Abstract
Rodenticides and pesticides pose a significant threat, not only to the environment, but also directly to humans by way of accidental and/or intentional exposure. Metal phosphides, such as aluminum, magnesium, and zinc phosphides, have gained popularity owing to ease of manufacture and application. These agents and their hydrolysis by-product, phosphine gas (PH3), are more than adequate for eliminating pests, primarily in the grain storage industry. In addition to the potential for accidental exposures in the manufacture and use of these agents, intentional exposures must also be considered. Ingestion of metal phosphides is a well-known suicide route, especially in Asia. An intentional release of PH3 in a populated area cannot be discounted. Metal phosphides cause a wide array of effects that include cellular poisoning, oxidative stress, cholinesterase inhibition, circulatory failure, cardiotoxicity, gastrointestinal and pulmonary toxicity, hepatic damage, neurological toxicity, electrolyte imbalance, and overall metabolic disturbances. Mortality rates often exceed 70%. There are no specific antidotes against metal phosphide poisoning. Current therapeutic intervention is limited to supportive care. The development of beneficial medical countermeasures will rely on investigative mechanistic toxicology; the ultimate goal will be to identify specific treatments and therapeutic windows for intervention.
Keywords: rodenticide, phosphine, systemic poison, reactive oxygen species, mitochondria
Introduction
Tens of thousands of toxic industrial compounds (TICs) are synthesized each year in chemical production facilities throughout the world. We come into contact with many of these TICs on a daily basis, risking exposures that range from nontoxic to highly toxic. Exposure can occur through dermal or ocular exposure, inhalation, ingestion, or a combination of these routes. However, research into the toxicological mechanisms and the development of specific medical countermeasures is problematic, simply because of the overwhelming numbers of TICs and the lack of technically trained personnel, facilities, and funding.
A major component of TIC exposures is the widespread use of pesticides, rodenticides, and fumigants that are vital for preservation of food, such as grains, that can be stored in confined spaces for days or weeks at a time. Globally, metal phosphide rodenticides, such as aluminum (AlP), magnesium (Mg3P2) and zinc phosphides (Zn3P2), are used to protect food from insects and rodents. The serious problem with metal phosphides is that they degrade under aqueous conditions, producing the deadly gas phosphine (PH3), although, unlike the previously popular pesticide methyl bromide, PH3 readily degrades to more environmentally favorable compounds and does not deplete the ozone layer.1
While most fatal PH3 exposures are unintentional, metal phosphide pellet ingestion has been used intentionally as a means of suicide in several Asian countries and in Europe. A recent report of phosphine poisonings in Europe indicated that approximately 28% were by intentional ingestion and 65% were by accidental inhalation.2 When phosphides or PH3 are ingested or inhaled, respectively, they can cause significant mortality, up to 90% according to some published accounts. For instance, a case report of 408 patients who attempted suicide ingesting AlP showed a mortality rate of over 77%.3 At the occupational level, PH3 or metal phosphide production workers are most likely to be exposed to higher levels of gas. PH3 is used in the production of pharmaceuticals, flame retardants, oil additives, and organophosphines that are used as a coating for silicon crystals in semiconductor manufacturing.4 Metal phosphides are also used in the fumigation of grain stores in ships, rail cars, and storage facilities. In a 2014 report, four counties in California used nearly 372 tons of phosphides from 2006 to 2010 to protect fruits, nuts, grasses, vegetables, and grains.5 In addition, toxic off-gassing, widespread use, and ease of retail access to the PH3-producing rodenticides have increased concern over its potential as a terrorist threat agent against targeted populations. Metal phosphide toxicity has been extensively reviewed by Proudfoot.6
Metal phosphides and their hydrolysis by-products are not only a grave health concern for exposed individuals. The emission of gases from the bodies and clothing of exposed individuals is also a major issue for medical staff and first responders. PH3 off-gassing from patients or cadavers following the ingestion of metal phosphides presents a potential occupational hazard and decontamination dilemma with regard to exposing medical personnel. Lawler and Thomas have reported the occurrence of “fumes” from cadavers up to 12 hours after the body was removed.7
PH3 is a highly toxic metabolic poison affecting all major organ systems (Table 1). The purpose of this review is to highlight the complexities of PH3 exposure as it relates to metabolic toxicity. Since the degradation of metal phosphides results in the liberation of PH3 gas, these terms will be used interchangeably in this review.
Table 1.
Clinical and pathological signs of metal phosphide ontoxication.
| Basic clinical signs |
Cardiac signs |
Pulmonary signs |
Systemic effects | Metabolic effects |
Neurological symptoms |
|---|---|---|---|---|---|
| Shortnes s of breath |
Tachycardia | Pulmonary edema |
↓ Blood pressure | ↑Lipid peroxidatio n |
Narcosis |
| Cough | Bradycardia | Acute respiratory distress syndrome |
Acute renal damage | ↑SODa | Excitotoxicity (hyperactivity ) |
| Jaundice | QRS broadening |
Atelectasis | Acute liver injury | ↓Catalasea | Lethargy |
| Fatigue | S-T segment elevation |
Emphysem a |
Hemolysis | ↓Serum AChE |
Tremor |
| Dizzines s |
Ventricular ectopic beats |
Hypokalemia | Dizziness | ||
| Vomiting | ↓Ejection fraction |
Hypomagnesemia | Parathesis | ||
| Nausea | Hypokinesi a |
Hypoxia | |||
| Diarrhea | ↑Multi-organ failure | ||||
| Hypoglycemia | |||||
| Hepato/splenomegal y |
NOTE: This table lists many of the exposure–response effects of metal phosphide toxicity. In general, not all signs are prominent in patients admitted to the clinic.
