Abstract
In humans, DNA vaccines have failed to demonstrate the equivalent levels of immunogenicity that were shown in smaller animals. Previous studies have encoded adjuvants, predominantly cytokines, within these vaccines in an attempt to increase antigen-specific immune responses. However, these strategies have lacked breadth of innate immune activation and have led to disappointing results in clinical trials.
Damage associated molecular patterns (DAMPs) have been identified as pattern recognition receptor (PRR) agonists. DAMPs can bind to a wide range of PRRs on dendritic cells (DCs) and thus our studies have aimed to utilize this characteristic to act as an adjuvant in a DNA vaccine approach. Specifically, HSP70 has been identified as a DAMP, but has been limited by its lack of accessibility to PRRs in and on DCs. Here, we discuss the promising results achieved with the inclusion of membrane-bound or secreted HSP70 into a DNA vaccine encoding HIV gag as the model immunogen.
Keywords: heat shock protein, adjuvant, cytolytic, DNA vaccine, protective immunity
Introduction
Vaccines used in HIV clinical trials have predominantly induced humoral responses, but failed to protect vaccinated individuals against infection.1,2 However, the STEP trial that aimed to induce T cell responses also failed to induce protection.3 More recently, the RV144 phase III trial is the only HIV vaccine that demonstrated some efficacy, albeit modest, and this has re-energised the field. The results of this trial failed to detect differences in T cell responses between individuals vaccinated with vaccine or placebo4 and the modest protection was attributed to IgG antibodies that bound to the V1 and V2 regions of HIV Env.5 Despite these promising results, further vaccine research is required to increase the levels of protection against HIV.
Interestingly, a live attenuated SIV6 and a replication-competent rhesus cytomegalovirus (CMV) vector encoding SIV genes7 were reported to elicit long-lived and broad T cell responses that protected macaques against SIV challenge. Although these strategies may be too risky for use in humans, they provide proof of principle that T cell responses can result in protection.
Although broadly neutralizing antibodies represent an efficient method to elicit protection, HIV generates an infection synapse, resulting in cell-to-cell spread and is thus able to evade neutralizing antibodies.8 Consequently, for the foreseeable future, it may be pertinent to pursue a vaccination strategy which is designed to elicit broadly neutralizing antibodies and robust cell-mediated immunity. The latter component could be elicited by vaccination with a DNA vaccine.
DNA Vaccines
DNA vaccines have numerous advantages over other vaccine strategies including ease of manufacture and stability that make them attractive vaccine candidates9 and ensures the intracellular expression of our choice of antigen, HIV gag, that mimics a natural HIV infection in this regard10 leading to MHC class I processing and presentation. Although DNA vaccines have performed well in small animal models, they have lacked immunogenicity in humans.11 Increasing the immune responses to immunogens encoded by DNA vaccines may require an increase in (1) the localized inflammatory response and (2) DNA uptake by dendritic cells.
Improving DNA Vaccines with Adjuvants
Previous studies have examined DNA vaccines that encode the antigen alongside a plasmid that encodes a cytokine.12 A recent study examined the efficacy of a DNA vaccine encoding IL-12 and HIV gag that progressed to clinical trials.13 However the results were disappointing as fewer than half the vaccinated individuals produced detectable gag-specific responses. The use of a single cytokine may restrict the breadth of immunity and this may be responsible for the limited responses. This possibility was confirmed in pre-clinical studies which compared DNA vaccines encoding 1 or 2 cytokines and showed that the latter vaccine, which encoded IL-15 and IL-21, increased the antigen-specific T cell responses.14 DNA vaccines are bacterial plasmids and thus have the advantage of naturally containing CpG motifs that are TLR9 agonists that result in the upregulation of pro-inflammatory cytokines.15 However, DNA vaccines encoding additional CpG motifs as a component of vaccines against infectious diseases predominantly targeted antibody responses,16,17 and similar cancer vaccines resulted in mixed responses as only a few vaccinated individuals produced tumor-specific responses.18,19 This may have resulted from saturation of the CpG-TLR9 interaction even by the vaccines which did not contain the additional CpG motifs.
