Abstract
Objective. Prescription opioid analgesics are commonly prescribed for moderate to severe pain. An unintended consequence of prescribing opioid analgesics is the abuse and diversion of these medications. Tapentadol ER is a recently approved centrally acting analgesic with synergistic mechanisms of action: μ-opioid receptor agonism and inhibition of norepinephrine reuptake. We assessed the amount of diversion and related cost of obtaining tapentadol IR (Nucynta®) and tapentadol ER (Nucynta ER®) as well as other Schedule II opioid medications in street transactions in the United States using the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS®) System.
Methods. The Drug Diversion Program measures the number of cases opened by 260 drug diversion investigators in 49 states. StreetRxTM uses a crowd-sourcing Website to collect the prices paid for licit or illicit drugs.
Results. The population-based rates of diversion were 0.003 (tapentadol IR), 0.001 (tapentadol ER), and 1.495 (other Schedule II opioid tablets) reports per 100,000 population. The tapentadol ER rate was lower than the other Schedule II opioid tablets (P < 0.001) and tapentadol IR (P= 0.004). Diversion rates based on drug availability were 0.03 (tapentadol IR), 0.016 (tapentadol ER), and 0.172 (other Schedule II opioid tablets) per 1,000 prescriptions dispensed. The median street price per milligram was $0.18 (tapentadol IR), $0.10 (tapentadol ER), and $1.00 (other Schedule II opioid tablets).
Discussion. Our results indicate that tapentadol ER is rarely sold illicitly in the United States. When sold illicitly, tapentadol ER costs less than other Schedule II opioid products.
Keywords: Tapentadol, Opioid, Abuse, Diversion, RADARS System, StreetRx
Introduction
The therapeutic use of prescription analgesics has increased substantially over the last two decades. In concert, the misuse, abuse and diversion of these medications has also increased [1]. The result has been increased contacts with poison centers, visits to emergency departments, admissions to substance abuse treatment centers, and deaths attributed to opioid analgesics [1–3]. The National Center for Health Statistics indicates deaths related to pharmaceutical opioid products reached 16,451 in 2010 [4].
Diversion of opioid analgesics is the transfer of a prescription drug from a lawful to an unlawful channel of distribution or use [5]. In 2003, the US Drug Enforcement Administration (DEA) estimated the value of diverted prescription drugs at $25 billion a year [6]. Despite its scope, the diversion of prescription drugs remains poorly understood. It is often assumed that the diverter abuses the drug; however, a diverter may sell the drug for profit (without abusing the drug) or abuse a portion of the prescription and sell the remainder [7].
Tapentadol is approved in the United States, Canada, and other countries for the relief of moderate to severe chronic pain. It is a centrally acting analgesic with two synergistic mechanisms of action: μ-opioid receptor agonism and inhibition of norepinephrine reuptake [8]. The US DEA categorizes tapentadol as a Schedule II drug in the same class as oxycodone and similar opioid analgesics. The immediate release (IR) form of tapentadol (Nucynta®, Depomed, Inc., Newark, CA, USA) was introduced in 2009. The extended release (ER) formulation was approved in 2011 and is available as Nucynta ER® (Depomed, Inc.) In the United States, the ER product utilizes a proprietary crush-resistant formulation [9].
The combined mechanism of action for tapentadol is hypothesized to produce effective pain relief with decreased potential for abuse. In preclinical models, the affinity of tapentadol for the mu opioid receptor was 50-fold lower than morphine although tapentadol was only 2- to 3-fold less potent than morphine in animal pain models [10]. In humans, tapentadol IR 50–100 mg provided analgesic efficacy similar to that of oxycodone 10–15 mg [11]. There are few studies evaluating the abuse of tapentadol and there are no reports concerning the diversion of tapentadol.
The Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS®) System measures misuse, abuse, and diversion of prescription opioids and stimulants from multiple perspectives. Two RADARS System programs assess street activity of prescription drugs. The Drug Diversion Program measures the number of cases opened by drug diversion investigators throughout the United States. StreetRxTM uses crowdsourcing to determine the price paid on the street for licit or illicit drugs.
