Abstract
Ruxolitinib is the only therapy with an approved indication for myelofibrosis (MF), a myeloproliferative neoplasm associated with progressive bone marrow fibrosis and extramedullary hematopoiesis. Although the pivotal phase 3 COMFORT studies included only patients with intermediate-2 or high-risk MF, the US indication includes all patients with intermediate- or high-risk disease. Data from recent nonrandomized studies confirm that the benefits of ruxolitinib established in the COMFORT studies in terms of spleen size reduction and symptom improvement also extend to patients with intermediate-1 risk MF, who tend to have less advanced disease than patients with higher-risk MF. Given the disease-modifying potential of ruxolitinib therapy, timely initiation of ruxolitinib therapy may not only improve patients’ current clinical status but also lead to better long-term outcomes. The decision of whether or when to initiate ruxolitinib treatment should be based on the expected benefit–risk ratio for each patient, specifically considering potential adverse effects.
Keywords: Intermediate-1 risk, International Prognostic Scoring System, myelofibrosis, ruxolitinib, splenomegaly
Myelofibrosis
Myelofibrosis (MF) is a chronic Philadelphia chromosome-negative myeloproliferative neoplasm (MPN) associated with progressive bone marrow fibrosis and extramedullary hematopoiesis.[1,2] MF may develop as primary MF (PMF) or evolve from polycythemia vera (PV) or essential thrombocythemia (ET) through myelofibrotic disease transformation.[3,4] MF pathogenesis is characterized by dysregulation of JAK-STAT signaling.[5] In addition to a driver mutation in JAK2, MPL, or CALR that is primarily responsible for overactive or constitutional JAK2-STAT signaling in the neoplastic clone, a patient may have subclonal mutations in members of other signaling pathways or in epigenetic modifiers.[6–10] The resulting mutation profile can be complex, may vary significantly from patient to patient, and may have a profound impact on a patient’s prognosis.[10,11] The main clinical manifestations of MF include splenomegaly, MF-related symptoms, and anemia.[1,12,13] The MF-associated symptom burden can be debilitating, particularly in patients with advanced disease.[14,15] Abnormally high circulating levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in patients with MF are believed to contribute to disease-associated symptoms and cachexia.[16,17]
Prognosis
MF is a progressive disease associated with shortened survival. Most patients die from consequences of disease progression, such as bone marrow or organ failure, thromboembolic complications, or leukemic transformation. A patient’s prognosis can be estimated using validated risk stratification systems. The different prognostic scoring systems developed in recent years reflect a gradual evolution in the understanding of factors that influence prognosis in MF.[2]
The International Prognostic Scoring System (IPSS) published in 2009 [18] was validated for patients with PMF before the availability of JAK-inhibitor therapy and considered the prognostic impact of age and various clinical characteristics at presentation [Table 1]. According to their risk scores, patients were classified as having low, intermediate-1, intermediate-2, or high risk of shortened survival, with median survival times of approximately 11, 8, 4, and 2 years, respectively.[18] Subsequently, more refined prognostic scoring systems have been developed to allow prognostication at any time after diagnosis and accommodate additional risk factors [Table 1].[12,18,19] Consequently, the risk categories of different scoring systems do not always align. For example, patients classified as having low- or intermediate-1 risk disease by IPSS and DIPSS (dynamic IPSS) may be classified in higher-risk categories by DIPSS plus if they have additional DIPSS plus risk factors such as thrombocytopenia or complex cytogenetics [Table 1].
Table 1.
Risk stratification of patients with MF according to the International Prognostic Scoring System (IPSS),[18] dynamic IPSS (DIPSS),[19] DIPSS plus,[12] and mutation-enhanced IPSS (MIPSS).[20]
| Risk category | Scale | Estimated survival (years) |
|---|---|---|
| IPSS | No. of risk factors a | Median (95% CI) |
| Low | 0 | 11.3 (9.8–15.1) |
| Intermediate-1 | 1 | 7.9 (6.6–9.5) |
| Intermediate-2 | 2 | 4.0 (3.6–4.9) |
| High | ≥ 3 | 2.3 (1.9–2.6) |
| DIPSS | Prognostic score b | Median |
| Low | 0 | NR |
| Intermediate-1 | 1 or 2 | 14.2 |
| Intermediate-2 | 3 or 4 | 4 |
| High | 5 or 6 | 1.5 |
| DIPSS plus | Prognostic score c | Median |
| Low | 0 | 15.4 |
| Intermediate-1 | 1 | 6.5 |
| Intermediate-2 | 2 or 3 | 2.9 |
| High | 4–6 | 1.3 |
| MIPSS | Prognostic score d | Median |
| Low | 0–0.5 | 17.6 |
| Intermediate-1 | 1–1.5 | 7.8 |
| Intermediate-2 | 2–3.5 | 4.3 |
| High | ≥ 4 | 1.6 |
NR: not reached.