Enzymes determined in rat brain experimental model.32
Physical characteristics
PH3 gas (MW = 34) is the hydrolysis by-product of the metal phosphide pesticides AlP, Mg3P2, and Zn3P2. When AlP, Mg3P2, or Zn3P2 pellets are spread and come into contact with water or acids they form PH3 gas (Eqs. 1–6]; a three-gram pellet can produce 1 gram of PH3 gas.8
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
In aqueous solutions, further oxidation products, such as phosphinic, phosphonic, and phosphoric acids, are formed (Eq. 7):9
| (7) |
PH3 is a colorless and odorless gas, but impurities in the pellet-manufacturing process have led to the odor of garlic or fish being ascribed to it. It is miscible in water, acids, oils, and fats. It is a reductant and forms coordinate bonds with metals. It is flammable when mixed with oxygen. The vapor pressure of PH3 at 25 °C is 22.93 × 104 mmHg. At 1 atm and 25 °C, 1 ppm PH3 is equivalent to 1.4 mg/m3 and is approximately 1.2 times heavier than air. See Table 2 for additional physical properties.
Table 2.
Physical properties of phosphine
| CAS number: | 7803-51-2 |
| Structural formula: | PH3 |
| Formula weight: | 34.00 |
| Boiling point: | −87.7 °C |
| Gas density, 20 °C: | 1.17 |
| Specific gravity, gas, 20 °C: | 1.185 |
| Vapor pressure: | 2.93 × 104 mmHg at 25 °C |
| Solubility: | Slightly soluble in water. Soluble in alcohol, ether, and cuprous chloride solution |
| Color: | Colorless |
| Odor: | Fish- or garlic-like odor due to impurities (Pure compound is odorless) |
| Odor threshold: | 0.03 ppm (200 ppm for pure) |
| Conversion factors: | 1 ppm = 1.41 mg/m3 1 mg/m3 = 0.71 ppm (20 °C, 760 mmHg) |
Reprinted, with permission, from Ref. 69..
Exposure physiology
Exposure to PH3 causes a host of systemic reactions that usually result in severe cellular, tissue, and organ damage and, ultimately, death. Health effects from inhaled PH3 may become evident at levels as low as 5.0–10.0 ppm over several hours,10 and in solid form a dose as low as 1.5 g is believed to be fatal to humans.11 Most deaths occur within the first 12–24 h after exposure. A significant number of exposures result from the suicidal use of fumigant pellets via ingestion. Published reports indicate that, following ingestion, metal phosphide fumigants produce PH3 gas in the gut, which is absorbed through intestinal mucosa and into the blood stream, thereby distributing throughout the body. Most of the experimental studies in rodents mimicked the most common route of human exposure, pellet ingestion. As such, much of the physiological responses mirror quite closely those observed in intentional human exposure situations.
Cardiac
The heart is thought to be one of the major target organs of PH3 toxicity, with circulatory failure and shock being very common. Work done in rats by Lall et al. with intragastric challenge with AlP showed severe myocardial necrosis.12 Both tachycardia and bradycardia were also observed. Pericarditis has also been observed in cases of metal phosphide exposure.13 The effects of metal phosphide exposures are demonstrated by shifts in cardiac conduction anomalies, such as ST segment elevation and QRS broadening. Dysrhythmic abnormalities can reflect disturbances in impulse initiation and propagation, resulting in conduction blockage and reentrant rhythms such as ectopic beats. Siwach et al. showed that metal phosphide ingestion caused ventricular fibrillation, 2° AV heart block, and, in some instances, complete 3° heart block.14 Case reports have provided data on marked depressed ejection fraction as low as 10–20% along with global hypokinesis.15,16 Additionally, systemic blood pressure drops within minutes of exposure as a result of vascular collapse and cardiac arrest.17 Postmortem reports from individuals exposed to phosphide off-gassing include descriptions of inflamed mitral and aortic valves, myocardial necrosis, and fragmented fibers.18
Neurological
Neurological changes have been associated with metal phosphide toxicity. Neuronal damage is manifested by ataxia, vomiting, nausea, tremor, and paresthesia.18 Phosphine exposure was reported to decrease serum acetylcholinesterase (AChE) activity, but not brain AChE activity, in patients after metal phosphide ingestion.19 The increased presence of acetylcholine could result in hyperactivity and possibly in excitotoxicity. Exposure to phosphine can also result in agitation, convulsions, and twitching in humans.20 To our knowledge, there have been no reports of long-term cognitive effects following metal phosphide exposures.
Pulmonary
Data on the direct consequences of an inhaled challenge of PH3 gas are limited. The effects of PH3 toxicity have been observed in the respiratory tract of exposed individuals, especially concerning shortness of breath and chest pain. Gregorakos et al. present two case reports on the effects of inhaled PH3.21 Chest-sound assessment of the lungs revealed diffuse bilateral mid- and end-expiratory rales. Chest X-rays showed diffuse infiltrates in the mid-lung and lung peripheral regions. Dyspnea, tachypnea, and, on occasion, pulmonary edema have been found to be dominant characteristics from metal phosphide exposures.22 Pulmonary edema, atelectasis, and emphysema have also been reported in patients following exposure.23
Systemic
Blood chemistries in patients admitted to the clinic display decreases in pH and plasma bicarbonate levels consistent with metabolic acidosis. Acute respiratory distress syndrome,24 hepatic damage and hypoglycemia,25 and multiple organ failure26 have also been described in patients following metal phosphide exposure. Hypoglycemia could be severe and continual, resulting from disrupted gluconeogenesis and glycogenolysis.25 Clinical presentations following metal phosphide poisoning indicated the presence of jaundice resulting from intravascular hemolysis and hyperbilirubinemia.27 Hypokalemia due to excessive vomiting is also a critical clinical factor after metal phosphide exposure.28 Hypomagnesemia has been reported in several cases, but not to the extent observed with metal phosphide–induced exposure on blood potassium levels.29
The biochemistry of exposure
Because of the availability, the widespread use of metal phosphides, and the devastating health outcome from exposure, numerous studies have attempted to focus on the precise mechanisms of metal phosphide toxicity, particularly on the direct effects of PH3 gas. The findings from these studies can be grouped into two broadly interrelated categories: oxidative stress and mitochondrial dysfunction, both of which are discussed below.