Thus, strategies which induce a broad innate immune response through binding of multiple TLRs that are more likely to culminate in a broad and protective adaptive immune response should be explored. Recently, the mechanism of the highly successful 17D yellow fever vaccine was described20; this study showed that the immune response is targeted through binding of multiple PRRs by pathogen associated molecular patterns (PAMPs) or DAMPs. A similar strategy to target multiple PRRs could represent an optimal HIV vaccine strategy. This approach, to broaden the immune by incorporating DAMPs into the strategy, is the hypothesized mechanism of our DNA vaccine regimen.
DAMPs and their Adjuvant Potential
Tissue resident innate immune cells recognize and bind to evolutionary conserved motifs on pathogens using PRRs. PRRs not only recognize PAMPs on invading pathogens,21 but also recognize endogenous host-related molecules, DAMPs, that are produced or released during tissue damage and cell necrosis.22 Molecules classified as DAMPs include HMGB1,23 uric acid24 and heat shock proteins.25 Binding of DAMPs to PRRs leads to further activation of antigen presenting cells, predominantly dendritic cells (DC), resulting in the upregulation of co-stimulatory molecules, an important signal required for the activation of naïve T cells.26 Host PRRs include Toll-like receptors (TLRs), nucleotide oligomerisation domain-like receptors (NLRs), retinoic acid-inducible gene-I-like receptors (RLRs), C-type lectin receptors (CLRs), and absent in melanoma 2-like receptors (AIM2).27
DNA Encoded PRR Agonists
We used 2 different strategies to facilitate the binding of DAMPs to PRRs after DNA vaccination. The heat shock protein 70 (HSP70) has been defined as a DAMP that binds to—and activates—DC.25 Previous studies with HSP70 focused predominantly on DNA vaccines which encoded an immunogenic antigen-bacterial HSP70 fusion protein.28,29 However, the use of mammalian HSP70, rather than the bacterial protein, may be beneficial as it is less likely to compete for the immune response. Furthermore, a direct comparison between DNA vaccines encoding bacterial or human HSP70 demonstrated that human HSP70 induced a stronger CD8+ T cell response.30
Our studies31 have focused on the use of DNA vaccines that encode the HIV gag protein as the antigen of choice and the inclusion of HSP70 permitted a comparison of the adjuvant properties of a cytoplasmic gag-HSP70 fusion protein, with 2 novel forms of HSP70 viz. membrane-bound or secreted HSP70. Gag and the latter 2 forms of HSP70 were encoded in bicistronic vectors containing the CMV and SV40 promoters, ensuring that HSP70-enhanced responses targeted gag-positive cells. We have previously shown that the CMV promoter is approximately 10-fold stronger than the SV40 promoter32 and thus these 2 promoters were used to evaluate the effect of differential gag and HSP70 expression on the immune responses in vaccinated mice.
Mice vaccinated with DNA encoding gag plus membrane-bound or secreted HSP70 resulted in significantly increased T cell functionality, multifunctionality, and proliferation compared with mice vaccinated with gag-only DNA.31 These responses, which are crucial for the quality and quantity of T cell immunity, also highlight the efficacy of these forms of HSP70 as novel adjuvants. To investigate the mechanism of action by the different forms of HSP70, naive bone marrow-derived DC were co-cultured in vitro with somatic cells transfected with one of the DNA vaccines, and gag-specific CD8 T cells were added subsequently. The inclusion of secreted or membrane-bound HSP70 in the DNA vaccine encoding gag resulted in increased T cell activation and proved that these forms of HSP70 significantly increased the cross-presentation of gag. Furthermore, in mice vaccinated with DNA encoding gag plus membrane-bound HSP70 or secreted HSP70, these responses resulted in significant reductions in the viral load after challenge with EcoHIV, suggesting that the significant increases in these T cell responses corresponded with increased protection. EcoHIV is a mouse model of HIV produced by substituting the envelope proteins of HIV with the gp80 envelope proteins from the murine leukemia virus.31-33 This results in a virus which can infect mouse but not human leukocytes.33 These results raise the possibility of using these forms of human HSP70 as an effective adjuvant in DNA vaccines in studies in larger animals. The optimum model for testing vaccine efficacy is the macaque, using SIV challenge. However, due to the expense, a more cost-effective animal model should be examined initially. The pig is an appropriate model as pigs possess similar organ function to humans, relatively similar immune responses, as well as similarities in the skin that are beneficial for intradermal vaccination.34 A major disadvantage of the pig model is the inability to examine the protective efficacy but nevertheless, successful vaccination of pigs may eliminate vaccine strategies which are unable to induce robust immune responses in large animals and thus reduce the time and cost of introducing an effective vaccine into macaques and eventually humans.