aRisk factors include age > 65 years, constitutional symptoms (defined as weight loss > 10% of baseline value in the year preceding diagnosis and/or unexplained fever or excessive sweats persisting for more than 1 month), hemoglobin <10 g/dL, white blood cell count > 25 × 109/L, and peripheral blood blasts ≥ 1%.
bRisk factors (score) include age >65 years (1), constitutional symptoms (1), hemoglobin < 10 g/dL (2), white blood cell count > 25 × 109/L (1), and peripheral blood blasts ≥ 1% (1).
cScoring is based on DIPSS risk categories (low risk, 0 points; intermediate-1 risk, 1 point; intermediate-2 risk, 2 points; high risk, 3 points) and additional risk factors (unfavorable karyotype, 1 point; platelet count < 100 × 109/L, 1 point; transfusion need, 1 point).
dRisk factors (score) include age >60 years (1.5), constitutional symptoms (0.5), hemoglobin < 100 g/L (0.5), platelet count < 200 × 109/L (1.0), triple-negative mutation status (1.5), JAK2 or MPL mutation (0.5), ASXL1 (0.5), and SRSF2 (0.5).
Most recently, recognition that prognosis can be strongly affected by a patient’s mutation profile has led to the development of other scoring systems, such as the mutation-enhanced IPSS (MIPSS), which combines clinical and genetic risk factors [Table 1],[20] and the genetics-based scoring system (GPSS), which relies exclusively on age and genetic risk factors.[21] However, MIPSS and GPSS require further validation before they can be widely adopted.
Treatment
Prognosis is an important consideration when weighing allogeneic hematopoietic cell transplantation (HCT) as a treatment option, as HCT is the only potentially curative treatment option to date.[22,23] Although HCT generally is not recommended for patients with advanced age, poor performance status, and/or prohibitive comorbidities, it may be the best option for those with a short life expectancy due to a high risk of leukemic transformation, anemia requiring transfusions, or an adverse mutation profile.[23] However, risk stratification has limited value in guiding treatment decisions primarily aimed at improving MF-related signs and symptoms and quality of life (QOL), as risk categories do not reflect the totality of disease burden. Although patients with higher risk scores tend to have more advanced MF, even patients with low- or intermediate-1 risk MF per IPSS may have symptoms or spleen size enlargements that require treatment.[24,25] The MPN symptom assessment form (MPN-SAF), which has been validated in patients with MPNs including MF,[26] is a convenient and comprehensive instrument for the quantitative assessment of disease-related symptom burden. Symptom cluster analysis using the MPN-SAF in 1470 patients with MPNs showed that many patients with intermediate-1 risk MF by DIPSS have a mild to moderate symptom burden characterized by fatigue, night sweats, insomnia, and/or concentration problems.[27]
The JAK1 and JAK2 inhibitor ruxolitinib is currently the only therapy with an approved indication in MF. The efficacy and safety of ruxolitinib were evaluated in two pivotal phase 3 studies, COMFORT-I and COMFORT-II, of patients with intermediate-2 or high-risk MF per IPSS.[28,29] The results demonstrated that ruxolitinib provided rapid reductions in splenomegaly and symptom burden compared with placebo or best available therapy (BAT), with concomitant improvements in QOL.[28–31] For example, in the placebo-controlled COMFORT-I study, 41.9% of patients in the ruxolitinib arm compared with 0.7% in the placebo arm had a ≥ 35% reduction in spleen volume at 24 weeks (p < 0.001), and 45.9% in the ruxolitinib arm compared with 5.3% in the placebo arm had a ≥ 50% decrease in Total Symptom Score (TSS) at 24 weeks (p < 0.001).[28] In both studies, ruxolitinib was not only safe and generally well tolerated but also associated with dose-limiting myelosuppression, most commonly thrombocytopenia and anemia, particularly in the first 3 months of therapy.[28,29,32] Although the results of the COMFORT studies showed that treatment-related cytopenias can be managed effectively with dose reductions or treatment interruptions,[28,29,32] these adjustments may eventually lead to loss of response with consequent treatment discontinuation in some patients. However, follow-up data from patients in the COMFORT trials showed that clinical benefits were generally maintained in patients remaining on therapy.[33–36] In addition, there is compelling evidence that ruxolitinib is associated with a survival advantage compared with placebo or conventional therapy.[33,35,37,38]
Efficacy of ruxolitinib in patients with intermediate-1 risk MF
The COMFORT studies are the only randomized controlled studies that assessed the efficacy of ruxolitinib in patients with MF, and these studies did not include patients with intermediate-1 risk MF.[28,29] Consequently, there are no randomized controlled trial data for this MF patient population. Based on the results of the COMFORT studies, the European Medical Association approved ruxolitinib ‘for the treatment of disease-related splenomegaly or symptoms in adult patients with PMF, post-PV MF or post-ET MF’.[39] In contrast, the risk-based indication approved in the US [40] includes all patients with intermediate- or high-risk MF, in recognition of the fact that even those with intermediate-1 risk MF may have symptoms that require treatment.[41] In fact, in real-world settings, a substantial proportion of patients with intermediate-1 risk MF are treated with ruxolitinib.[42–45] Here we review recent data regarding the efficacy of ruxolitinib in patients with intermediate-1 risk MF from a number of nonrandomized, uncontrolled studies.