The role of oxidative stress
ROS and lipid peroxidation
Basic toxicological disturbances have been described in relationship to the effects of metal phosphides and their off-gas product PH3 on oxidative stress. Chemical models have shown that the effects of phosphine gas are tied to the generation of reactive oxygen species (ROS) as well as to the inhibition of detoxifying enzyme systems. In vitro studies have shown that PH3 can reduce Fe3+ to Fe2+ in both cytochrome oxidase and cytochrome c.30 Fe2+ in the presence of hydrogen peroxide (H2O2) yields the ROS hydroxyl free radical (•OH), which is an important reactant and initiator of lipid peroxidation in Fenton reactions and critical in the formation of highly unstable superoxide anions, •O2−.
Phosphine-induced oxidative damage in rats has been shown to cause a decrease in reduced glutathione (GSH) levels and increases in lipid peroxidation in the brain, lung, and liver.31 The presence of lipid peroxidation products in the brain is supported by studies in AlP-exposed rats. Dua and Gill showed decreases in total and nonprotein sulfhydryls in the rat cerebellum, cerebrum, and brain stem.32 Intragastric administration of AlP in rats has resulted in significant increases in malondialdehyde (MDA), a marker of lipid peroxidation, in rat cardiac tissue.12 Mouse liver MDA was also increased following an intraperitoneal exposure to PH3.11 The authors suggest that, after ingestion of Mg3P2, the observed lipid peroxidation may have been derived from direct reaction of PH3 with H2O2. In a separate study, rat liver, lung, and brain had significantly higher MDA concentrations following an intraperitoneal injection of PH3 gas.33 These effects were attenuated by pretreatment with the antioxidant melatonin 30 min before to PH3 challenge.
Detoxifying systems
In addition to the decrease in GSH, elements of the GSH redox cycle, such as GSH reductase activity, were also reduced after metal phosphide challenge in rats. However, in a separate rat study by these same groups, there was no change in catalase or glutathione peroxidase.32,33 Activities of the serum enzymes catalase and superoxide dismutase (SOD), which detoxify •O2−, forming H2O2, were decreased and enhanced, respectively, in patients following exposure to AlP.34
DNA damage
An interesting effect of PH3 toxicity is that 8-hydroxydeoxyguanosine (8-OH-dGuo), an oxidation by-product of DNA guanine, was increased by about 70% in the brain and 39% in the liver.33 Because 8-OH-dGuo is a sensitive marker of the presence of ROS and an important mutagen in DNA replication, its increased presence suggests that the effects of metal phosphide toxicity reach further than just simple target organ, tissue, or cellular damage, but to nuclear and mitochondrial DNA (mtDNA) as well. Electrophilic compounds can become inherently mutagenic following their metabolic conversion to reactive nucleophiles. In the case of metal phosphide–induced mutagenesis, the long-term effects on the downstream gene expression have not been epidemiologically and experimentally addressed.
The role of mitochondria
Mitochondria are well known for their role in energy production, redox control, calcium homeostasis, and intermediary metabolism. Many of the overwhelming toxic effects of metal phosphide exposure may result from compromised metabolic function. For example, several studies have shown that toxicity may be caused by the disruption or inhibition of mitochondrial function involving inhibition of cytochrome c oxidase activity.35,36
Mitochondria and ROS
In addition to their role in energy metabolism, mitochondria play a significant role in the production of ROS and in the activation of processes leading to cell death.37,38 Mitochondrial signaling is involved in what is termed the intrinsic pathway of programmed cell death, as opposed to the extrinsic pathway activated mainly by extracellular signals. A wealth of published work in recent years indicates that ROS play an important role in pathophysiological processes, especially with regard to the role of mitochondrial cell signaling and biological outcome.38–40 ROS are by-products of cellular respiratory activity and, for the most part, are cleared or rendered nontoxic by innate detoxification enzymatic processes, such as manganese SOD (MnSOD). In fact, the conversion of •O2− to H2O2 via the matrix enzyme MnSOD takes place in intact mitochondria. Loss of control of •O2− and H2O2 production has been shown to be fatal in MnSOD knockout mice.41 If active electron flow ceases through the respiratory chain, increases in ROS production can easily overwhelm the detoxification system. According to Cadenas and Davies, steady-state •O2− can be up to 10-fold higher in the mitochondria than in the cytosol and nuclear spaces.39 This is an important reservoir of ROS readily available for discharge during ill-timed signaling conditions.
