Abstract
Background:
Ganglioglioma is a common seizure associated tumor. The goal of this study was to observe the postoperative outcome in patients with gangliogliomas.
Material and Methods:
A total 24 patients with gangliogliomas who underwent surgery at our institute from 2008 to 2011 were included. There were 13 males (54%) in our study. A retrospective analysis for the demographic profile, surgery and outcome was performed using STATA software. Literature on this subject was also reviewed, MEDLINE and PUBMED databases were searched.
Observations:
Sixteen patients presented with signs and symptoms of raised intracranial pressure and 12 patients had seizure disorder. Average age at surgery was 20 years (range 7-50 years). Twelve each were located in the temporal lobe and extra-temporal location. Intra-operative electrocorticography (ECoG) alone in three and image guidance alone were used in two patients, respectively. Both ECoG and image guidance were used in one patient and none of them was used in 18 patients. Gross total resection was achieved in 17 patients. After a mean follow-up of 1.6 years (range 3 months to 2.5 years), out of 12 patients with preoperative seizures, 10 (83.3%) were seizure free (Engel class-I) and 2 (16.6%) belonged to Engel class-II. None of the factors, including age at surgery, seizure duration prior to surgery, type of seizures, use of intra-operative ECoG and image guidance, extent of tumor resection, and surgical strategy proved to have significant correlation with postoperative seizure outcome.
Conclusions:
Surgical treatment is effective and safe for patients with gangliogliomas. Neither intra-operative ECoG nor image guidance necessarily leads to better seizure control, although they are useful adjunct for achieving safe and complete tumor resection.
Key words: Epilepsy surgery, ganglioglioma, intra-operative electrocorticography, neuronavigation
Introduction
Gangliogliomas are rare central nervous system tumors, which pathologically consist of low-grade neuronal–glial elements. They are considered as benign and slow-growing neoplasms with low morbidity and mortality.[1,2,3] They are common in children and young adults,[4,5] with a predilection for temporal lobe.[2,6] They frequently present with seizures and account for approximately 40% of all epileptogenic tumors.[1,3,7,8] Complete and timely resection of ganglioglioma is known to achieve better seizure control and outcome.[2,3,9,10] We retrospectively analyzed data pertaining to 24 patients who underwent surgical resection for ganglioglioma at our centre. The objective of our study was to analyze this subgroup of patients, with this rare central nervous system pathology for their demographic profile, clinical features, management, and outcome.
Materials and Methods
Patient population
We retrospectively analyzed data pertaining to 24 patients, with biopsy-proven ganglioglioma. All patients had undergone preoperative magnetic resonance (MR) imaging and who had history of seizure were subjected to one or more electroencephalography studies. The aim of the surgery was complete resection of the tumor whenever possible. The surgical approach was chosen depending on the site of the lesion. A postoperative brain MR imaging scan was performed in all patients, most of them at 6 months after surgery. The extent of resection was confirmed by reviewing the postoperative MR imaging and operation records.
Resection was defined as 'gross total' if residual tumor was not seen on postoperative contrast MR imaging; 'subtotal' if more than 80% of the tumor was removed; and 'partial' if less than 80% of the tumor was removed. Seizure outcome was assessed periodically in the outpatient department and with telephone interviews. The classification proposed by Engel et al.[11] was used to grade postoperative seizure outcome.
Results
Clinical findings
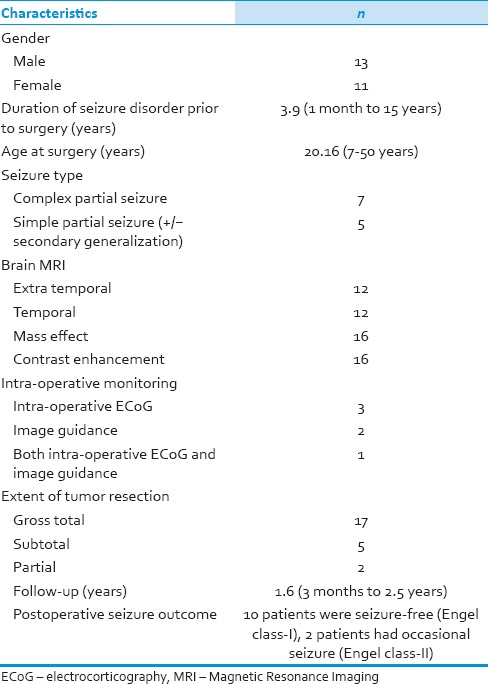
In our study, 13 (54%) patients were male and 11(46%) female. Signs and symptoms of raised ICP were present in 16 (66.6%) patients. Twelve patients (50%) presented with seizure disorder (seven patients with complex partial seizure with temporal lobe mass and five patients with simple complex seizure and secondary generalization with extra-temporal lesions) and four patients with pyramidal signs and five with cranial nerve paresis; 1 patient also had cerebellar signs. The mean interval between the onset of seizures and surgery was 3.9 years (range 1 month to 15 years) and average age at surgery was 20.2 years (range 7-50 years) [Table 1].