JUMP
JUMP is an international, open-label, expanded-access study of ruxolitinib in patients with intermediate-2 or high-risk MF (per IPSS) with or without splenomegaly, or with intermediate-1 risk MF and a palpable spleen length ≥ 5 cm (from the left costal margin).[45] With an enrollment of 2233 patients from 26 countries before January 2015, this ongoing study is the largest observational study of ruxolitinib in patients with MF. Recent data from 1869 patients who started treatment ≥ 1 year before the data cutoff date (January 1, 2015; median exposure, 13.6 months) showed that 62.0% of evaluable patients achieved a ≥ 50% reduction from baseline in palpable spleen length at week 48, and an additional 19.0% had a 25–50% reduction in palpable spleen length.[45]
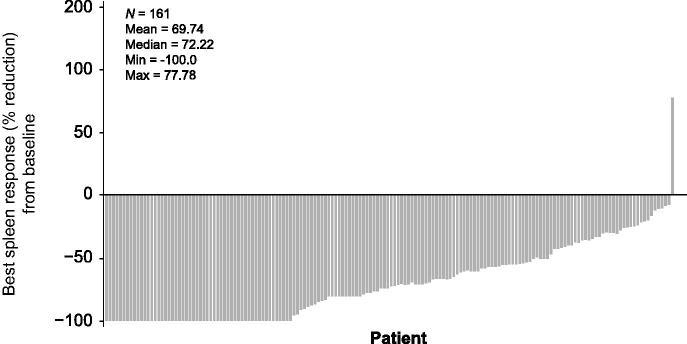
In a subgroup analysis of 163 patients in the JUMP study with intermediate-1 risk MF (median treatment exposure, 14.4 months), 64% and 61% of evaluable patients had a ≥ 50% decrease in palpable spleen length at weeks 24 and 48, respectively.[46] By week 72, best responses included a ≥ 50% decrease in palpable spleen length in 77.6% of patients, including complete resolution of splenomegaly in 21% of patients [Figure 1]. In addition, 30% of evaluable patients had clinically meaningful symptom improvement at week 48, as determined by Functional Assessment of Cancer Therapy–Lymphoma (FACT-Lym) total scores.[46] Although the FACT-Lym scale was originally developed for response evaluation in patients with non-Hodgkin lymphoma, it has been used successfully to measure treatment benefit of ruxolitinib compared with BAT in patients with MF in the COMFORT-II study.[31] In addition to a generic 27-item QOL questionnaire, it contains a cancer-specific 15-item questionnaire that addresses common symptoms of MF, including pain, swelling, fever, night sweats, itching, insomnia, fatigue, weight loss, and loss of appetite.[31]
Figure 1.
Best percentage reduction from baseline in spleen length by week 72 in patients with intermediate-1 risk MF in the JUMP trial Reproduced from [46].
Safety data in the subgroup of patients with intermediate-1 risk MF in the JUMP study [46] were consistent with findings for the total study population [45] and the results of the COMFORT studies.[28,29] Twenty-five percent of patients with intermediate-1 risk MF experienced grade 3 or 4 anemia, and 11% had grade 3 or 4 thrombocytopenia. However, 21% of the patients already had anemia (hemoglobin < 10 g/dL) at baseline, and 8% had a baseline platelet count < 100 × 109/L, although no patient had a platelet count < 75 × 109/L. Only one patient discontinued treatment for anemia, whereas three patients discontinued for thrombocytopenia. In the overall patient population (N = 1869), grade 3 or 4 anemia or thrombocytopenia occurred in 34.0% and 14.9% of patients, respectively, leading to treatment discontinuation in 2.2% and 3.3% of patients, respectively.[45] Rates of nonhematologic grade 3 or 4 adverse events in patients with intermediate-1 risk MF were < 2%, except for asthenia, which occurred in 2.5% of the patients.[46] Overall the findings from JUMP suggest that ruxolitinib provides meaningful clinical benefits with acceptable tolerability in patients with intermediate-1 risk MF who have splenomegaly.