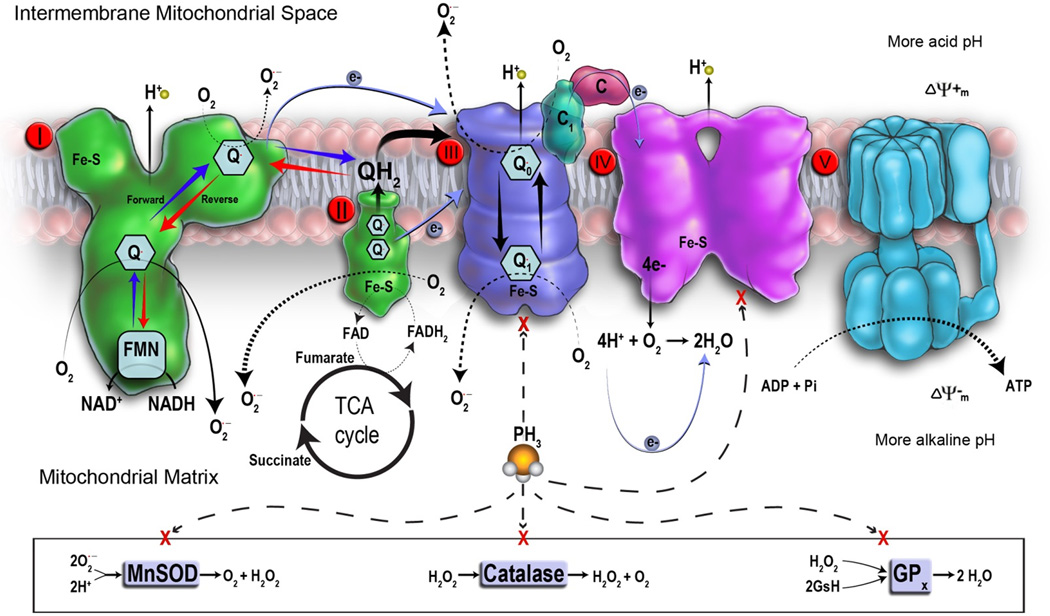
Not only are mitochondria one of the major sources of H2O2 and •O2−, but they serve as targets as well (Fig. 1).36,38,42 Resulting oxidative damage can alter mitochondrial proteins, lipids, and DNA, which gives rise to bioenergetic disorders and the initiation of cell death.43 Oxidative damage in specific regions of the respiratory chain, known as Rieske Fe–S clusters, embedded in complexes I/II/III can increase •O2− production in some cases by up to 4-fold.44–46 Mitochondrial matrix •O2− is rapidly transmutated by MnSOD into H2O2, which can diffuse through membranes into the cytoplasm, where it can act as a second messenger in NF-κB regulation. NF-κB has been shown to be critical in the role of TNFα, MIP-2, and IL-8, which are elements of the proinflammatory cascade.47 In humans, defects in oxidation, phosphorylation, and/or abnormalities along any portion of the respiratory chain complex mentioned above can result in serious metabolic disorders that ultimately threaten survival.48 Chen and Zweier provide an excellent and extensive review of mitochondrial and ROS generation.49
Figure 1.
A schematic representation of the mitochondrial respiratory chain, ROS formation, and phosphine toxicity. The respiratory chain consists of specialized metal heme complexes such as complexes II, III, IV, and an ATP synthase known as complex V. Complex I, ubiquinone, cytochrome c, proton pumps, membrane potential, and proton motive force are critical parts of electron flow in the respiratory chain. Oxidative phosphorylation is carried out in the inner membrane by respiratory assemblies. From these assemblies, two electrons from NADH are transferred to O2 by a series of electron carriers, starting with NADH dehydrogenase. Electrons are transferred to the ubiquinone (also known as coenzyme Q (CoQ)) and then to the cytochrome a group of hemeproteins. There are five cytochromes between CoQ and molecular O2: cytochromes b, c1, c, a, and a3. Cytochromes a and a3 are also known as cytochrome oxidase. The production of ATP occurs at 3 sites: (1) between NADH and CoQ, (2) between cytochromes b and c, and (3) between cytochrome c and O2. The inner membrane is impermeable to most molecules because of its high proportion of phospholipids and cardiolipin. Transport proteins embedded in the inner membrane selectively incorporate metabolites into the matrix and export ATP for biological processes. The matrix is the site of high energy–yielding reactions from the metabolism of pyruvate and fatty acids derived from carbohydrates and other nutrients. Pyruvate and fatty acids that are transported into the matrix help to generate a pair of electrons that have a high energy transfer potential. These are carried by NADH and FADH2 (flavin adenine dinucleotide reduced form), which are formed in glycolysis, fatty acid oxidation, and the citric acid cycle. The released high energy from this reaction is used to produce ATP through oxidative phosphorylation, which is a major metabolic reaction in aerobic organisms. Oxidative phosphorylation is strongly coupled to fatty acid oxidation, which forms acetyl CoA and the citric acid cycle to produce the energy needed for aerobic cell metabolism. Respiratory assemblies are an integral part of the inner membrane, whereas fatty acid oxidation and the citric acid cycle activity occur in the matrix. Flow of electrons causes an electrochemical proton gradient under the control of the proton motive force for the production of ATP. During active respiration, mitochondria produce a proton motive force across the inner membrane. This results in a negative charge inside, thereby producing a pH gradient. Electron leakage from the respiratory chain from enhanced ROS production causes additional oxidative insult. If active electron flow ceases through the respiratory chain, proton motive force collapses and ATP production discontinues. This causes loss of membrane potential and release of additional ROS. The dashed arrows indicate the putative inhibition of critical components of the respiratory electron transport chain by phosphine.
Mitochondria and cell death
Mitochondrial membrane permeability is regulated through channels known as voltage-dependent anion channels, which form a complex with adenine nucleotide translocase in the inner membrane and with cyclophilin D. This complex forms the mitochondrial permeability transition pore, MPTP (Δψm). The role of the Δψm is to exchange metabolites through the inner and outer mitochondrial membranes. Compromised Δψm opening is linked to the initiation of apoptotic and necrotic processes. The integrity of mitochondrial function and the regulation of the MPTP opening are highly dependent on several factors. As cells become stressed under conditions of toxicant biological attack, BH3-only signaling proteins are transcriptionally upregulated. BH3-only proteins, such as BAD and BMF, are considered sensors of apoptosis and target antiapoptotic proteins such as BCL-2. Cytochrome c and apoptotic proteins are released, which activates caspases that cleave complex I of the respiratory chain. Complex I is considered to be a primary source of electron leak, with the subsequent release of •O2−.47 As increased concentrations of ROS and/or intracellular Ca2+ outpace neutralizing processes, they can have an undesirable impact on the modulation of MPTP. As more ROS are formed as a result of Δψm opening, apoptotic proteins are released, and cytochrome c activates additional caspases into the cellular milieu.