The purpose of our analysis was to assess the diversion and related street prices of tapentadol ER in the United States compared with tapentadol IR and other Schedule II opioids.
Methods
Our analysis included Drug Diversion and StreetRx programs. Each program provides drug product-specific information every 3 months for postmarketing surveillance in the United States. The medications included were tapentadol IR, tapentadol ER, and the group of other Schedule II opioids (oxycodone, hydromorphone IR and ER tablets, oxymorphone, morphine IR and ER tablets, and methadone tablets). The Drug Diversion Program provides systematic surveillance data on diversion of drugs. In 2013, there were 260 participating agencies in 49 states and DC covering 38.2% of the US population. Drug diversion officers submit data quarterly on the number of documented drug diversion cases within their jurisdiction for specific prescription drugs of interest. A case is one that results in a written complaint or report. Drug diversion officers represent municipal police departments, multi-jurisdictional drug task forces, county sheriffs’ departments, regulatory agencies such as state medical and pharmacy boards, state police agencies, prosecutors’ offices, and departments of health. When necessary, repeated contacts are made with participants to verify information. Detailed methods have been published previously [12].
As an adjunct to the ongoing Drug Diversion Program, we conducted a one-time, semi-structured telephone interview with participating investigators in the third quarter of 2013. Given that tapentadol is a relatively new drug with few reports of abuse or diversion in the scientific literature or lay press, the purpose was to explore diversion investigators’ field experiences with illicitly obtained tapentadol. A total of 30 drug diversion investigators in 23 states were interviewed to assess their awareness and approach to detection of tapentadol. The sample comprised a variety of agency types, most often sheriff’s offices, drug task forces, and local police units. Investigators were stratified into two groups based on prior reporting of tapentadol diversion and 15 investigators were randomly selected from each list. The 15-minute interview asked general questions about the investigator’s unit, caseload information, primary sources of diversion, and the primary drugs diverted in their jurisdictions. In addition, investigators responded to a series of questions specific to tapentadol diversion, including cases investigated, general awareness of the drug, awareness of street or other illicit activity related to tapentadol even if not officially investigated, and current diversion status. The interview guide included pictures of tapentadol IR and ER products to ensure accurate identification.
StreetRx (StreetRx.com) is an anonymous public Website that enables real-time collection and display of street price data on diverted pharmaceutical products. Site users anonymously submit prices that they paid, received, or heard were paid for diverted prescription drugs. A visual photo identification feature for each formulation aids accuracy of reporting. StreetRx averages roughly 2,000 visitors and 70 street price submissions per day. The average price calculated by StreetRx has been validated using two other independent sources: Silk Road (a hidden online marketplace that sells illicit items including prescription drugs) and the RADARS System drug diversion investigators [13].
Analysis
Drug Diversion rates were calculated by dividing the number of diversion cases in a jurisdiction by the population of the same jurisdiction (population rate) and dividing by drug availability as represented by the number of prescriptions (IMS Health, Danbury, CT, USA) dispensed in the jurisdiction (prescription rate).The population was calculated by assuming linear growth between the 2000 and 2010 US census and interpolating for each quarter from July 1, 2009 through June 30, 2010. The population was extrapolated using this same rate of change from July 1, 2010 through September 30, 2014. Poisson regression with a Bonferroni multiple comparisons adjustment was used to compare the average rates over the study period (July 1, 2011 through September 30, 2014). The variance was calculated separately for each drug group.
StreetRx submissions for opioid analgesics that were received from the United States between January 1, 2011 and September 30, 2014 and contained data about formulation and dose strength were analyzed for this report. The median street price per milligram from reports submitted between October 1, 2011 and September 30, 2014 for tapentadol ER, tapentadol IR, and other Schedule II opioid tablets were calculated and boxplots were generated. The median street prices were also compared for the three groups using Wilcoxon’s rank-sum test.