Mechanism
The secreted form of HSP70 was produced by fusing the secretory leader sequence from the tissue plasminogen activator (t-PA) to the N-terminus of HSP70 that is normally cleaved to produce the mature t-PA protein (NCBI GenBank Accession number: D01096.1) The signal sequence of t-PA is inserted into the ER membrane and proteins in the constitutive secretory pathway are dispatched from the ER to the golgi where the signal sequences are cleaved by proprotein convertases.35 The secretory proteins are then transported within secretory vesicles, which translocate to and fuse with the plasma membrane resulting in the release of secretory proteins.
Membrane-bound HSP70 can translocate to the surface of tumor cells, but the exact mechanism is unknown. In our study,31 a membrane-bound form of HSP70 was produced using a type II integral membrane fusion to ensure that the N-terminus was fused to a transmembrane domain anchor, while the C-terminus of HSP70 was free to act as an extracellular ligand. We generated this structure by using the transmembrane domain of human transferrin receptor (hTfR)36 and this enabled the C-terminal region of HSP70 to bind and activate DCs via TLR2/4 as shown previously.37 A schematic of the mechanism of DC activation by membrane-bound or secreted HSP70 is shown in Figure 1. DCs will be recruited to the antigen positive cell by the membrane-bound or secreted HSP70 resulting from expression of HSP70 and gag from the same plasmid. The secreted form of HSP70 may also recruit tissue resident DCs through chemotaxis.
Figure 1.
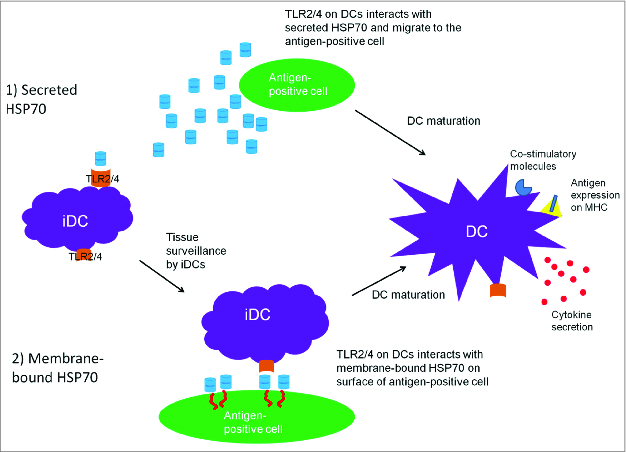
A schematic of the proposed mechanism of adjuvanticity of membrane bound and secreted HSP70. Tissue surveillance by immature DCs (iDCs) may result in recognition and binding to (1) secreted HSP70 or (2) membrane-bound HSP70, which represent danger signals (DAMPs), and thus the DCs are attracted to the antigen-positive cells. HSP70/TLR ligation results in DC maturation and co-stimulatory molecule upregulation and cytokine secretion. In turn, the mature DC expressing these co-stimulatory molecules process and present antigen in a MHC-restricted manner, migrate to the lymph nodes and interact with naive T cells to generate antigen-specific T cells.
Our second approach incorporates a cytolytic gene within a DNA vaccine encoding the HIV gag antigen. We hypothesize that after antigen expression, the cytolytic gene will induce cell death resulting in release of DAMPs from cells targeted by the vaccine. It is proposed that this will result in recruitment of additional DCs and culminate in DC activation through PRR binding. We have demonstrated that the cytolytic gene technology is able to increase antigen-specific immune responses, and that mouse perforin represents a suitable cytolytic protein.32 The proposed mechanism of action for mouse perforin encoded in a DNA vaccine is shown in Figure 2.
Figure 2.