Table 1.
Clinical profile of patients (n=24)
On MR imaging 12 tumors (50%) were located in the temporal lobe [Figures 1 and 2] 1 in the frontal lobe; 2 each in the parietal and occipital lobe, cerebellum, and posterior third ventricle region; and 3 patients presented with a suprasellar mass with chiasmal involvement and brainstem compression. Contrast enhancement of the tumor was observed in 16 patients.
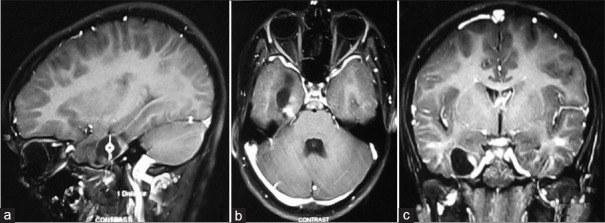
Figure 1.
Post-contrast T1-W (T1-weighted) axial (a) Sagittal; (b) And coronal; (c) Images showing contrast enhancing solid cystic mass in the right medial temporal region
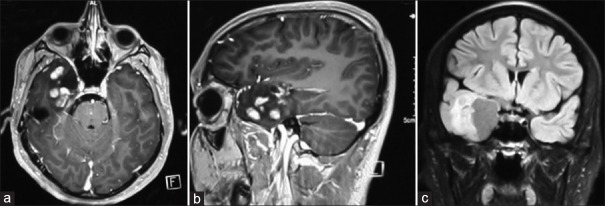
Figure 2.
Post-contrast T1-W axial (a) Sagittal; (b) And T2-Flair coronal; (c) Images showing contrast enhancing solid cystic mass in the right anterior temporal region
Surgical results
Intra-operative electrocorticography (ECoG) alone was used in three patients of temporal lobe lesion (2 with epilepsy and 1 without epilepsy) and image guidance alone was used in two patients (1 with temporal lobe lesion without epilepsy and 1 with suprasellar tumor with chiasmal involvement). Both ECoG and image guidance were used in one patient with temporal lobe lesion with epilepsy. In all three temporal lobe ganglioglioma patients with epilepsy in which intra-operative ECoG was used, we removed the adjacent neocortex from where the active spikes or pronounced attenuation were obtained. We did not use intra-operative ECoG and image guidance for 18 (75%) patients (2 patients with temporal lobe lesion with epilepsy and 16 with extra-temporal lesions).
Gross total resection was achieved in 17 (70.8%), subtotal resection in 5 (20.8%), and partial resection in 2 (8.3%) patients. No tumor recurrence or progression was observed during the mean follow-up of 1.6 years.
Pathological findings
All tumors presented features of WHO grade-I ganglioglioma. They were composed of clusters of large polymorphic ganglion cells and neoplastic astrocytes. Vascular endothelial proliferation or mild peri-vascular chronic inflammation was observed.
Seizure outcome
After a mean follow-up of 1.6 years (range 3 months to 2.5 years), out of 12 patients with pre-operative seizures, 10 (83.3%) were seizure-free (Engel class-I) and 2 (16.6%) belonged to Engel class-II [Table 2].
