor
Preprocessing consists of inputting a fluid flow problem into a CFD program. Most of the time spent is devoted to this process.
Solver
Numerical solution techniques are available such as finite difference, finite element, finite volume, and spectral methods.
Postprocessor
The object of this process is to visualize the computational results. The changes in blood flow profiles, pressure distribution, WSS, oscillating shear index, and shear rate can be visualized using color rendering techniques. The WSS and pressure at various places in the aneurysm sac can be calculated.
WSS is the frictional force applied by flowing blood tangentially on the vessel wall. TAWSS is the time-averaged WSS taken for one cardiac cycle as WSS can change with cardiac systole and diastole. The WSS mentioned below is the TAWSS.
The ages of the patients ranged from 49 to 75 with mean of 66.5. There were eight female patients and one male patient. Six out of eight cases were internal carotid artery aneurysms, two cases was of anterior communicating artery (Acom) aneurysm and another of middle cerebral artery aneurysm [Table 1].
Table 1.
Aneurysm data
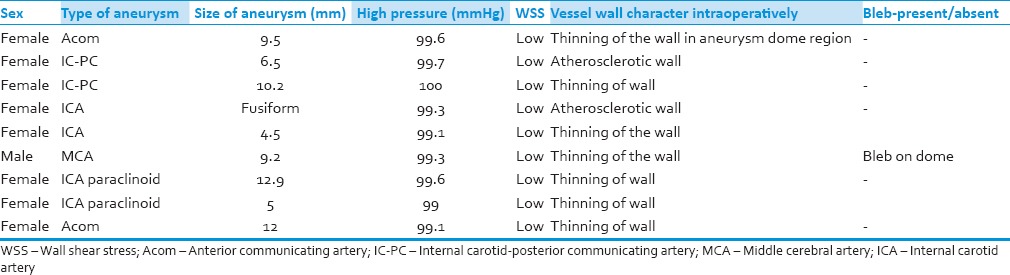
The patients were operated, and the aneurysm wall was observed with a microscope. Intraoperatively decreased vessel wall thinness was determined by the transparency of the vessel wall, the presence of a bleb on the aneurysm wall. Yellowish color of the vessel wall indicates atherosclerosis. Ability to see blood flow in the aneurysm or thinning of the vessel wall, i.e., translucency, and bleb on aneurysm wall were predictors for future rupture. Intraoperatively indocyanine video angiography (ICG-VA) was also used. The areas of thinning had higher fluorescence and flow of blood could also be noted with ICG-VA.
Results
In 7/9 cases of intracranial aneurysms that were studied by us using CFD software and surgery, there was thinning of the wall of the aneurysm, suggestive of increased risk of rupture in future [Figure 1]. Two cases had atherosclerotic plaques involving most of aneurysm wall. In all cases, the pressure in the aneurysm sac was increased whereas the WSS was decreased [Figure 2].
Figure 1.
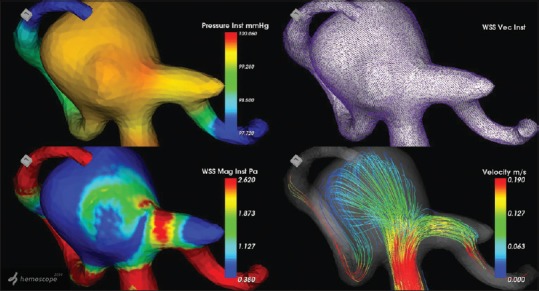
Computational fluid dynamics image of a middle cerebral artery aneurysm with high pressure in aneurysm dome and low wall shear stress
Figure 2.
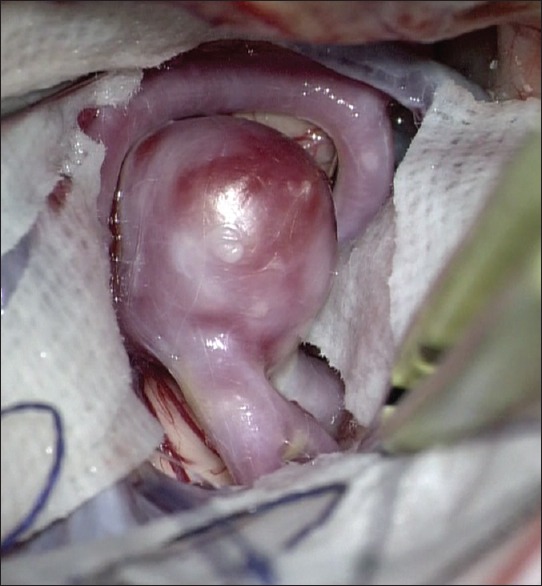
Intraoperative image of the same aneurysm with thinned wall
Comparison of the CFD images were done with the operative microscope images. The analysis was done to see if it correlates. The CFD parameters used were pressure in the aneurysm and WSS. Increased pressure in CFD correlated with increased rupture risk in 9/9 cases. 7/9 cases had vessel wall thinning and was at risk of rupture. Low WSS with the risk of rupture correlated in 7/9 cases according to Xiang et al. In 8/9 cases, clipping of aneurysm was done. In one case of aneurysm with the patchy atherosclerotic wall, the clipping was done avoiding the atherosclerotic patch. In another case, only wrapping could be done as the wall was very atheroclerotic. All cases had a normal postoperative course without any complications.