Results
Drug Diversion Program
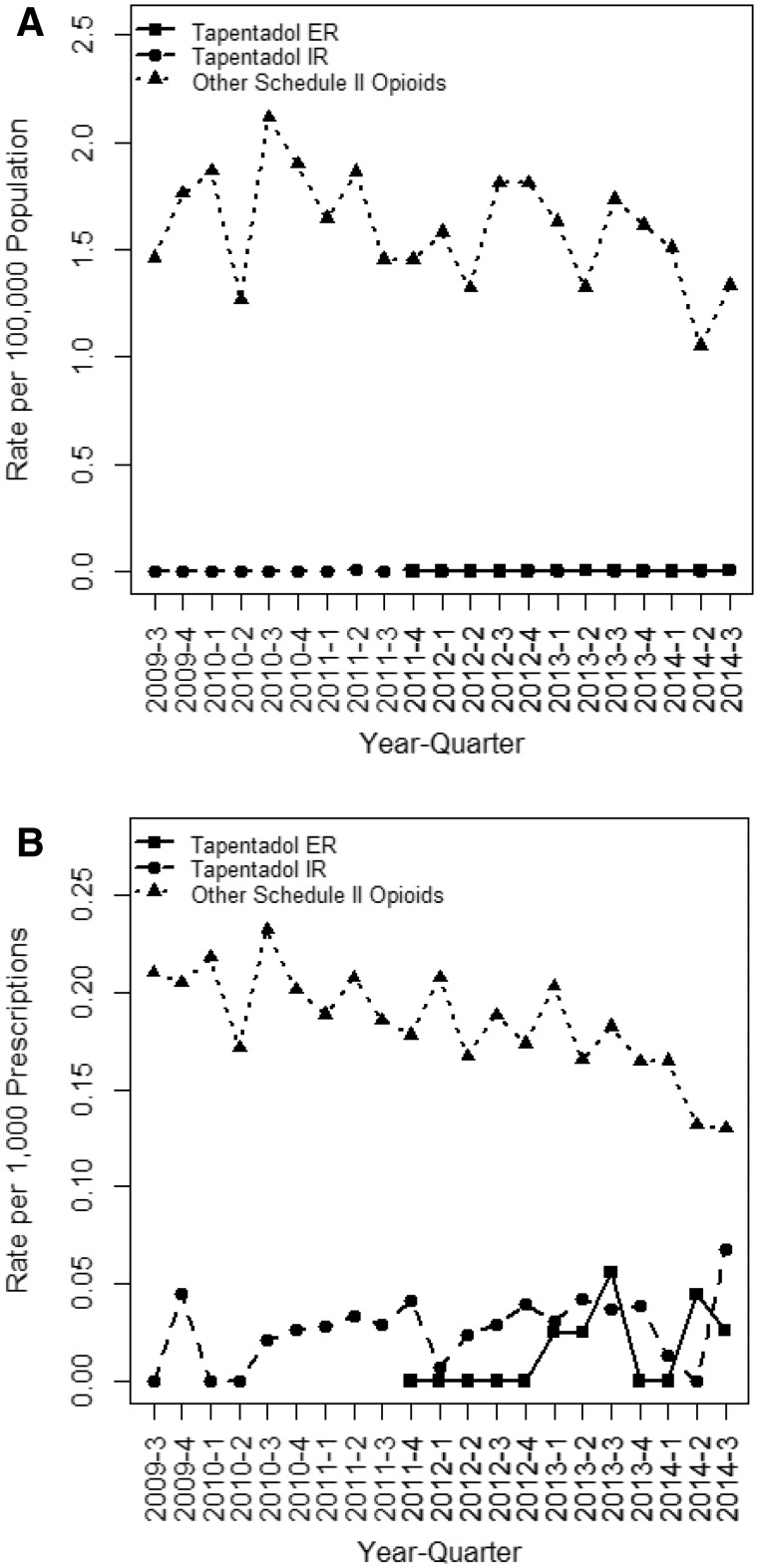
We detected 56 diversion cases for tapentadol IR, seven for tapentadol ER, and 38,325 for other Schedule II opioids for the period July 1, 2009 through September 30, 2014 (Table 1). The average quarterly population based rates were 0.003 (tapentadol IR), 0.001 (tapentadol ER), and 1.495 (other Schedule II opioid tablets) reports per 100,000 population for the period of October 1, 2011 through September 30, 2014 when tapentadol IR, tapentadol ER, and Schedule II opioids were available simultaneously. The average quarterly population rate for tapentadol ER was much lower than the other Schedule II opioid tablets (adjusted P < 0.001) and tapentadol IR (adjusted P = 0.004) (Figure 1).