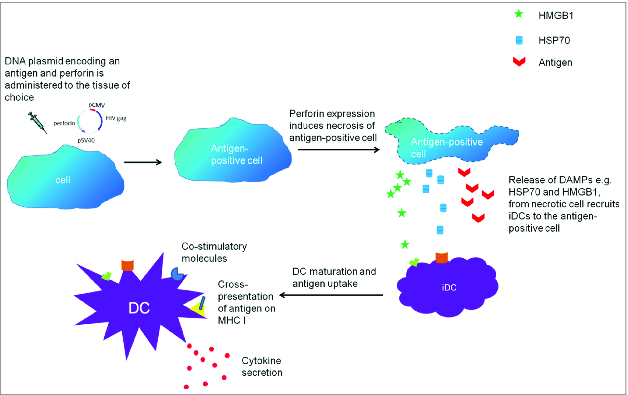
The proposed mechanism for perforin-induced cell death which results in increased antigen-specific immune responses.
Summary
We have described 2 strategies which increase the efficacy of DNA vaccines. Although multi-dose DNA vaccine regimens may generate protective immunity, DNA vaccines are most commonly used in a prime/boost setting in which the DNA prime is followed by a viral vector boost.38 Human adenovirus replication-defective vaccine vectors have been used previously,39 however concerns were raised about their use in humans due to pre-existing immunity.40 Nevertheless, adenoviruses are transmitted via the respiratory tract and consequently, intranasal delivery of a vaccine is likely to elicit pan-mucosal immunity41 and thus prevent mucosal transmission of HIV at the site of infection. An adenovirus from an alternative species such as bovine, porcine, or chimpanzee adenovirus may overcome the concerns associated with human adenovirus, and these have shown promising results in small animal studies42,43 and in clinical trials.44,45 Therefore, a vaccination strategy consisting of a systemic prime using a DNA vaccine encoding gag plus membrane-bound HSP70 that can induce a strong immune response followed by intranasal delivery of an adenovirus vector encoding HIV specific antigens as a boost has the potential to elicit robust systemic and mucosal immunity, similar to that suggested for DNA vaccines in a previous study.46
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Prof. John Hayball for his advice regarding the in vitro cross presentation assay. This work was supported by grants 543143 and APP1026293 from the National Health and Medical Research Council (NHMRC) of Australia and by grant number BF040005 from the Australia-India Biotechnology Fund awarded to E.J.G., who is a Senior Research Fellow of the NHMRC.
References
- 1. Montefiori DC, Graham BS, Kliks S, Wright PF; NIAID AIDS Vaccine Clinical Trials Ne2rk. Serum antibodies to HIV-1 in recombinant vaccinia virus recipients boosted with purified recombinant gp160. J Clin Immunol 1992; 12:429-39; PMID:1287035; http://dx.doi.org/ 10.1007/BF00918855 [DOI] [PubMed] [Google Scholar]
- 2. Dolin R, Graham BS, Greenberg SB, Tacket CO, Belshe RB, Midthun K, Clements ML, Gorse GJ, Horgan BW, Atmar RL, et al.; NIAID AIDS Vaccine Clinical Trials Network. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. Ann Intern Med 1991; 114:119-27; PMID:1984386; http://dx.doi.org/ 10.7326/0003-4819-114-2-119 [DOI] [PubMed] [Google Scholar]
- 3. Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med 2008; 205:7-12; PMID:18195078; http://dx.doi.org/ 10.1084/jem.20072681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wren L, Kent SJ. HIV Vaccine efficacy trial: glimmers of hope and the potential role of antibody-dependent cellular cytotoxicity. Hum Vaccin 2011; 7:466-73; PMID:21389779; http://dx.doi.org/ 10.4161/hv.7.4.14123 [DOI] [PubMed] [Google Scholar]
- 5. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. . Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275-86; PMID:22475592; http://dx.doi.org/ 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD 3rd, et al. . Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med 2012; 18:1673-81; PMID:22961108; http://dx.doi.org/ 10.1038/nm.2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. . Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473:523-7; PMID:21562493; http://dx.doi.org/ 10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiffner T, Sattentau QJ, Duncan CJ. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine 2013; 31:5789-97; PMID:24140477; http://dx.doi.org/ 10.1016/j.vaccine.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 9. Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet 2008; 9:776-88; PMID:18781156; http://dx.doi.org/ 10.1038/nrg2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perlman M, Resh MD. Identification of an intracellular trafficking and assembly pathway for HIV-1 gag. Traffic 2006; 7:731-45; PMID:16683918; http://dx.doi.org/ 10.1111/j.1398-9219.2006.00428.x [DOI] [PubMed] [Google Scholar]