In studies with pesticide organophosphates, the translocation of cytochrome c by parathion activated caspases 9 and 3.50 Xu et al. demonstrated that trichlorfon caused cytochrome c release from mitochondria, activating caspase 3 in cultured hepatocytes.51 Caspases are not the only toxic molecules released during oxidative damage to cells or tissues. The cytokine TNFα can act on specific receptors to promote either cell death or survival. Following treatment with TNFα, mitochondrial ROS activity is increased in a human embryonic kidney cell line, suggesting that the extramitochondrial formation of inflammatory cytokines can contribute to failed mitochondrial metabolism.52 Inhibition along parts of the respiratory chain can also trigger the accumulation of electrons from highly reactive carriers and load the cell with additional ROS. Additional ROS can cause the oxidation of the mitochondrial lipid cardiolipin, which is generated in response to TNFα-induced caspase 8 activation.
Normally, mitochondria amass and gradually release Ca2+ to maintain specific gradients of cytosolic Ca2+, a function that is critical to cell survival. However, very high intramitochondrial Ca2+, when released into the cytosol as a result of damage, can overwhelm the remaining intact mitochondria, resulting in the collapse of the electrochemical gradient across the inner membrane and the loss of the ability to produce ATP. It is thought that mitochondria-derived ROS induce Ca2+ release from ryanodine receptors on the endoplasmic reticulum in endothethial cells.53 This series of events eventually signals cells to self-destruct through apoptotic or necrotic pathways. ATP is critical in the regulation of apoptosis. The inability to control programmed cell death leads to caspase-activated necrosis and inflammation. For a more thorough review of mitochondrial dysfunction, see Ref. 54.
Effects of PH3 on mitochondria
Numerous reports show that mitochondrial metabolic homeostasis and detoxification processes are targets for metal phosphides/PH3 assault.36,55–58 PH3 has been shown to inhibit complex IV (cytochrome c oxidase) of the respiratory chain in isolated mouse liver mitochondria and lessen the strength of Δψm.55, 58 Metal phosphide exposure has been shown to alter glucose homeostasis in the brain and liver. The brain requires high metabolic rates and is dependent on glucose for the maintenance of neural activity. Dua et al. show that, in acute AlP exposure in rats, lactate dehydrogenase (LDH) activity is increased in the brain.35 This is highly indicative of the conversion of aerobic metabolism to anaerobic metabolism and signifies severe mitochondrial dysfunction. According to Dua and colleagues, increases in LDH activity result in the reversible conversion of pyruvate to lactate and reoxidation of NADH (nicotinamide adenine dinucleotide reduced form) to NAD+ (NAD oxidized form) independent of mitochondrial electron transport. Under these conditions, lactate accumulates in the brain and may predispose animals to disturbances in central nervous system–derived functions.
Studies indicate that PH3 has a substantial effect on the function of liver mitochondria in both rodents and insects. Both produce H2O2 following PH3 challenge. In rat liver mitochondria under anoxic conditions and when ADP is diminished, enough H2O2 can be formed to account for about 2% of the oxygen uptake.59 Inhibiting respiratory chain activity using myxothiazol and antimycin in insect mitochondria indicated that glycerophosphate dehydrogenase auto-oxidation is the source of H2O2 production following PH3 challenge.60 Although PH3 affects the electron transport in the cytochrome c cascade, another well-known metabolic poison, cyanide, which is a classic example of a respiratory chain toxicant, blocks electron flow from cytochrome a and a3 (which make up cytochrome c oxidase) to oxygen.61 However, earlier work by Kashi and Chefurka has indicated that, under certain conditions, PH3 primarily inhibits one component of the cytochrome aa3 complex (complex IV), such as cytochrome a, possibly by changing the valence state of heme iron.30
The toxicity of PH3 has been closely linked to the availability and metabolic uptake of oxygen. Toxicity is increased in the presence of oxygen and decreases under anoxic conditions. Bolter and Chefurka showed that the toxicity of PH3 increases in more aerobic environments as opposed to anaerobic conditions.55 This is not a new phenomenon and has been described in metal phosphide studies using insects or PH3 resistant nematodes.57,61,62 A partial explanation for the correlation of increased toxicity in normoxic environments may be the mass action relationship between the rate of •O2− formation and the concentration of electron donors [R•] in the presence of oxygen. This is mathematically described in Equation 8.43
| (8) |
The balance between oxidant versus antioxidant pathways determines the biological consequences of the response. The intrinsic capacity of metabolically compromised mitochondria to drive Equation 8 to the left during aerobic conditions may be the basic thrust required for progressive PH3 toxicity and cell death.