Discussion
Management of unruptured intracranial aneurysms is a challenge. More so with International Study of Unruptured Intracranial Aneurysms (ISUIA) recommendations which says that the rupture chance in aneurysms <7 mm in the anterior circulation, which have not bled, is very less. The ISUIA concluded that aneurysms <7 mm in size in the anterior circulation have an annual rupture risk of 0–0.1% per year.[7,9] However in practice it is different. Small aneurysms in the anterior circulation, particularly in the Acom region is notorious for bleeding.[10] Other than the size, the location of the aneurysm is also very important. Small aneurysms in the posterior circulation, posterior communicating artery and Acom region have a higher risk of rupture.[11]
Improvement in the imaging and screening modalities has contributed to the increase in detection of unruptured aneurysms. In some countries, the incidence of intracranial aneurysms is more than the average mentioned in the studies. There is a need to quantify objectively the risk of rupture in the cohort of people with incidentally found aneurysms.
With this dilemma and the need for definite management in unruptured aneurysms smaller than 5 mm arose the need for prediction of rupture risk of unruptured aneurysm and that too in minimally or noninvasive way.
CFD which was earlier a purely engineering tool has come into vogue in biomedical engineering with its usefulness in knowing the flow dynamics in an aneurysm noninvasively. CFD helps in calculating the velocity of blood flowing in the aneurysm sac, the pressure in the aneurysm sac and the WSS.
There are many laboratory studies of CFD but they have a limitation that the wall in their studies is rigid and thereby change the outcome of studies. In vivo studies have been few.
However, there were no studies which could directly see if the results shown in CFD analysis could be correlated intraoperatively. In our series of nine cases we could see the thinning of the aneurysmal wall in seven cases which is a predictor for future rupture.[11] Increased pressure in the aneurysm sac in all cases shows that hemodynamic factors have been responsible for the initiation and growth of the aneurysm.[12,13,14,15]
There has been an ongoing debate regarding whether low WSS or high WSS contributes to aneurysm formation.[12] Meng et al. proposed that high WSS correlates with Type 1 aneurysm formation, i.e., small and transparent aneurysms whereas low WSS contributed to Type 2 aneurysm formation, i.e., thick walled atherosclerotic type.[12] Cebral et al. reported that ruptured aneurysms have higher WSS compared with unruptured aneurysms.[13] Xiang et al. have proposed in their study that lower WSS contributes to rupture of aneurysm.[14]
Yoichi et al. in their study proposed that lower shear stress is associated with increased risk of rupture.[15] This controversy has mainly been due the low sample sizes in the studies and a small number of studies so far.
In our study, all cases had low WSS and only two were of thick wall type i.e. atherosclerotic. Seven cases with low WSS had thinning of the vessel wall. One aneurysm had a bleb. Studies have shown that thinning of vessel wall identified by the transparency of vessel wall or bleb formation increases the risk of rupture.[16]
This is in contrast to what Meng et al. have proposed. They proposed that higher WSS leads to thin wall and lower WSS leads to thick wall aneurysms.[12] So this shows that the mechanisms proposed so far in aneurysm initiation, growth, and rupture are far from accurate. However in all the cases, it has shown that the pressure in the aneurysm sac is high, which denotes increased risk. There has been no controversy as far the pressure parameter in CFD is considered. The WSS component is controversial with regard to the interpretation. We accept the limitations of our study due to the small number of patients. While the controversies regarding WSS are not resolved, CFD does hold promise as an innovative tool in the management of aneurysm patients. Single center studies may be biased. Multicentric studies involving larger series are needed to settle the WSS controversy and form a uniform hypothesis.