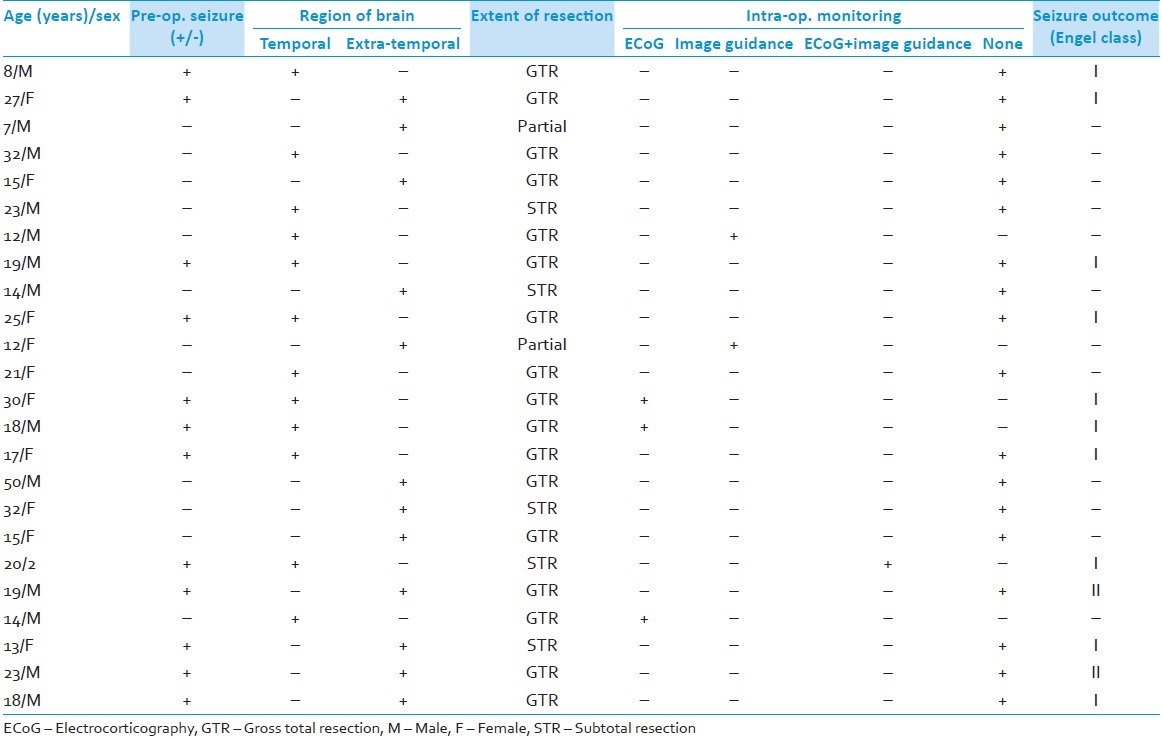
Table 2.
Summary of patients (n=24)
Statistical analysis
As previously mentioned, patients were divided into a seizure-free group (Engel class-I) and a non-seizure-free group (Engel class II-IV). Gender, age at surgery, and seizure duration prior to surgery did not differ between the two groups. Presence of seizure type or secondary generalization was not significantly correlated with postoperative seizure outcome. MR imaging findings, including mass effect and contrast enhancement, were not correlated with seizure outcome. Use of intra-operative ECoG or intra-operative ultrasonography IOUS did not affect postoperative seizure outcome. Simple lesionectomy and tailored epilepsy surgery led to similar seizure outcome. Gross total resection tends to be better achieved in patients operated on under Intraoperative ultrasound (IOUS) monitoring. However, extent of resection was not related with a better postoperative seizure outcome. The seizure outcome difference between temporal resection and extra-temporal resection was not statistically significant.
Complications
There was no operative mortality and none of the patients died during the mean follow-up period of 1.6 years. Four patients had postoperative complications: Two patients had seizure in the immediate postoperative period and one each developed inter-nuclear opthalmoplegia and urinary incontinence. All patients recovered completely prior to discharge from hospital.
Discussion
Ganglioglioma is a common epileptogenic tumor, and surgery is currently considered as the treatment of choice. It is still unclear whether tumor resection (lesionectomy) alone is sufficient[2,12] or additional resection of the epileptogenic zone is required for seizure control.[13,14]
We believe in complete resection of the tumor along with additional resection of the epileptogenic zone whenever possible. We find ECoG to be very helpful in identifying the irritative zone adjacent to tumor. Use of intra-operative CT/ultrasound/MR imaging guidance has helped us in accurate localization of the tumor. However, the available literature on usage of these adjuncts is limited and does not show a positive correlation with the final outcome.
During the follow-up period, all nine patients for whom primary indication for operation was intractable epilepsy became seizure-free. These data indicated that surgical treatment might result in excellent seizure control for patients with ganglioglioma. Whether early surgical intervention, short duration of epilepsy and absence of secondary generalization are positive predictors of seizure outcome is controversial. Morris et al.[2] observed a better seizure outcome in patients with a shorter duration of epilepsy and younger age at operation. These findings were supported by Aronica et al.[15] However, Im et al.[10] failed to find any correlation between improved seizure outcome and the above-mentioned factors.
Intra-operative ECOG is traditionally used during epilepsy surgery to identify epileptogenic zone, thus aiding in resection of epileptic foci, which can be away from the tumor itself.[16,17] However, it has limitations, including absence of ictal information and susceptibility to anesthesia disturbance.[18] Due to its short duration and absence of ictal recording, intra-operative ECoG is not a substitute for pre-surgical evaluation.[19] Some investigators have demonstrated satisfactory seizure control achieved by lesionectomy alone without intra-operative ECoG monitoring.[20,21,22] Based on the above facts and our own experience, we believe that intra-operative ECoG is a useful aid in the armamentarium of an epilepsy surgeon, but in its absence also, adequate tumor resection is likely to provide good outcome in terms of recurrence of seizure and tumor itself.