Previous experimental and clinical data have shown that the heart is a significant target of metal phosphide toxicity. Whether this is a direct effect of the ingested metal ligands, such as zinc, aluminum, or the magnesium constituents of the phosphide, or the result of toxic off-gas products has not been experimentally determined. It is well established that cardiac muscle is very rich in mitochondria as a result of the high oxygen and energy demand of heart tissue. This is opposite from skeletal muscle, which contains fewer mitochondria. Since these muscles can operate anaerobically for long periods of time, they build up a significant oxygen debt. What is not known is whether the primary cause of cardiac dysfunction is inhibition of electron transport at the level of cytochrome c oxidase and consequent loss of ATP production or oxidative conditions that lead to ischemic and/or reperfusion injury. One report indicated that a patient with PH3 poisoning was favorably treated by enhancing the preservation of oxidative metabolism and decreasing MDA with the myocardial protective anti-ischemic drug trimetazidine.63 Chen and Zweier comment that oxidative impairment of complex I during ischemia has been identified in several animal studies.49
In contrast to the reports in rodents that PH3 affects mitochondrial respiratory chain activity at the level of cytochrome c oxidase (complex III), other studies have shown that this may be dependent on species. Earlier findings with the PH3-treated insects Rhyzopertha dominica indicated that inhibition of cytochrome c oxidase activity does not occur in vivo.64 This was supported by Jian et al. and Price and Dance, investigating the effects of PH3 on adult copra mites and beetles, respectively.65,66 There may be a link between in vitro and in vivo PH3 uptake and resistance to fumigation. This may be related to the capacity of PH3 to penetrate the double mitochondrial membrane. An effective breach would have a greater inhibitory effect on cytochrome c oxidase.66 As noted earlier, ROS can damage or alter nuclear and mitochondrial DNA. Zuryn et al. determined that this could be the direct result of a dysfunctional respiratory complex affecting genes that encode resistance against PH3 challenge, especially in Caenorhabditis elegans.57 The specific site was isolated to genes in respiratory chain complex III using RNA interference following exposure to PH3. Suppression of mitochondrial respiratory chain genes has been shown to also lead to longevity and phosphine resistance, nullifying the intended use for fumigation.67,68 Specifically, longevity in nematodes may be a result of the activation of genes that encode components of the respiratory chain, such as complexes I (NADH/ubiquinone oxidoreductase), III (cytochrome c reductase), and IV (cytochrome c oxidase).67 This would be a major concern for end users in the application of PH3 as a fumigant for insect control, making its use obsolete and leading to the future development of more intense pesticides. However, before newer, more toxic pesticides are developed, more precise mechanistic toxicology needs to be applied, using consistent and reliable animal models to understand the mechanisms of PH3 resistance.
Conclusions
PH3 toxicity has been shown to involve irreversibly compromised metabolism. Mechanistically, this can occur through metabolic crisis and/or the indirect effect of metabolism-generated increased ROS production, ultimately leading to cellular/target organ collapse. In totality, this has had a profoundly deleterious effect on survival rates in exposed individuals. The extensive and global use of fumigants such as metal phosphides can and does trigger serious health consequences whether through accidental or intentional exposure. As we are all consumers of food products stored in various ways, exposure to pesticides affects nearly every human as a result of their widespread use to protect nuts, fruits, vegetables, and grains. Basically, these pesticides and rodenticides protect a multibillion dollar industry. The insidious exposure–response consequences, coupled to a > 70% mortality rate, present a major challenge to healthcare providers. This is not only from a medical countermeasure vantage point, but also from potential off-gassing airborne contamination. Knowledge of mechanistic toxicology is critical for understanding how metal phosphides and/or their by-products affect exposed individuals. It is evident that, in some instances, toxicity is directly aligned with failed metabolic function and the inability to maintain cellular homeostasis. Treatment of metal phosphide exposure is generally supportive at this time because of the broad systemic effects of toxicity coupled to the direct effects on failed metabolism. Further investigation into the precise relationships between exposure, metabolic, and systemic toxicities will aid in developing a more rational approach to treatment and the determination of temporal therapeutic windows.
Acknowledgments
Special thanks to the National Institute of Health (NIH) Countermeasures Against Chemical Threats (CounterACT) program for support (Inter-Agency Agreement: AOD13017-001). The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. government. We wish to thank James Abraham for his illustrative expertise designing Figure 1 and Ms Jaclyn Andres for administrative support. Motivation for writing this review was provided by Joshua M. Novak and Nathan A. Jones.
Footnotes
Conflicts of interest
The authors report no conflict of interests in preparing this work.
References
- 1.M.B.T.O.C. Report of the methyl bromide technical options committee:2006 assessment. Nairobi: United Nations Environment Programme; 2006. [Google Scholar]
- 2.Lauterbach M, Solak E, Kaes J, et al. Epidemiology of hydrogen phosphide in humans reported to the poison center in Mainz, Germany, 1983–2003. Clin Toxicol. 2005;43:575–581. doi: 10.1081/clt-200068847. [DOI] [PubMed] [Google Scholar]