- 11. Liu MA, Ulmer JB. Human clinical trials of plasmid DNA vaccines. Adv Genet 2005; 55:25-40; PMID:16291211; http://dx.doi.org/ 10.1016/S0065-2660(05)55002-8 [DOI] [PubMed] [Google Scholar]
- 12. Morrow MP, Weiner DB. Cytokines as adjuvants for improving anti-HIV responses. AIDS 2008; 22:333-8; PMID:18195559; http://dx.doi.org/ 10.1097/QAD.0b013e3282f42461 [DOI] [PubMed] [Google Scholar]
- 13. Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, Hural J, Lubeck M, Eldridge J, Cardinali M, et al.; NIAID HIV Vaccine Trials Network. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One 2012; 7:e29231; PMID:22242162; http://dx.doi.org/ 10.1371/journal.pone.0029231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li W, Li S, Hu Y, Tang B, Cui L, He W. Efficient augmentation of a long-lasting immune responses in HIV-1 gag DNA vaccination by IL-15 plasmid boosting. Vaccine 2008; 26:3282-90; PMID:18472194; http://dx.doi.org/ 10.1016/j.vaccine.2008.03.081 [DOI] [PubMed] [Google Scholar]
- 15. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004; 5:190-8; PMID:14716310; http://dx.doi.org/ 10.1038/ni1028 [DOI] [PubMed] [Google Scholar]
- 16. Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, Fay MP, Saul A, Zhu D, Rausch K, et al. . Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS One 2008; 3:e2940; PMID:18698359; http://dx.doi.org/ 10.1371/journal.pone.0002940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol 2004; 24:693-701; PMID:15622454; http://dx.doi.org/ 10.1007/s10875-004-6244-3 [DOI] [PubMed] [Google Scholar]
- 18. Haining WN, Davies J, Kanzler H, Drury L, Brenn T, Evans J, Angelosanto J, Rivoli S, Russell K, George S, et al. . CpG oligodeoxynucleotides alter lymphocyte and dendritic cell trafficking in humans. Clin Cancer Res 2008; 14:5626-34; PMID:18765557; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0526 [DOI] [PubMed] [Google Scholar]
- 19. Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, et al. . In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 2010; 28:4324-32; PMID:20697067; http://dx.doi.org/ 10.1200/JCO.2010.28.9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 2006; 203:413-24; PMID:16461338; http://dx.doi.org/ 10.1084/jem.20051720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 1997; 9:4-9; PMID:9039775; http://dx.doi.org/ 10.1016/S0952-7915(97)80152-5 [DOI] [PubMed] [Google Scholar]
- 22. Tournier JN, Quesnel-Hellmann A. Host-pathogen interactions: a biological rendez-vous of the infectious nonself and danger models? PLoS Pathog 2006; 2:e44; PMID:16733542; http://dx.doi.org/ 10.1371/journal.ppat.0020044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 2007; 220:35-46; PMID:17979838; http://dx.doi.org/ 10.1111/j.1600-065X.2007.00574.x [DOI] [PubMed] [Google Scholar]
- 24. Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest 2010; 120:1939-49; PMID:20501947; http://dx.doi.org/ 10.1172/JCI40124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 2000; 6:435-42; PMID:10742151; http://dx.doi.org/ 10.1038/74697 [DOI] [PubMed] [Google Scholar]
- 26. Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol 1994; 152:5208-19; PMID:7514631 [PubMed] [Google Scholar]
- 27. Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A 2000; 97:13766-71; PMID:11095740; http://dx.doi.org/ 10.1073/pnas.250476497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res 2000; 60:1035-42; PMID:10706121 [PubMed] [Google Scholar]
- 29. Li H, Ou X, Xiong J. Modified HPV16 E7/HSP70 DNA vaccine with high safety and enhanced cellular immunity represses murine lung metastatic tumors with downregulated expression of MHC class I molecules. Gynecol Oncol 2007; 104:564-71; PMID:17081598; http://dx.doi.org/ 10.1016/j.ygyno.2006.09.027 [DOI] [PubMed] [Google Scholar]
- 30. Zong J, Peng Q, Wang Q, Zhang T, Fan D, Xu X. Human HSP70 and modified HPV16 E7 fusion DNA vaccine induces enhanced specific CD8+ T cell responses and anti-tumor effects. Oncol Rep 2009; 22:953-61; PMID:19724878 [DOI] [PubMed] [Google Scholar]