Table 1.
Rates of diversion for tapentadol IR, tapentadol ER, and other Schedule II opioids, July 1, 2011 through September 30, 2014
| Tapentadol immediate release (IR) |
Tapentadol extended release (ER) |
Other Schedule II opioids |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Year-quarter | Cases | Population rate (95% CI) | Prescription rate (95% CI) | Cases | Population rate (95% CI) | Prescription rate (95% CI) | Cases | Population rate (95% CI) | Prescription rate (95% CI) |
| 2009-3 | 0 | 0(0,0.002) | 0(0,0.106) | NA | NA | NA | 2,208 | 1.459(1.399,1.522) | 0.21(0.202,0.219) |
| 2009-4 | 2 | 0.002(0,0.006) | 0.045(0.005,0.163) | NA | NA | NA | 2,010 | 1.759(1.683,1.838) | 0.205(0.196,0.214) |
| 2010-1 | 0 | 0(0,0.003) | 0(0,0.065) | NA | NA | NA | 2,138 | 1.866(1.788,1.947) | 0.218(0.209,0.227) |
| 2010-2 | 0 | 0(0,0.002) | 0(0,0.044) | NA | NA | NA | 1,918 | 1.264(1.208,1.321) | 0.171(0.164,0.179) |
| 2010-3 | 2 | 0.002(0,0.006) | 0.021(0.003,0.077) | NA | NA | NA | 2,433 | 2.116(2.032,2.201) | 0.232(0.223,0.242) |
| 2010-4 | 3 | 0.003(0.001,0.008) | 0.026(0.005,0.077) | NA | NA | NA | 2,068 | 1.896(1.816,1.98) | 0.201(0.193,0.21) |
| 2011-1 | 4 | 0.003(0.001,0.008) | 0.028(0.008,0.073) | NA | NA | NA | 1,998 | 1.645(1.574,1.719) | 0.188(0.18,0.197) |
| 2011-2 | 5 | 0.004(0.001,0.01) | 0.033(0.011,0.078) | NA | NA | NA | 2,214 | 1.86(1.783,1.939) | 0.207(0.199,0.216) |
| 2011-3 | 4 | 0.003(0.001,0.007) | 0.029(0.008,0.074) | NA | NA | NA | 2,041 | 1.454(1.391,1.518) | 0.185(0.177,0.194) |
| 2011-4 | 6 | 0.005(0.002,0.01) | 0.041(0.015,0.09) | 0 | 0(0,0.003) | 0(0,0.161) | 1,906 | 1.452(1.388,1.519) | 0.178(0.17,0.186) |
| 2012-1 | 1 | 0.001(0,0.004) | 0.007(0,0.041) | 0 | 0(0,0.003) | 0(0,0.115) | 2,259 | 1.582(1.517,1.648) | 0.207(0.199,0.216) |
| 2012-2 | 3 | 0.002(0,0.007) | 0.023(0.005,0.068) | 0 | 0(0,0.003) | 0(0,0.097) | 1,755 | 1.32(1.259,1.383) | 0.167(0.159,0.175) |
| 2012-3 | 3 | 0.003(0.001,0.009) | 0.029(0.006,0.085) | 0 | 0(0,0.004) | 0(0,0.101) | 1,681 | 1.811(1.726,1.9) | 0.188(0.179,0.197) |
| 2012-4 | 4 | 0.005(0.001,0.012) | 0.04(0.011,0.102) | 0 | 0(0,0.004) | 0(0,0.094) | 1,532 | 1.81(1.72,1.903) | 0.174(0.165,0.183) |