- 3.Chugh SN, Dushyant RS, Malhotra AB, et al. Incidence and outcome of aluminum phosphide poisoning in a hospital study. Indian J. Med. Res. 1991;94:232–235. [PubMed] [Google Scholar]
- 4.World Health Organization. Phosphine and selected metal phosphides. Environ. Health Criteria. 1998:73.
- 5.Reeve I. Estimation of Exposure to Persons in California to Phosphine due to Use of Aluminum Phosphide, Magnesium Phosphide, and Cylinderized Phosphine Gas. California EPA: Dept. Pesticide Regulation; 2014. Jun 12, p. 124. [Google Scholar]
- 6.Proudfoot AT. Aluminium and zinc phosphide poisoning. Clin. Tox. 2009;47:89–100. doi: 10.1080/15563650802520675. [DOI] [PubMed] [Google Scholar]
- 7.Lawler JM. Off Gassing” following fatal aluminium phosphide ingestion [abstract] Clin. Toxicol. 2007;45:362. [Google Scholar]
- 8.Anand R, Binukumar BK, Gill KD. Aluminum phosphide poisoning: an unsolved riddle. J. Appl Toxicol. 2011;31:499–505. doi: 10.1002/jat.1692. [DOI] [PubMed] [Google Scholar]
- 9.Lam WW, Toia RF, Casida J. Oxidatively initiated phosphorylation reactions of phosphine. J. Agric. Food Chem. 1991;39:2274–2278. [Google Scholar]
- 10.Beliles RP. Phosphorus, Selenium, and Tellurium. In: Clayton GD, Clayton FE, editors. Patty’s Industrial Hygiene and Toxicology. 3rd. New York: John Wiley & Sons; 1978. pp. 2121–2141. Volume 2A Toxicology. [Google Scholar]
- 11.Quistad GB, Sparks SE, Casida JE. Chemical model for phosphine-induced lipid peroxidation. Pest Manage Sci. 2000;56:779–783. [Google Scholar]
- 12.Lall SB, Sinha K, Mittra S, et al. An experimental study on cardiotoxicity of aluminum phosphide. Indian J. Exp. Biol. 1997;35.10:1060–1064. [PubMed] [Google Scholar]
- 13.Wander GS, Han B-C, Liu M-Y, et al. Acute pericarditis in aluminum phosphide poisoning. J. Assoc. Physicians India. 1990;38:675. [PubMed] [Google Scholar]
- 14.Siwach SB, Singh H, Jagdish, et al. Cardiac arrhythmias in aluminium phosphide poisoning studied by on continuous holter and cardioscopic monitoring. J. Assoc. Physicians India. 1998;46:598–601. [PubMed] [Google Scholar]
- 15.Vohra RB, Schwarz KA, Williams SR, et al. Phosphine Toxicity with Echocardiographic Signs in Railcar Stowaways [abstract] Clin. Toxicol. 2006;44:719–720. [Google Scholar]
- 16.Akkaoui M, Achour S, Abidi K, et al. Reversible myocardial injury associated with aluminum phosphide poisoning. Clin. Toxicol. 2007;45:728–731. doi: 10.1080/15563650701517350. [DOI] [PubMed] [Google Scholar]
- 17.Mittra S, Peshin SS, Lall SB. Cholinesterase inhibition by aluminum phosphide poisoning in rats and effects of atropine and pralidoxime chloride. Acta Pharmacol. Sin. 2001;22:37–39. [PubMed] [Google Scholar]
- 18.Wilson R, Lovejoy FH, Jaeger RJ, et al. Acute phosphine poisoning aboard a grain freighter. Am. J. Foren. Med. Pathol. 1981;2:284. [PubMed] [Google Scholar]
- 19.Rastogi P, Raman R, Rastogi VK. Serum cholinesterase and brain acetylcholinesterase activity in aluminium phosphide poisoning. Med. Sci. Res. 1990;18:783–784. [Google Scholar]
- 20.Nath N, Bhatttacharya I, Tuck AG, et al. Mechanisms of phosphine toxicity. J. Toxicol. 2011;2011:494168. doi: 10.1155/2011/494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregorakos L, Sakayianni K, Harizopoulou V. Recovery from severe inhalation phosphine poisoning. Report of two cases. Clin Intensive Care. 2002;13.4:177–179. [Google Scholar]
- 22.Chugh SN, Aggarwal KK, Mahajan SK. Zinc phosphide intoxication symptoms: analysis of 20 cases. In. J. Pharmacol. Ther. 1998;7:406–407. [PubMed] [Google Scholar]
- 23.Heyndrieckx A, Peteghem CV, Heede MV, et al. A double fatality with children due to fumigated wheat. Eur. J. Toxicol. 1976;9:113–118. [PubMed] [Google Scholar]
- 24.Chugh SN, Ram S, Mehta LK, et al. Adult Respiratory distress syndrome following aluminum phosphide ingestion. Report of 4 cases. J. Assoc. Physicians India. 1989;37:271–272. [PubMed] [Google Scholar]
- 25.Patial RK, Bansal SK, Kashyap S, et al. Hypoglycaemia following zinc phosphide poisoning. J. Assoc. Physicians India. 1990;38.4:306. [PubMed] [Google Scholar]
- 26.Gupta V, Singh J, Doodan SS, et al. Multisystem organ failure (MSOF) in aluminum phosphide (AlP) poisoning. Clin. Toxicol. 2000;47.2:287–288. [Google Scholar]
- 27.Aggarwal P, Handa R, Wig N, et al. Intravascular hemolysis in aluminum phosphide poisoning. Am. J. Emerg. Med. 1999;17:488–489. doi: 10.1016/s0735-6757(99)90255-3. [DOI] [PubMed] [Google Scholar]
- 28.Kochar DK, Jain N, Sharma BV, et al. Successful management of hypokalaemia related conduction disturbances in acute aluminum phosphide poisoning. JIMA. 2000;98.8:461–462. [PubMed] [Google Scholar]
- 29.Chugh SN, Kamar P, Chugh K, et al. Magnesium status and parenteral magnesium sulphate in acute aluminum phosphide intoxication. Magnes Res. 1994;7:289–294. [PubMed] [Google Scholar]
- 30.Kashi KP, Chefurka W. The effect of phosphine on the absorption and circular dichroic spectra of cytochrome c and cytochrome oxidase. Pest. Biochem. and Physiol. 1976;6:350–362. [Google Scholar]
- 31.Hsu CH, Chi BC, Liu MY, et al. Phosphine-induced oxidative damage in rats: role of glutathione. Toxicology. 2002;179.1:1–8. doi: 10.1016/s0300-483x(02)00246-9. [DOI] [PubMed] [Google Scholar]
- 32.Dua R, Gill KD. Aluminum phosphide exposure: implication on rat brain lipid peroxidation and antioxidant defence system. Pharmacol., & Toxicol. 2001;89.6:315–319. doi: 10.1034/j.1600-0773.2001.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 33.Hsu CH, Han BC, Liu MY, et al. Phosphine-induced oxidative damage in rats: attenuation by melatonin. Free Rad. Bio. Med. 2000;28.4:636–642. doi: 10.1016/s0891-5849(99)00277-4. [DOI] [PubMed] [Google Scholar]
- 34.Chugh SN, Arora V, Sharma A, et al. Free radical scavengers and lipid peroxidation in acute aluminum phosphide poisoning. India J. Med. Res. 1996;104:190–193. [PubMed] [Google Scholar]