ROBUST
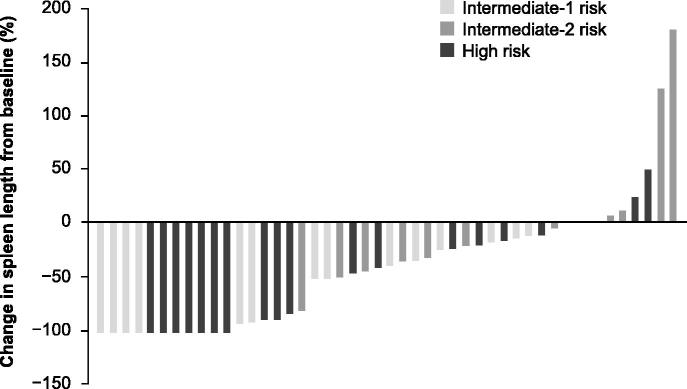
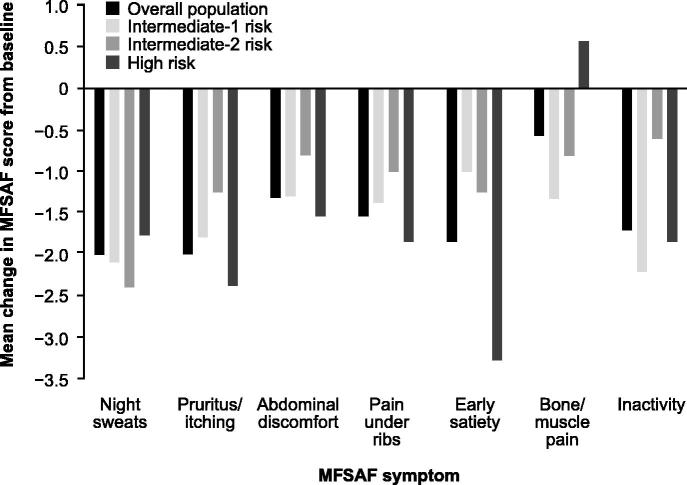
The ROBUST trial (N = 48) was an open-label phase 2 study of ruxolitinib conducted in the UK.[47] The study included 14, 13, and 21 patients with intermediate-1, intermediate-2, and high-risk MF, respectively. Efficacy was reported for each risk category separately and for the entire study population. At week 48, 57% of patients with intermediate-1 risk disease and 50% of all patients achieved the composite primary endpoint of ≥ 50% reduction in palpable spleen length and/or a ≥ 50% reduction in Myelofibrosis Symptom Assessment Form (MFSAF) TSS from baseline, with no significant differences between risk groups (p = 0.599 by χ 2 test). A ≥ 50% reduction in palpable spleen length at week 48 was observed in 50% of patients with intermediate-1 risk MF and 39.6% of all patients. The mean reduction in palpable spleen length was 51.6% among patients with intermediate-1 risk disease and 46.7% in the overall population. At the last available assessment, 79% of the patient population, including all patients with intermediate-1 risk disease, had some reduction in palpable spleen length; 4/14 patients with intermediate-1 risk disease, and 7/21 patients with high-risk disease had a complete resolution of palpable splenomegaly [Figure 2]. At week 48, 20.8% of all patients enrolled, including 21.4% of patients with intermediate-1 risk MF, had a ≥ 50% decrease in MFSAF TSS, with absolute improvements in individual symptom scores reflecting corresponding improvements in the overall study population [Figure 3]. A contributing factor for the low rates of symptom response in the intent-to-treat population (N = 48) compared with the COMFORT studies was the small percentage of patients in the ROBUST study who had available MFSAF data for week 48 (18/48). Among evaluable patients, 55.6% had a ≥ 50% decrease in TSS. At last available assessment (n = 39), 80.0% (8/10) of intermediate-1 risk, 72.7% (8/11) of intermediate-2 risk, and 72.2% (13/18) of high-risk patients had improved TSS.[47]
Figure 2.
Change from baseline in palpable spleen length to last available assessment by risk group in the ROBUST trial.[47] Last available measurement plotted. Data are presented for patients with: intermediate-1 risk disease, n = 14; intermediate-2 risk disease, n = 12; high-risk disease, n = 21. Reproduced with permission from Mead AJ, et al. [47]. © 2015 John Wiley & Sons Ltd.
Figure 3.
Mean change from baseline in MFSAF individual symptom scores at week 48 for all patients and by risk group in the ROBUST trial. Reproduced with permission from Mead AJ, et al. [47]. © 2015 John Wiley & Sons Ltd.