| 2013-1 | 3 | 0.003(0.001,0.008) | 0.031(0.006,0.089) | 1 | 0.001(0,0.005) | 0.025(0.001,0.141) | 1,866 | 1.628(1.555,1.703) | 0.203(0.194,0.212) |
| 2013-2 | 4 | 0.003(0.001,0.009) | 0.042(0.011,0.107) | 1 | 0.001(0,0.005) | 0.025(0.001,0.139) | 1,564 | 1.325(1.26,1.392) | 0.165(0.157,0.174) |
| 2013-3 | 3 | 0.003(0.001,0.01) | 0.037(0.008,0.109) | 2 | 0.002(0,0.008) | 0.056(0.007,0.202) | 1,580 | 1.732(1.647,1.819) | 0.182(0.174,0.192) |
| 2013-4 | 3 | 0.004(0.001,0.01) | 0.039(0.008,0.113) | 0 | 0(0,0.004) | 0(0,0.105) | 1,376 | 1.612(1.528,1.699) | 0.164(0.156,0.173) |
| 2014-1 | 1 | 0.001(0,0.006) | 0.013(0,0.071) | 0 | 0(0,0.004) | 0(0,0.099) | 1,384 | 1.509(1.431,1.591) | 0.165(0.156,0.174) |
| 2014-2 | 0 | 0(0,0.003) | 0(0,0.041) | 2 | 0.002(0,0.006) | 0.045(0.005,0.161) | 1,263 | 1.051(0.994,1.111) | 0.132(0.125,0.139) |
| 2014-3 | 5 | 0.006(0.002,0.014) | 0.068(0.022,0.158) | 1 | 0.001(0,0.007) | 0.026(0.001,0.144) | 1,131 | 1.332(1.256,1.412) | 0.13(0.122,0.138) |
| Total cases/Mean rates* | 56 | 0.003(0.002,0.004) | 0.03(0.021,0.042) | 7 | 0.001(0,0.001) | 0.016(0.007,0.034) | 38,325 | 1.495(1.366,1.637) | 0.172(0.158,0.187) |
*Mean rates were calculated using data from 2011-4 to 2013-4 as data for all products are available during this time. Total cases are calculated since 2009-3; NA - Not applicable, drug not available for sale at during this period.
Figure 1.
Rate of diversion reports for tapentadol IR, tapentadol ER, and other Schedule II opioid tablets adjusted population (A) and for number of prescriptions dispensed (B), July 1, 2009 through September 30, 2014.
To estimate diversion rates relative to drug availability, we adjusted for the number of prescriptions filled during the period of analysis. The rates based on drug availability were 0.030 (tapentadol IR), 0.016 (tapentadol ER), and 0.172 (other Schedule II opioid tablets) per 1,000 prescriptions dispensed (Table 1). The tapentadol ER prescriptions dispensed rate was less than the other Schedule II opioid prescriptions dispensed rate (adjusted P < 0.001) but not different from tapentadol IR (adjusted P = 0.408) (Figure 1).