- 35.Dua R, Kumar V, Sunkaria A, et al. Altered glucose homeostasis in response to aluminum phosphide induced cellular oxygen deficit in rat. Ind. J. Exp. Biol. 2010;48:722–730. [PubMed] [Google Scholar]
- 36.Chefurka W, Kashi KP, Bond EJ. The effect of phosphine on electron transport in mitochondria. Pesticide Biochemistry and Physiology. 1976;6:65–84. [Google Scholar]
- 37.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchi S, Giorgi C, Agnoletto JM, et al. Mitochondrial-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012;2012:1–17. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos JH, Meyer JN, Van Houten B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum. Mol. Genet. 2006;15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- 40.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 42.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 45.Panov A, Dikalov S, Shalbuyeva N, et al. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short-term systemic rotenone intoxication. J. Biol. Chem. 2005;280:42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 46.Kirkinezos IG, Moraes CT. Reactive oxygen species and mitochondrial diseases. Semin. Cell Dev. Biol. 2011;12:449–457. doi: 10.1006/scdb.2001.0282. [DOI] [PubMed] [Google Scholar]
- 47.Zmijewski JW, Lorne E, Zhao X, et al. Mitochondrial respiratory complex 1 regulates neutrophil activation and severity of lung injury. Am J. Resp. Crit Care Med. 2008;178:168–179. doi: 10.1164/rccm.200710-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.diMauro S, De Vivo DC. Diseases of carbohydrate, fatty acid, and mitochondrial metabolism. In: Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. 6th. Chapter 42. Philadelphia: Lippicott Williams & Wilkens; 1999. [Google Scholar]
- 49.Chen Y-R, Zweier JL. Cardiac mitochondrial and reactive oxygen species generation. Circ. Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleh AM, Vijayasarathy C, Masoud L, et al. Paraoxon induces apoptosis in EL4 cells via activation of mitochondrial pathways. Toxicol. Appl. Pharmacol. 2003;190:47–57. doi: 10.1016/s0041-008x(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 51.Xu WN, Liu WB, Liu ZP. Trichlorfon-induced apoptosis in hepatocyte primary cultures of Carassius auratus gibelio. Chemosphere. 2009;77:895–901. doi: 10.1016/j.chemosphere.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 52.Chandel NS, Schumacker PT, Arch RH. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J. Biol. Chem. 2001;276(16):42728–42736. doi: 10.1074/jbc.M103074200. [DOI] [PubMed] [Google Scholar]
- 53.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Cir Res. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ninomiya-Tsuji J. Mitochondrial dysfunction. In: Smart RC, Hodgson E, editors. Molecular and Biochemical Toxicology. Hoboken: John Wiley & Sons; 2008. p. 4. [Google Scholar]
- 55.Nakakita H, Katsumata Y, Ozawa T. The effect of phosphine on respiration of rat liver mitochondria. J. Biochem. 1971;69:589–593. [PubMed] [Google Scholar]
- 56.Bolter C, Chefurka W. The effect of phosphine treatment on superoxide dismutase, catalase and peroxidase in the granary weevil, Sitophilus granaries. Pest. Biochem. Physiol. 1990;36:52–60. [Google Scholar]
- 57.Zuryn S, Kuang J, Ebert P. Mitochondrial modulation of phosphine toxicity and resistance in Caenorhabditis elegans. Toxicol. Sci. 2008;102:179–186. doi: 10.1093/toxsci/kfm278. [DOI] [PubMed] [Google Scholar]
- 58.Valmas N, Zuryn S, Ebert PR. Mitochondrial uncouplers act synergistically with the fumigant phosphine to disrupt mitochondrial membrane potential and cause cell death. Toxciol. 2008;252:33–39. doi: 10.1016/j.tox.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 59.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolter C, Chefurka W. Extramitochondrial release of hydrogen peroxide from insect and mouse liver mitochondria using the respiratory inhibitors phosphine, myxothiazol, and antimycin and spectral analysis of inhibited cytochromes. Arch. Biochem. Biophys. 1990;278(1):65–72. doi: 10.1016/0003-9861(90)90232-n. [DOI] [PubMed] [Google Scholar]
- 61.Price NR, Walter CM. A comparison of some effects of phosphine, hydrogen cyanide and anoxia in the lesser grain borer, Rhyzopertha dominica (F.) (Coleopteran: Bostrychidae) Comp. Biochem. Physiol. 1987;86.1:33–36. doi: 10.1016/0742-8413(87)90139-3. [DOI] [PubMed] [Google Scholar]
- 62.Bond EJ, Monro HAU, Buckland CT. The influence of oxygen on the toxicity of fumigants to Sitophilus granaries L. J. Stored Prod. Res. 1967;3:289–294. [Google Scholar]
- 63.Dueñas A, Pérez-Castrillon JL, Cobos MA, et al. Treatment of the cardiovascular manifestations of phosphine poisoning with trimetazidine, a new antiischemic drug. Am. J. Emerg. Med. 1999;17(2):219–220. doi: 10.1016/s0735-6757(99)90075-x. [DOI] [PubMed] [Google Scholar]
- 64.Price NR. Some aspects of inhibition of cytochrome-c oxidase by phosphine in susceptible and resistant strains of Rhyzopertha dominica . Insect Biochem. 1980;10:147–150. [Google Scholar]
- 65.Jian FD, Jayas D, White NDG. Toxic action of phosphine on the adults of the copra mite Tyrophagus putrescentiae [Astigmata: Acaridae] Phytoprotection. 2000;81:23–28. [Google Scholar]
- 66.Price NR, Dance SJ. Some biochemical aspects of phosphine action and resistance in three species of stored product beetles. Comp. Biochem. Physiol. 1983;76C(2):277–281. doi: 10.1016/0742-8413(83)90078-6. [DOI] [PubMed] [Google Scholar]
- 67.Dillin A, Hsu AL, Arantes-Oliveira N, et al. Rates of behavior and aging specified by mitochondrial function during development. Sci. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 68.Lee SS, Lee RY, Fraser AG, et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nature genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 69.Perkins MW, Wong B, Olivera D, et al. Phosphine. In: Harbison RD, Bourgeois MM, Johnson GT, editors. Hamilton & Hardy’s Industrial Toxicology. 6th. Chapter 118. Hoboken, NJ: Wiley; 2015. [Google Scholar]