Abstract
Purpose: Duchenne muscular dystrophy (DMD) is a rapidly progressive neuromuscular disorder causing weakness of the skeletal, respiratory, cardiac and oropharyngeal muscles with up to one third of young men reporting difficulty swallowing (dysphagia). Recent studies on dysphagia in DMD clarify the pathophysiology of swallowing disorders and offer new tools for its assessment but little guidance is available for its management. This paper aims to provide a step-by-step algorithm to facilitate clinical decisions regarding dysphagia management in this patient population.
Methods: This algorithm is based on 30 years of clinical experience with DMD in a specialised Centre for Neuromuscular Disorders (Inkendaal Rehabilitation Hospital, Belgium) and is supported by literature where available.
Results: Dysphagia can worsen the condition of ageing patients with DMD. Apart from the difficulties of chewing and oral fragmentation of the food bolus, dysphagia is rather a consequence of an impairment in the pharyngeal phase of swallowing. By contrast with central neurologic disorders, dysphagia in DMD accompanies solid rather than liquid intake. Symptoms of dysphagia may not be clinically evident; however laryngeal food penetration, accumulation of food residue in the pharynx and/or true laryngeal food aspiration may occur. The prevalence of these issues in DMD is likely underestimated.
Conclusions: There is little guidance available for clinicians to manage dysphagia and improve feeding for young men with DMD. This report aims to provide a clinical algorithm to facilitate the diagnosis of dysphagia, to identify the symptoms and to propose practical recommendations to treat dysphagia in the adult DMD population.
Implications for Rehabilitation
Little guidance is available for the management of dysphagia in Duchenne dystrophy.
Food can penetrate the vestibule, accumulate as residue or cause aspiration.
We propose recommendations and an algorithm to guide management of dysphagia.
Penetration/residue accumulation: prohibit solid food and promote intake of fluids.
Aspiration: if cough augmentation techniques are ineffective, consider tracheostomy.
Keywords: Duchenne, dysphagia, swallowing
Introduction
Duchenne muscular dystrophy (DMD) is a rapidly progressive neuromuscular disorder (NMD). With increasing age, DMD progressively affects skeletal, respiratory and cardiac muscles. Without assisted ventilation, death is predictable before 25 years of age.[1] The combination of cardiac, respiratory and orthopaedic care prolongs survival probability until 30–35 years of age.[2,3] During chest infections, ageing DMD patients have increasing difficulty managing airway encumbrance and clearance. In addition, oropharyngeal muscles weaken with age and young men experience increasing difficulties with swallowing which can worsen the condition of these patients.[4] Our own experience has shown that swallowing saliva can be challenging for patients with DMD, irrespective of the presence of chest infections.
The term dysphagia refers to difficulty with swallowing and covers all the presenting symptoms in patients in whom swallowing function is impaired. Dysphagia may have various neurologic aetiologies,[5] mainly central (e.g. Stroke, Parkinson, Multiple Sclerosis, Cerebral Palsy) or neuromuscular (e.g. DMD). The central forms of dysphagia are related to poor neurological coordination of swallowing function which is often a risk factor for aspiration during fluid intake.[6] Instead the neuromuscular form of dysphagia present in DMD results from progressive muscle weakness and accompanies solid rather than liquid intake.[5,7] In these patients, the laryngeal innervation by cranial nerves IX, XI and XII remains intact.
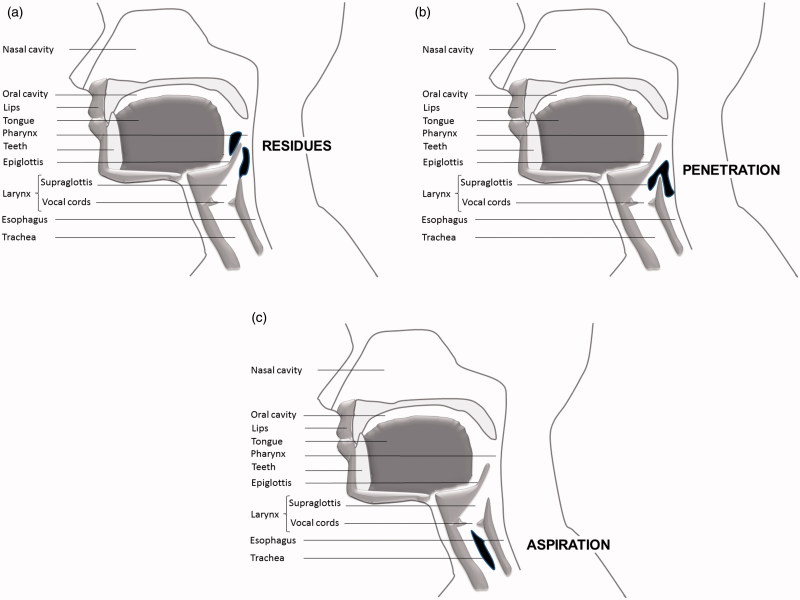
Swallowing is a complex physiological mechanism of which the primary function is to protect the lungs from any food or saliva intrusion.[8] Swallowing conventionally includes three successive stages: the oral stage (bolus chewing and fragmentation), the pharyngeal stage (bolus transition from the mouth to the upper opening of the oesophagus) and the oesophageal stage (bolus progression towards the stomach). Depending on the severity of the impairment, the bolus can meet unrelaxed muscles (pain), stop in the throat (blocking) or be directed to the airways causing post swallowing accumulation in the pharynx (piriform sinus, above the oesophageal sphincter or at the pharyngeal wall) which is termed ‘residue’ (Figure 1a), laryngeal vestibule penetration (Figure 1b), or, worse, reach the subglottic area and induce laryngeal aspiration and asphyxiation (Figure 1c) with or without cough reflex. Swallowing difficulties may also lead to decreased oral intake causing weight loss that can be spectacular in end-stage DMD patients.[9]
Figure 1.