Street Price of Tapentadol IR and ER
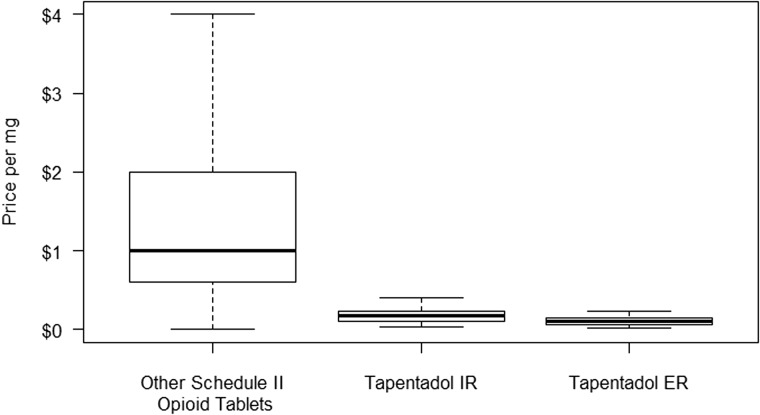
The StreetRx Website received 44 reports of tapentadol IR, 12 reports of tapentadol ER, and 11,539 reports of other Schedule II opioid tablets during the study period (Table 2). The median street price was $0.18 per milligram for tapentadol IR, $0.10 per milligram for tapentadol ER, and $1.00 per milligram for other Schedule II opioid tablets (Figure 2). The median street price of other Schedule II opioid tablets ($1.00, IQR: 0.60–2.00) is significantly greater than the median street prices of tapentadol IR ($0.18, IQR: 0.10–0.23, adjusted P < 0.0001) and tapentadol ER ($0.10, IQR: 0.06, 0.15, adjusted P < 0.0001). The median street prices of tapentadol ER and IR were similar (adjusted P = 0.163).
Table 2.
Median reported street price of tapentadol immediate release, tapentadol extended release, and other Schedule II opioid drugs from January 1, 2011 through September 30, 2014
| Tapentadol immediate release (IR) |
Tapentadol extended release (ER) |
Other Schedule II opioid tablets |
||||
|---|---|---|---|---|---|---|
| Year-quarter | N | Median price per mg (IQR) | N | Median price per mg (IQR) | N | Median price per mg (IQR) |
| 2011-1 | 1 | $1.00($1.00, $1.00) | NA | NA | 101 | $0.71($0.50, $1.50) |
| 2011-2 | 0 | NA | NA | NA | 53 | $1.00($0.63, $2.50) |
| 2011-3 | 1 | $0.60($0.60, $0.60) | NA | NA | 42 | $1.00($0.60, $2.00) |
| 2011-4 | 1 | $0.14($0.14, $0.14) | 0 | NA | 146 | $1.00($0.50, $1.55) |
| 2012-1 | 3 | $0.27($0.20, $0.60) | 0 | NA | 414 | $1.00($0.59, $2.00) |
| 2012-2 | 6 | $0.10($0.06, $0.20) | 0 | NA | 524 | $1.00($0.60, $2.22) |
| 2012-3 | 2 | $0.05($0.04, $0.07) | 0 | NA | 521 | $1.00($0.60, $2.33) |
| 2012-4 | 6 | $0.40($0.20, $1.00) | 3 | $0.10($0.10, $0.20) | 608 | $1.00($0.61, $2.00) |
| 2013-1 | 7 | $0.20($0.20, $0.40) | 0 | NA | 520 | $1.00($0.60, $2.00) |
| 2013-2 | 3 | $0.04($0.04, $0.10) | 0 | NA | 605 | $1.00($0.67, $2.50) |
| 2013-3 | 2 | $0.15 ($0.10, $0.20) | 2 | $0.07($0.04, $0.10) | 745 | $1.00($0.60, $2.00) |
| 2013-4 | 3 | $0.13($0.06, $0.20) | 1 | $0.06($0.06, $0.06) | 771 | $1.00($0.67, $2.50) |
| 2014-1 | 2 | $0.11($0.10, $0.12) | 2 | $1.00($0.06, $1.93) | 1,470 | $1.00($0.60, $2.00) |
| 2014-2 | 4 | $0.15($0.10, $0.60) | 1 | $0.03($0.03, $0.03) | 2,793 | $1.00($0.60, $2.00) |
| 2014-3 | 5 | $0.16($0.10, $1.20) | 3 | $0.10($0.08, $0.24) | 2,422 | $1.00($0.50, $1.60) |
| Total* | 44 | $0.18($0.10, $0.23) | 12 | $0.10($0.06, $0.15) | 11,539 | $1.00($0.60, $2.00) |
NA = Not applicable; either drug not available or no reports received.