- 31. Garrod TJ, Grubor-Bauk B, Gargett T, Li Y, Miller DS, Yu W, Major L, Burrell CJ, Wesselingh S, Suhrbier A, et al. . DNA vaccines encoding membrane-bound or secreted forms of heat shock protein 70 exhibit improved potency. Eur J Immunol 2014; (Forthcoming); PMID:24723366; http://dx.doi.org/ 10.1002/eji.201343983 [DOI] [PubMed] [Google Scholar]
- 32. Gargett T, Grubor-Bauk B, Garrod TJ, Yu W, Miller D, Major L, Wesselingh S, Suhrbier A, Gowans EJ. Induction of antigen-positive cell death by the expression of perforin, but not DTa, from a DNA vaccine enhances the immune response. Immunol Cell Biol 2014; 92:359-67; PMID:24323081; http://dx.doi.org/ 10.1038/icb.2013.93 [DOI] [PubMed] [Google Scholar]
- 33. Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A 2005; 102:3760-5; PMID:15728729; http://dx.doi.org/ 10.1073/pnas.0500649102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol 2012; 20:50-7; PMID:22153753; http://dx.doi.org/ 10.1016/j.tim.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steiner DF. The proprotein convertases. Curr Opin Chem Biol 1998; 2:31-9; PMID:9667917; http://dx.doi.org/ 10.1016/S1367-5931(98)80033-1 [DOI] [PubMed] [Google Scholar]
- 36. Martyn JC, Cardin AJ, Wines BD, Cendron A, Li S, Mackenzie J, Powell M, Gowans EJ. Surface display of IgG Fc on baculovirus vectors enhances binding to antigen-presenting cells and cell lines expressing Fc receptors. Arch Virol 2009; 154:1129-38; PMID:19557497; http://dx.doi.org/ 10.1007/s00705-009-0423-8 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol 2002; 169:2422-9; PMID:12193710; http://dx.doi.org/ 10.4049/jimmunol.169.5.2422 [DOI] [PubMed] [Google Scholar]
- 38. Robinson HL, Montefiori DC, Villinger F, Robinson JE, Sharma S, Wyatt LS, Earl PL, McClure HM, Moss B, Amara RR. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology 2006; 352:285-94; PMID:16740288; http://dx.doi.org/ 10.1016/j.virol.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 39. Uberla K. HIV vaccine development in the aftermath of the STEP study: re-focus on occult HIV infection? PLoS Pathog 2008; 4:e1000114; PMID:18769723; http://dx.doi.org/ 10.1371/journal.ppat.1000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murphy CG, Lucas WT, Means RE, Czajak S, Hale CL, Lifson JD, Kaur A, Johnson RP, Knipe DM, Desrosiers RC. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol 2000; 74:7745-54; PMID:10933680; http://dx.doi.org/ 10.1128/JVI.74.17.7745-7754.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol 2009; 183:6883-92; PMID:19923474; http://dx.doi.org/ 10.4049/jimmunol.0901466 [DOI] [PubMed] [Google Scholar]
- 42. Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, Hill AV, Cottingham MG. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One 2012; 7:e40385; PMID:22808149; http://dx.doi.org/ 10.1371/journal.pone.0040385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharma A, Bangari DS, Tandon M, Hogenesch H, Mittal SK. Evaluation of innate immunity and vector toxicity following inoculation of bovine, porcine or human adenoviral vectors in a mouse model. Virus Res 2010; 153:134-42; PMID:20659505; http://dx.doi.org/ 10.1016/j.virusres.2010.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O’Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, Goodman AL, Edwards NJ, Reyes-Sandoval A, Bird P, et al. . Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 2012; 205:772-81; PMID:22275401; http://dx.doi.org/ 10.1093/infdis/jir850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, Williams AR, Halstead FD, Moretz SE, Miura K, et al. . Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol Ther 2011; 19:2269-76; PMID:21862998; http://dx.doi.org/ 10.1038/mt.2011.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ranasinghe C, Ramshaw IA. Genetic heterologous prime-boost vaccination strategies for improved systemic and mucosal immunity. Expert Rev Vaccines 2009; 8:1171-81; PMID:19722891; http://dx.doi.org/ 10.1586/erv.09.86 [DOI] [PubMed] [Google Scholar]