Safety data in ROBUST were not stratified by risk group.[47] However, the overall safety findings were consistent with those reported in the COMFORT studies.[28,29,32] The most common adverse events were anemia (45.8%) and thrombocytopenia (37.5%), resulting in one and three discontinuations, respectively. Mean platelet levels decreased by approximately 40% from baseline to week 4 and then stabilized. Mean hemoglobin decreased by 10% from baseline to week 12 and then recovered to 5% below baseline by week 48.[47] Infections were more common in ROBUST than in the COMFORT trials, including mostly urinary tract infections (16.7%) and lower (14.6%) or upper (10.4%) respiratory tract infections. Of note, one patient developed progressive multifocal encephalopathy,[48] and two patients developed staphylococcal sepsis. However, no cases of herpes zoster, hepatitis B, or tuberculosis occurred during the trial.[47]
US chart review
A retrospective observational review of medical records collected by 49 hematologists and oncologists in the US evaluated the effects of ruxolitinib in 108 patients with low- (n = 25) or intermediate-1 risk (n = 83) MF per IPSS.[49] To be included in the study, patients had to have received ruxolitinib therapy for at least 3 months before the medical record abstraction date. Median exposure to ruxolitinib was 8 months, and 77% of patients with intermediate-1 risk MF were still on therapy at last follow-up. Among patients with intermediate-1 risk MF, most (80%) were aged ≤ 65 years, and most had splenomegaly at diagnosis. During treatment with ruxolitinib, the proportion of patients with intermediate-1 risk MF and a palpable spleen length ≥ 10 cm decreased from 51% at treatment initiation to 10% at best response. Similarly, most patients with intermediate-1 risk disease experienced a reduction in symptom severity during ruxolitinib treatment. For example, the proportion of patients with moderate or severe fatigue among patients who provided symptom data decreased from 76% at diagnosis to 42% at best response. However, a substantial reduction in the overall percentage of patients experiencing a specific symptom was only observed for spleen-related abdominal pain. These findings have to be interpreted with caution as symptom information was based on available medical records, with the possibility that some patients had missing data.
Another limitation of the study is that actual changes in palpable spleen length and symptom scores were not reported. During ruxolitinib treatment, 23% and 6% of patients with intermediate-1 risk disease experienced grade ≥ 3 anemia or thrombocytopenia, respectively. However, although 19% of patients in this risk category required dose reductions for adverse events, anemia and thrombocytopenia were not a cause for treatment interruption or discontinuation.[49]
Other studies
Results of JUMP,[46] ROBUST,[47] and the US chart review by Davis et al.[49] support the efficacy and safety of ruxolitinib in patients with intermediate-1 risk MF by IPSS, including those with marked splenomegaly and/or substantial symptom burden. Two other studies provided further data in support of these findings. In one study, 25 patients with low-risk or intermediate-1 risk MF per DIPSS and a median palpable spleen length of 13 cm who were treated with ruxolitinib for 12 months at two US institutions had a median 73% reduction in MPN-SAF TSS (p < 0.001 for change from baseline) and a 64% reduction in palpable spleen length from baseline.[24] Furthermore, interim results of a phase 2 study [50] of ruxolitinib in patients with intermediate- or high-risk MF per DIPSS showed that the median percentage change from baseline to week 24 in spleen volume was similar for patients with intermediate-1 risk MF (−23.3% [range −40.6% to 38.5%]; n = 8) and all evaluable patients (−24.2% [range −55.8% to 38.5%]; n = 30) [unpublished data on file]. Median reduction in TSS at week 24 was 57.5% (n = 8) in patients with intermediate-1 risk MF and 43.8% (n = 32) in all evaluable patients [unpublished data on file].
In summary, findings from independent studies provide convincing evidence suggesting that patients with intermediate-1 risk MF derive similar benefits from ruxolitinib therapy as do higher-risk patients in terms of spleen size reduction and symptom alleviation. However, compared with the COMFORT studies, these studies have considerable limitations. Most importantly, all studies were uncontrolled, nonrandomized studies, and some were retrospective. In addition, none provide detailed information on long-term outcomes.
Who should receive ruxolitinib therapy?
Unlike the US indication of ruxolitinib, which favors a risk-based treatment model,[40] the European label, indicating ruxolitinib for patients with MF-related splenomegaly and/or symptoms, reflects the limitations of current risk models intended to classify patients based on their prognosis rather than capturing disease burden.[39] However, prognosis remains an important criterion when weighing ruxolitinib against other therapies, especially HCT as the only potentially curative treatment option. In this context, it is important to note that long-term follow-up of the ruxolitinib registration trials, COMFORT-I and COMFORT-II, demonstrated a ruxolitinib-associated survival advantage compared with placebo and standard therapy in patients with intermediate-2 or high-risk MF (by IPSS).[33,35,37,51] Although this survival benefit has not been investigated for patients with intermediate-1 risk MF, and its underlying mechanism is not entirely clear, the findings raise the possibility that early intervention has the potential to improve outcomes. The overall improvement in clinical status with ruxolitinib, including the reversal of cachexia and the mitigation of MF-related symptoms related to systemic inflammation,[16,28,30,52] likely contributed to the observed effects of ruxolitinib on survival, as weight loss, constitutional symptoms, and proinflammatory cytokines are known for their negative impact on prognosis.[18,53] In addition, long-term therapy with ruxolitinib may halt bone marrow fibrosis in some patients,[54] and is associated with gradual decreases in mutant allele burden.[35,55]
Of note, complete histopathologic and/or molecular remission has been observed in individual patients.[55–58] Together, these findings suggest that ruxolitinib has the potential to alter the natural history of the disease in some patients. However, although ruxolitinib-treated patients in the COMFORT studies who had greater allele burden reductions tended to also have greater reductions in spleen size,[35,55] allele burden reduction was not a prerequisite for clinical benefit.[28,29] The potential of ruxolitinib to prolong survival needs to be taken into account when weighing ruxolitinib as a treatment option against the background of current dynamic risk models [Table 1] because these models were developed before the era of JAK inhibitors and may not accurately capture risk in patients on ruxolitinib therapy.