*Total median (IQR) calculated from 2011-4 to 2014-3.
Figure 2.
Median street price of tapentadol IR, tapentadol ER, and other Schedule II opioid tablets, January 1, 2011 through September 30, 2014.
Drug Diversion Investigator Interviews
When asked specifically about tapentadol diversion, 22/30 (73.3%) investigators responded that tapentadol (either IR or ER) is not diverted in their area to their knowledge; 7/30 (23.3%) responded that tapentadol (IR or ER) is occasionally diverted in their area; and, 1/30 (3.3%) responded that tapentadol (IR or ER) is commonly diverted. When asked to elaborate, the respondent endorsing common diversion clarified that he had not worked a case involving tapentadol, but assumed there was a problem with this drug as he was asked about it.
Most interviewees (n = 23, 76.7%) reported never having a diversion case involving tapentadol during their tenure as investigators. None had ever purchased tapentadol illicitly nor had it been offered for purchase in undercover operations. Two respondents mentioned that they believed tapentadol was not prescribed in their area, and hence, not available for diversion.
Seven (23.3%) interviewees endorsed experience with at least one tapentadol diversion case. In two cases individuals sold their own prescriptions. Two other individuals obtained tapentadol from doctor shopping during the process of acquiring oxycodone. There was also a report of one street purchase involving tapentadol incidental to the sale of buprenorphine-naloxone combination tablets. In two additional cases individuals possessed tapentadol among other drugs during a drug raid.
Limitations
Limitations of our research include that the drug diversion program is not nationally representative. Nevertheless, with investigators reporting in 49 states, it seems unlikely that a substantial presence of tapentadol diversion was missed. StreetRx is limited by self-report that cannot be independently confirmed.
Discussion
Our results indicate that illicit transactions involving tapentadol ER are relatively rare in the United States. We detected few reports of tapentadol ER as compared with tapentadol IR and other Schedule II opioids. In the two years since its introduction, diversion rates for tapentadol ER have remained low despite the increased number of prescriptions for the drug. In contrast, illicit distribution of other Schedule II opioids is much higher and has persisted for many years.
The black market activity and price of tapentadol ER are low, with only 12 purchase reports in the StreetRx database. The median price for tapentadol ER was 0.10 per milligram compared with $1.00 for the group of Schedule II opioids. A previous report from StreetRx found that the mean price paid for opioid analgesics ranged from $3.29 per milligram for hydromorphone down to $0.05 per milligram for tramadol [13].
Interviews of drug diversion investigators produced results consistent with the small number of diversion cases and street price reports, and the low price of tapentadol in the illicit market. We verified that diversion investigators are aware of the drug and consider it an opioid of abuse. Despite their regular activities in interdiction of prescription drugs, none of the investigators had encountered tapentadol ER as the primary drug in street transactions.
Other sources also indicate low diversion activity in regards to tapentadol. We previously reported low rates of abuse for tapentadol IR during the first 24 months after its approval [14]. Vosburg et al. examined whether a tamper resistant formulation of tapentadol ER could be converted to a form suitable for snorting or injection in experienced oxycodone extended release abusers. They found that compared with an immediate release formulation of oxycodone, abusers could not convert the tablets into powder and were unwilling to snort the result of their tapentadol ER tampering. The amount of drug extracted for the tampered tapentadol ER was 10% the amount extracted from oxycodone immediate release tablets [15].