However, to our present knowledge, no previous study has investigated the significance of image guidance in postoperative seizure control among patients with gangliogliomas. Use of image guidance significantly increased the rate of complete tumor resection. The surgical strategy (simple lesionectomy or tailored epilepsy surgery) for epileptogenic ganglioglioma remains debatable. Some investigators suggested that simple lesionectomy was sufficient for favorable seizure control,[20] whereas others advocated tailored epilepsy surgery.[7,23] Basic studies also revealed that there were some mechanisms underlying tailored epilepsy surgery for epileptogenic tumors, which included morphological, metabolic, pH, amino acid, neuroreceptor, and neuro-immunological abnormalities in the adjacent cortex.[24] However, no previous report is available dealing with both simple lesionectomy and tailored epilepsy surgery or compared the seizure outcome of two different surgical strategies. In the present study, all patients were treated with simple lesionectomy, which indicated simple lesionectomy was sufficient for good seizure control. However, additional studies are required to provide more convincing evidence in this regards. Senior author advocates use of ECoG and wherever possible and required intraoperative co-registration of MRI, positron emission tomography and ECoG for improving the localization of the epileptogenic focus.25,[26]
Conclusions
In conclusion, surgical treatment is effective in achieving good seizure outcome with low complication rates in patients with epileptogenic gangliogliomas. Although early surgical intervention appears not to be correlated with better seizure outcome, it is necessary for achieving early seizure control. Intra-operative ECoG and neuronavigation are useful adjuncts in the armamentarium of an epilepsy surgeon. Simple lesionectomy is sufficient for favorable postoperative seizure control.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Zentner J, Wolf HK, Ostertun B, Hufnagel A, Campos MG, Solymosi L, et al. Gangliogliomas: Clinical, radiological, and histopathological findings in 51 patients. J Neurol Neurosurg Psychiatry. 1994;57:1497–502. doi: 10.1136/jnnp.57.12.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris HH, Matkovic Z, Estes ML, Prayson RA, Comair YG, Turnbull J, et al. Ganglioglioma and intractable epilepsy: Clinical and neurophysiologic features and predictors of outcome after surgery. Epilepsia. 1998;39:307–13. doi: 10.1111/j.1528-1157.1998.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 3.Haddad SF, Moore SA, Menezes AH, VanGilder JC. Ganglioglioma: 13 years of experience. Neurosurgery. 1992;31:171–8. doi: 10.1227/00006123-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Casazza M, Avanzini G, Broggi G, Fornari M, Franzini A. Epilepsy course in cerebral gangliogliomas: A study of 16 cases. Acta Neurochir Suppl (Wien) 1989;46:17–20. doi: 10.1007/978-3-7091-9029-6_4. [DOI] [PubMed] [Google Scholar]
- 5.Chintagumpala MM, Armstrong D, Miki S, Nelson T, Cheek W, Laurent J, et al. Mixed neuronal–glial tumors (gangliogliomas) in children. Pediatr Neurosurg. 1996;24:306–13. doi: 10.1159/000121060. [DOI] [PubMed] [Google Scholar]
- 6.Radhakrishnan A, Abraham M, Radhakrishnan VV, Sarma SP, Radhakrishnan K. Medically refractory epilepsy associated with temporal lobe ganglioglioma: Characteristics and postoperative outcome. Clin Neurol Neurosurg. 2006;108:648–54. doi: 10.1016/j.clineuro.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Zentner J, Hufnagel A, Wolf HK, Ostertun B, Behrens E, Campos MG, et al. Surgical treatment of neoplasms associated with medically intractable epilepsy. Neurosurgery. 1997;41:378–86. doi: 10.1097/00006123-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Silver JM, Rawlings CE, 3rd, Rossitch E, Jr, Zeidman SM, Friedman AH. Ganglioglioma: A clinical study with long-term follow-up. Surg Neurol. 1991;35:261–6. doi: 10.1016/0090-3019(91)90002-q. [DOI] [PubMed] [Google Scholar]