The observations detailed above strongly argue for timely initiation of therapy in patients with intermediate- or high-risk MF to maximize the potential long-term benefits of ruxolitinib therapy. However, patient selection for ruxolitinib therapy and timing of intervention should not be based only on nominal risk scores, but on a careful evaluation of the entire benefit–risk ratio. This evaluation should consider all known risk factors, including, but not limited to, age and mutation status, the degree of clinical burden from splenomegaly and symptoms, the expected hematologic toxicity of treatment in patients with preexisting cytopenias, and the likelihood of treatment success, which appears to diminish with the presence of multiple mutations.[59] In those patients with intermediate-1 risk MF who are often in the proliferative phase of MF and have no clinically significant symptoms and no or only minor spleen enlargement, alternative treatments (eg, interferon-α) [60] may be considered.
Age is an important prognostic factor that affects treatment decisions. For example, watchful waiting rather than starting ruxolitinib therapy is likely to be appropriate for patients who are older than 65 years but have no other known risk factors and no clinically significant symptoms. In contrast, patients aged < 65 years with intermediate-1 risk MF by IPSS should be considered as candidates for HCT if they have refractory anemia requiring transfusions, > 2% of peripheral blasts, or detrimental cytogenetics.[22] Given the observation that median duration of spleen response in patients with intermediate- or high-risk MF has been estimated to be less than 4 years,[36,61] the risk of losing response should also be considered before deciding on the initiation of ruxolitinib therapy in younger patients with intermediate-1 risk MF who may also qualify for HCT.
The presence of anemia, or thrombocytopenia, may worsen with ruxolitinib therapy in some patients and thus could lead to a burden of treatment from adverse hematologic effects that needs to be weighed against the expected treatment benefit. However, anemia per se is not a contraindication for the use of ruxolitinib and often may be mitigated with red blood cell transfusions, dose adjustments, and/or erythropoiesis-stimulating agents.[32,62] Management of thrombocytopenia may be more difficult, requiring the use of defined starting doses and timely dose modifications or treatment interruptions based on platelet count.[32,40] Another factor to be considered during patient selection is the immunosuppressive properties of ruxolitinib,[63] as there have been reports of serious infections in patients receiving ruxolitinib therapy.[48,64–69] Thus, the risk of infections from comorbidities that compromise the immune system or from concomitant immunosuppressant therapies should be taken into account when considering ruxolitinib as a treatment option. In particular, safety warnings in the prescribing information should be consulted before considering ruxolitinib for patients suspected to be at risk of potentially dangerous infections, such as tuberculosis.[39,40]
Conclusions
Patients with intermediate-1 risk MF may have a substantial disease burden that requires treatment. Results from several independent studies confirm that the benefits of ruxolitinib established in the COMFORT studies in terms of spleen size reduction and symptom improvement extend to patients with intermediate-1 risk MF. Timely initiation of ruxolitinib therapy may not only improve patients’ overall clinical status but also maximize the disease-modifying potential of long-term therapy. However, the decision on and timing of intervention should be based on an overall assessment of prognostic and clinical factors and the expected benefit–risk ratio for each patient. Therefore, it is important to gain a better understanding of the mechanisms by which ruxolitinib affects prognosis, and, specifically whether ruxolitinib can improve long-term outcomes of patients with adverse genetic risk factors. To this end, a double-blind, placebo-controlled phase 3 study has been designed to evaluate the effects of ruxolitinib treatment in delaying disease progression in patients with early-stage nonsymptomatic-MF who have at least one high-risk mutation.[70]
Supplementary Material
Acknowledgments
Medical writing support was provided by Roland Tacke, PhD, of Evidence Scientific Solutions, Philadelphia, Pennsylvania, USA, and funded by Incyte Corporation.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at http://dx.doi.org/10.1080/10428194.2016.1195501.