Consistent with reports from other sources, we found that the rate of diversion for other prescription opioids is decreasing. The cause for the decrease in other prescription opioids is not well documented, but presumably is related to the many different interventions created throughout the United States in the last few years: Potential solutions include: enhanced law enforcement; improved prescription practices by physicians (especially the use of prescription monitoring plans), as well as prescribing guidelines and restrictions; drug take-back days, and “take-home” naloxone. Perhaps the most controversial concept; however, is abuse-deterrent formulations (ADFs), which in Canada are termed tamper-resistant formulations (TRFs). Further monitoring of all opioids including tapentadol is needed as Com abusers appear to be switching from drugs like oxycodone to other alternatives. These alternatives can include heroin, but would logically also potentially include other less desirable opioid analgesics.
In conclusion, the low rate of diversion events combined with independently documented low street price collected from numerous jurisdictions throughout the US indicates that substantial diversion and abuse of tapentadol ER has not emerged in the United States during the first 36 months after introduction. However, further surveillance is warranted because of dramatic changes in supply and demand of Schedule II opioids in the United States.
Acknowledgments
This analysis was funded by Janssen Pharmaceuticals, Inc., Titusville, NJ, USA. The sponsor reviewed the manuscript for proprietary information. The sponsor did not participate in the conception, execution, or reporting of the results. The authors thank Chris Menone for his help on comments on an earlier draft of this manuscript.
References
- 1.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. Rockville, MD: Available at: http://www.samhsa.gov/data/2k13/DAWN127/sr127-DAWN-highlights.htm (accessed March 30, 2014). [PubMed] [Google Scholar]
- 2.Davis JM, Severtson SG, Bucher-Bartelson B, Dart RC. Using poison center exposure calls to predict prescription opioid abuse and misuse-related emergency department visits. Pharmacoepidemiol Drug Saf 2014;23:18–25. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend 90(1):64–71. [DOI] [PubMed] [Google Scholar]
- 4.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA 2013;309:657–9. [DOI] [PubMed] [Google Scholar]
- 5.Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain 2013;154(11):2287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Drug Satety Network. Prescription drug abuse rivals illicit drug abuse. Some see double standard in law enforcement. News Briefs–Drug use Trends. [December 6, 2006]. Available at: http://www.ndsn.org/oct96/prescrip.html (accessed November 5, 2015).
- 7.Rigg KK, Kurtz SP, Surratt HL. Patterns of prescription medication diversion among drug dealers. Drugs 2012;19:144–55. doi:10.3109/09687637.2011.631197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schröder W, Tzschentke TM, Terlinden R, et al. Synergistic interaction between the two mechanisms of action of tapentadol in analgesia. J Pharmacol Exp Ther 2011;337(1):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartholomäus J, Schwier S, Brett M, et al. New abuse deterrent formulation (ADF) technology for immediate-release opioids. Drug Develop Deliv 2013;13:76–81. [Google Scholar]
- 10.Tzschentke TM, Christoph T, Kogel B, et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): A novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exper Ther 2007;323(1):265–76. [DOI] [PubMed] [Google Scholar]
- 11.Vorsanger G, Xiang J, Okamoto A, et al. Evaluation of study discontinuations with tapentadol immediate release and oxycodone immediate release in patients with low back or osteoarthritis pain. J Opioid Manag 2010;6(3):169–79. [DOI] [PubMed] [Google Scholar]
- 12.Inciardi JA, Surratt HL, Stivers Y, Cicero TJ. FDA approvals of generic drugs: Impact on the diversion of opioid analgesics with a potential for abuse. J Opioid Manag 2009;5(2):81–7. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta N, Freifeld C, Brownstein JS, et al. Crowdsourcing black market prices for prescription opioids. J Med Internet Res 2013;15:e178. doi:10.2196/jmir.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dart RC, Cicero TJ, Surratt HL, et al. Assessment of the abuse of tapentadol immediate release: The first 24 months. J Opioid Manag 2012;8:395–402. doi: 10.5055/jom.2012.0139. [DOI] [PubMed] [Google Scholar]
- 15.Vosburg SK, Jones JD, Manubay JM, et al. A comparison among tapentadol tamper-resistant formulations (TRF) and OxyContin (non-TRF) in prescription opioid abusers. Addiction 2013;108:1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]