- 9.Yang I, Chang EF, Han SJ, Barry JJ, Fang S, Tihan T, et al. Early surgical intervention in adult patients with ganglioglioma is associated with improved clinical seizure outcomes. J Clin Neurosci. 2011;18:29–33. doi: 10.1016/j.jocn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Im SH, Chung CK, Cho BK, Wang KC, Yu IK, Song IC, et al. Intracranial ganglioglioma: Preoperative characteristics and oncologic outcome after surgery. J Neurooncol. 2002;59:173–83. doi: 10.1023/a:1019661528350. [DOI] [PubMed] [Google Scholar]
- 11.Engel J, Jr, Van Ness P, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993. pp. 609–21. [Google Scholar]
- 12.Giulioni M, Gardella E, Rubboli G, Roncaroli F, Zucchelli M, Bernardi B, et al. Lesionectomy in epileptogenic gangliogliomas: Seizure outcome and surgical results. J Clin Neurosci. 2006;13:529–35. doi: 10.1016/j.jocn.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Park YS, Kim DS, Shim KW, Kim JH, Choi JU. Factors contributing to resectability and seizure outcomes in 44 patients with ganglioglioma. Clin Neurol Neurosurg. 2008;110:667–73. doi: 10.1016/j.clineuro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Morioka T, Hashiguchi K, Nagata S, Miyagi Y, Yoshida F, Shono T, et al. Additional hippocampectomy in the surgical management of intractable temporal lobe epilepsy associated with glioneuronal tumor. Neurol Res. 2007;29:807–15. doi: 10.1179/016164107X223566. [DOI] [PubMed] [Google Scholar]
- 15.Aronica E, Leenstra S, van Veelen CW, van Rijen PC, Hulsebos TJ, Tersmette AC, et al. Glioneuronal tumors and medically intractable epilepsy: A clinical study with long-term follow-up of seizure outcome after surgery. Epilepsy Res. 2001;43:179–91. doi: 10.1016/s0920-1211(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 16.Awad IA, Rosenfeld J, Ahl J, Hahn JF, Luders H. Intractable epilepsy and structural lesions of the brain: Mapping, resection strategies, and seizure outcome. Epilepsia. 1991;32:179–86. doi: 10.1111/j.1528-1157.1991.tb05242.x. [DOI] [PubMed] [Google Scholar]
- 17.Gelinas JN, Battison AW, Smith S, Connolly MB, Steinbok P. Electrocorticography and seizure outcomes in children with lesional epilepsy. Childs Nerv Syst. 2011;27:381–90. doi: 10.1007/s00381-010-1279-7. [DOI] [PubMed] [Google Scholar]
- 18.Kuruvilla A, Flink R. Intraoperative electrocorticography in epilepsy surgery: Useful or not? Seizure. 2003;12:577–84. doi: 10.1016/s1059-1311(03)00095-5. [DOI] [PubMed] [Google Scholar]
- 19.Zumsteg D, Wieser HG. Presurgical evaluation: Current role of invasive EEG. Epilepsia. 2000;41(Suppl 3):S55–60. doi: 10.1111/j.1528-1157.2000.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 20.Giulioni M, Galassi E, Zucchelli M, Volpi L. Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurg. 2005;102:288–93. doi: 10.3171/ped.2005.102.3.0288. [DOI] [PubMed] [Google Scholar]
- 21.Kim SK, Wang KC, Hwang YS, Kim KJ, Cho BK. Intractable epilepsy associated with brain tumors in children: Surgical modality and outcome. Childs Nerv Syst. 2001;17:445–52. doi: 10.1007/s003810000431. [DOI] [PubMed] [Google Scholar]
- 22.Yeon JY, Kim JS, Choi SJ, Seo DW, Hong SB, Hong SC. Supratentorial cavernous angiomas presenting with seizures: Surgical outcomes in 60 consecutive patients. Seizure. 2009;18:14–20. doi: 10.1016/j.seizure.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Pilcher WH, Silbergeld DL, Berger MS, Ojemann GA. Intraoperative electrocorticography during tumor resection: Impact on seizure outcome in patients with gangliogliomas. J Neurosurg. 1993;78:891–902. doi: 10.3171/jns.1993.78.6.0891. [DOI] [PubMed] [Google Scholar]
- 24.Beaumont A, Whittle IR. The pathogenesis of tumour associated epilepsy. Acta Neurochir (Wien) 2000;42:1–15. doi: 10.1007/s007010050001. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi M, Garg A, Gaikwad S, Bal CS, Chitra S, Prasad K, et al. Intra-operative electrocorticography in lesional epilepsy. Epilepsy Res. 2010;89:1133–41. doi: 10.1016/j.eplepsyres.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Chandra SP, Bal CS, Jain S, Joshua SP, Gaikwad S, Garg A, et al. Intraoperative Coregistration of Magnetic Resonance Imaging, Positron Emission Tomography, and Electrocorticographic Data for Neocortical Lesional Epilepsies May Improve the Localization of the Epileptogenic Focus: A Pilot Study. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.02.057. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]