References
- Reilly JT, McMullin MF, Beer PA, et al. Guideline for the diagnosis and management of myelofibrosis. Br J Haematol. 2012;158:453–471. doi: 10.1111/j.1365-2141.2012.09179.x. [DOI] [PubMed] [Google Scholar]
- Bose P, Verstovsek S. The evolution and clinical relevance of prognostic classification systems in myelofibrosis. Cancer. 2016;122:681–692. doi: 10.1002/cncr.29842. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Green A, Barosi G, et al. MPN-associated myelofibrosis (MPN-MF). Leuk Res. 2011;35:12–13. doi: 10.1016/j.leukres.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437–438. doi: 10.1038/sj.leu.2404914. [DOI] [PubMed] [Google Scholar]
- Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123:e123–e133. doi: 10.1182/blood-2014-02-554634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. . N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123:2220–2228. doi: 10.1182/blood-2013-11-537167. [DOI] [PubMed] [Google Scholar]
- Hobbs GS, Rampal RK. Clinical and molecular genetic characterization of myelofibrosis. Curr Opin Hematol. 2015;22:177–183. doi: 10.1097/MOH.0000000000000122. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Guglielmelli P, Lasho TL, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28:1494–1500. doi: 10.1038/leu.2014.57. [DOI] [PubMed] [Google Scholar]
- Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the Mayo Clinic experience. Mayo Clin Proc. 2012;87:25–33. doi: 10.1016/j.mayocp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa RA, Shields A, Hare T, et al. Progressive burden of myelofibrosis in untreated patients: assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study. Leuk Res. 2013;37:911–916. doi: 10.1016/j.leukres.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–4103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer HL, Dueck AC, Scherber RM, et al. Impact of inflammation on myeloproliferative neoplasm symptom development. Mediators Inflamm. 2015;2015:284706. doi: 10.1155/2015/284706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Guglielmelli P, Rotunno G, et al. Mutation-enhanced International Prognostic Scoring System (MIPSS) for primary myelofibrosis: an AGIMM & IWG-MRT project [abstract]. Blood. 2014;124:405. [Google Scholar]
- Tefferi A, Guglielmelli P, Finke C, et al. Integration of mutations and karyotype towards a Genetics-based Prognostic Scoring System (GPSS) for primary myelofibrosis [abstract]. Blood. 2014;124:406. [Google Scholar]
- Kroger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–2133. doi: 10.1038/leu.2015.233. [DOI] [PubMed] [Google Scholar]
- Viswabandya A, Devlin R, Gupta V. Myelofibrosis – when do we select transplantation or non-transplantation therapeutic options? Curr Hematol Malig Rep. 2016;11:6–11. doi: 10.1007/s11899-015-0296-8. [DOI] [PubMed] [Google Scholar]
- Zarzour A, Tabarroki A, Visconte V, et al. Burden of disease and clinical responses in low and intermediate-1 risk myelofibrosis patients treated with ruxolitinib [abstract]. Blood. 2014;124:1834. [Google Scholar]
- Mitra D, Kaye JA, Piecoro LT, et al. Symptom burden and splenomegaly in patients with myelofibrosis in the United States: a retrospective medical record review. Cancer Med. 2013;2:889–898. doi: 10.1002/cam4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. doi: 10.1182/blood-2011-01-328955. [DOI] [PubMed] [Google Scholar]
- Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014;123:3803–3810. doi: 10.1182/blood-2013-09-527903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31:1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CN, Mesa RA, Kiladjian JJ, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol. 2013;162:229–239. doi: 10.1111/bjh.12375. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. Onco Targets Ther. 2013;7:13–21. doi: 10.2147/OTT.S53348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98:1865–1871. doi: 10.3324/haematol.2013.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015;100:479–488. doi: 10.3324/haematol.2014.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- Harrison CN, Vannucchi AM, Kiladjian J-J, et al. Long-term efficacy and safety in COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for the treatment of myelofibrosis: 5-year final study results [abstract]. Blood. 2015;126:59. [Google Scholar]
- Passamonti F, Maffioli M, Cervantes F, et al. Impact of ruxolitinib on the natural history of primary myelofibrosis: a comparison of the DIPSS and the COMFORT-2 cohorts. Blood. 2014;123:1833–1835. doi: 10.1182/blood-2013-12-544411. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Kantarjian HM, Kiladjian JJ, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100:1139–1145. doi: 10.3324/haematol.2014.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . JAKAVI: summary of product characteristics. London: European Medicines Agency; 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002464/WC500133223.pdf [Google Scholar]
- Incyte Corporation . Jakafi (ruxolitinib) tablets [prescribing information] Wilmington (DE): Incyte Corporation; 2014. http://www.jakafi.com/pdf/prescribing-information.pdf [Google Scholar]
- Deisseroth A, Kaminskas E, Grillo J, et al. U.S. Food and Drug Administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012;18:3212–3217. doi: 10.1158/1078-0432.CCR-12-0653. [DOI] [PubMed] [Google Scholar]
- Barosi G, Agarwal M, Zweegman S, et al. An individual patient supply program for ruxolitinib for the treatment of patients with primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF), or post-essential thrombocythemia myelofibrosis (PET-MF) [abstract]. Blood. 2012;120:2844. [Google Scholar]