There are many advantages when considering CFD. CFD provides a means of simulating blood flow, in cerebral vasculature noninvasively. It provides detailed visual and comprehensive information colorfully. It provides the ability to know the pressure, WSS, streamlines and vector at various points in the aneurysm and helps us to know the character of blood flow in the aneurysm.
However, it has some disadvantages. There is difficulty in interpreting the results, software is available only at a few centers, insufficient resolution when using low-resolution magnetic resonance imaging or CT, numerical errors can occur during the test leading to incorrect conclusions and wrong management. Cooperation between the surgeon and the biomedical engineer is also difficult at times.
Overcoming the limitations with simplification of software and the analyses will help in the wider use of this modality. Integration of this software with the bigger studies like ISUAI and UCAS Japan will help in the prediction accuracy.
Conclusion
CFD as a diagnostic tool is noninvasive to the patient and helps in assessing the hemodynamic parameters in the aneurysm sac. It gives us an idea about the rupture risk of aneurysm. However with the software available so far no definite conclusion can be made. Adding to this is the controversy regarding high or low WSS contributing to aneurysmal rupture. Multicentric trials involving a larger subset of the population are needed before drawing any definite conclusions. Improvement of the software in a clinician-friendly manner in interpreting results will help. On-going development in the CFD analysis may help to predict the rupture chances accurately in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Komotar RJ, Mocco J, Solomon RA. Guidelines for the surgical treatment of unruptured intracranial aneurysms: The first annual J. Lawrence pool memorial research symposium – Controversies in the management of cerebral aneurysms. Neurosurgery. 2008;62:183–93. doi: 10.1227/01.NEU.0000311076.64109.2E. [DOI] [PubMed] [Google Scholar]
- 2.Bonneville F, Sourour N, Biondi A. Intracranial aneurysms: An overview. Neuroimaging Clin N Am. 2006;16:371–82, vii. doi: 10.1016/j.nic.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Curtis SL, Bradley M, Wilde P, Aw J, Chakrabarti S, Hamilton M, et al. Results of screening for intracranial aneurysms in patients with coarctation of the aorta. AJNR Am J Neuroradiol. 2012;33:1182–6. doi: 10.3174/ajnr.A2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, et al. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: A possible index for surgical treatment of intracranial aneurysms. Neurosurgery. 1999;45:119–29. doi: 10.1097/00006123-199907000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Weir B, Amidei C, Kongable G, Findlay JM, Kassell NF, Kelly J, et al. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg. 2003;99:447–51. doi: 10.3171/jns.2003.99.3.0447. [DOI] [PubMed] [Google Scholar]
- 6.Unruptured intracranial aneurysms – Risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725–33. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 7.King JT, Jr, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: A meta-analysis. J Neurosurg. 1994;81:837–42. doi: 10.3171/jns.1994.81.6.0837. [DOI] [PubMed] [Google Scholar]
- 8.Tu J, Yeoh GH, Liu C. Introduction. In: Yeoh GH, editor. Computational Fluid Dynamics: A Practical Approach. 2nd ed. Oxford: Elesevier; 2013. pp. 1–29. [Google Scholar]
- 9.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 10.UCAS Japan Investigators. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–82. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 11.Kadasi LM, Dent WC, Malek AM. Cerebral aneurysm wall thickness analysis using intraoperative microscopy: Effect of size and gender on thin translucent regions. J Neurointerv Surg. 2013;5:201–6. doi: 10.1136/neurintsurg-2012-010285. [DOI] [PubMed] [Google Scholar]
- 12.Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35:1254–62. doi: 10.3174/ajnr.A3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cebral JR, Mut F, Weir J, Putman C. Quantitative characterization of the hemodynamic environment in ruptured and unruptured brain aneurysms. AJNR Am J Neuroradiol. 2011;32:145–51. doi: 10.3174/ajnr.A2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. 2011;42:144–52. doi: 10.1161/STROKEAHA.110.592923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura Y, Ishida F, Umeda Y, Tanemura H, Suzuki H, Matsushima S, et al. Low wall shear stress is independently associated with the rupture status of middle cerebral artery aneurysms. Stroke. 2013;44:519–21. doi: 10.1161/STROKEAHA.112.675306. [DOI] [PubMed] [Google Scholar]
- 16.Cebral JR, Sheridan M, Putman CM. Hemodynamics and bleb formation in intracranial aneurysms. AJNR Am J Neuroradiol. 2010;31:304–10. doi: 10.3174/ajnr.A1819. [DOI] [PMC free article] [PubMed] [Google Scholar]