- Alzate MA, Mela Osorio MJ, Barreyro P, et al. Ruxolitinib In myelofibrosis (MF) patients through compassionate use program (CUP). Argentinian experience [abstract] Blood. 2013;122:5247. [Google Scholar]
- Jabbour E, Kantarjian HM, Garcia-Manero G, et al. Outcome of patients (pts) with myelofibrosis (MF) after ruxolutinib (Rux) therapy [abstract]. Blood. 2013;122:1584. [Google Scholar]
- Tavares R, Palumbo GA, Le Coutre P, et al. Safety and efficacy of ruxolitinib in an 1869-patient cohort of JUMP: an open-label, multicenter, single-arm, expanded-access study in patients with myelofibrosis [abstract]. Blood. 2015;126:2799. [Google Scholar]
- Giraldo P, Palandri F, Palumbo GA, et al. 2015;100:265. [Google Scholar]
- Mead AJ, Milojkovic D, Knapper S, et al. Response to ruxolitinib in patients with intermediate-1-, intermediate-2-, and high-risk myelofibrosis: results of the UK ROBUST Trial. Br J Haematol. 2015;170:29–39. doi: 10.1111/bjh.13379. [DOI] [PubMed] [Google Scholar]
- Wathes R, Moule S, Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N Engl J Med. 2013;369:197–198. doi: 10.1056/NEJMc1302135. [DOI] [PubMed] [Google Scholar]
- Davis KL, Cote I, Kaye JA, et al. Real-world assessment of clinical outcomes in patients with lower-risk myelofibrosis receiving treatment with ruxolitinib. Adv Hematol. 2015;2015:848473. doi: 10.1155/2015/848473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpaz M, Paquette R, Afrin L, et al. Interim analysis of safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts. J Hematol Oncol. 2013;6:81. doi: 10.1186/1756-8722-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas J, Hoffman R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood. 2013;121:4832–4837. doi: 10.1182/blood-2013-02-482232. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Verstovsek S, Gupta V, et al. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with myelofibrosis from COMFORT-I. Clin Lymphoma Myeloma Leuk. 2015;15:214–221. doi: 10.1016/j.clml.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Vaidya R, Caramazza D, et al. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. Exploratory analysis of the effect of ruxolitinib on bone marrow morphology in patients with myelofibrosis. J Clin Oncol. 2013;31:7030. [Google Scholar]
- Deininger M, Radich J, Burn TC, et al. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015;126:1551–1554. doi: 10.1182/blood-2015-03-635235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BS, Radia D, Woodley C, et al. Resolution of bone marrow fibrosis in a patient receiving JAK1/JAK2 inhibitor treatment with ruxolitinib. Haematologica. 2013;98:1872–1876. doi: 10.3324/haematol.2013.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molica M, Serrao A, Saracino R, et al. Disappearance of fibrosis in secondary myelofibrosis after ruxolitinib treatment: new endpoint to achieve? Ann Hematol. 2014;93:1951–1952. doi: 10.1007/s00277-014-2096-y. [DOI] [PubMed] [Google Scholar]
- Al-Ali HK, Hubert K, Lange T, et al. Complete clinical, histopathologic and molecular remission of primary myelofibrosis with long-term treatment with the JAK1/2 inhibitor ruxolitinib [abstract]. Blood. 2014;124:1836. [Google Scholar]
- Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126:790–797. doi: 10.1182/blood-2015-03-633404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianotto JC, Boyer-Perrard F, Gyan E, et al. Efficacy and safety of pegylated-interferon α-2a in myelofibrosis: a study by the FIM and GEM French cooperative groups. Br J Haematol. 2013;162:783–791. doi: 10.1111/bjh.12459. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120:1202–1209. doi: 10.1182/blood-2012-02-414631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin MF, Harrison CN, Niederwieser D, et al. The use of erythropoiesis-stimulating agents with ruxolitinib in patients with myelofibrosis in COMFORT-II: an open-label, phase 3 study assessing efficacy and safety of ruxolitinib versus best available therapy in the treatment of myelofibrosis. Exp Hematol Oncol. 2015;4:26. doi: 10.1186/s40164-015-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122:1192–1202. doi: 10.1182/blood-2013-03-484642. [DOI] [PubMed] [Google Scholar]
- Shamil E, Cunningham D, Wong BL, et al. Ruxolitinib associated tuberculosis presenting as a neck lump. Case Rep Infect Dis. 2015;2015:284168. doi: 10.1155/2015/284168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest. 2013;143:1478–1479. doi: 10.1378/chest.12-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmason R, Linden O, Richter J. Case-report: EBV driven lymphoproliferative disorder associated with ruxolitinib. BMC Hematol. 2015;15:10. doi: 10.1186/s12878-015-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Feenstra J, Georghiou PR. Pneumocystis jiroveci pneumonitis complicating ruxolitinib therapy. BMJ Case Rep. 2014;2014:204950. doi: 10.1136/bcr-2014-204950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong LX, Jackson J, Kerstetter J, et al. Reactivation of herpes simplex virus infection in a patient undergoing ruxolitinib treatment. J Am Acad Dermatol. 2014;70:e59–e60. doi: 10.1016/j.jaad.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Caocci G, Murgia F, Podda L, et al. Reactivation of hepatitis B virus infection following ruxolitinib treatment in a patient with myelofibrosis. Leukemia. 2014;28:225–227. doi: 10.1038/leu.2013.235. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Kiladjian J-J, Vannucchi AM, et al. J Clin Oncol (suppl) 2016;34:TPS7080. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.