Oropharynx lateral view. (a) Accumulation of residues. (b) Penetration of bolus. (c) Aspiration of bolus.
Studies on swallowing difficulties in patients with NMDs are relatively recent.[5–12] These studies clarify the pathophysiology of swallowing disorders and offer new tools for the assessment of dysphagia and its complications such as weight loss and aspiration. However, there is little guidance available for the management of dysphagia in NMD’s. With increasing survival, the population of DMD is changing and the challenging management of dysphagia is a prominent feature.
The Neuromuscular Excellency Centre in Inkendaal Rehabilitation Hospital (Belgium) takes care of almost 20% of all Belgian boys with DMD. Inkendaal specialises in NMDs in advanced stages of progression and has managed dysphagia in patients with NMDs from the 1980s. In 2013, Inkendaal cared for 205 patients with NMDs out of which 48 were patients with DMD (mean age: 26.7 ± 7.1 years) using respiratory support. Among this group, 32 used ventilation 24/24 h and a majority of them required a specific management of dysphagia.
The purpose of the current article is to provide a clear algorithm to facilitate clinical decisions regarding dysphagia management in patients with DMD. This algorithm is based on our 30 years of experience with DMD at Inkendaal Rehabilitation Hospital and is supported by relevant evidence where available.
Diagnosis of dysphagia
Dysphagia is common in patients with NMDs. However, the timing of occurrence and evolution is poorly documented. Dysphagia affects about one third of all patients with NMDs.[9] In addition to increasing difficulties with mastication and oral fragmentation of the food bolus with disease progression leading to prolonged meals, dysphagia is mainly a consequence of an impairment in the weak pharyngeal phase of swallowing: the weak tongue and pharyngeal muscles lead to slower and effortful bolus transportation,[10,11] poor muscle synchronisation and reduced breathe–swallow interaction.[12] In addition, muscles from the submental group are also affected, leading to reduced opening of the upper oesophageal sphincter. The pharyngeal phase is generally well triggered, but clearance is incomplete and leaves pharyngeal residue (Figure 1). Residue increases the risk of aspiration pneumonia.[10] Dysphagia and aspiration are reported to increase in DMD with age and can interfere with quality of life.[13]
Symptoms of dysphagia
The experience of symptoms is sufficient to state the presence of dysphagia.[10] Complaints include discomfort during swallowing, sensation of food blocked in the throat, difficulty swallowing saliva, coughing during and after meals,[14] increased meal duration,[15] difficulty starting swallowing,[7] loss of appetite, unintentional weight loss, and increased occurrence of chest infections and choking episodes.[16,17]
It is important to systematically question patients on their potential symptoms because patients will not report them spontaneously.[18] This is an advantage of questionnaires such as the Sydney Swallowing Questionnaire (SSQ), which assesses the presence of dysphagia based on 17 questions (http://stgcs.med.unsw.edu.au/stgcsweb.nsf/resources/SSQ/$file/SSQ.pdf). The SSQ is useful because it is simple to conduct, quickly performed in 10 min and validated in patients with DMD.[7] In general, a videofluoroscopic swallow study (VFSS), a manometry and a fiberoptic evaluation of swallowing (FEES) solely confirm and quantify the expected diagnosis of dysphagia.[10] These tests are expensive and provide a single snapshot of the situation. However, in case of choking episodes, VFSS may distinguish supraglottic penetration from subglottic aspiration (Figure 1). Because of the danger associated with their use in weak DMD patients at risk of aspiration, these examinations must take place in a specialised unit where effective cough assistance techniques are available.
As with all elements of care for patients with DMD, multidisciplinary team management is essential to the optimal management of dysphagia.[19] Health professionals that should be considered as a part of the multidisciplinary team include: the patient’s primary physician, physiotherapist, speech pathologist and dietitian. Medical specialties may also need to be included at different times such as respiratory, Ear Nose Throat and gastroenterology.
Algorithm to manage dysphagia
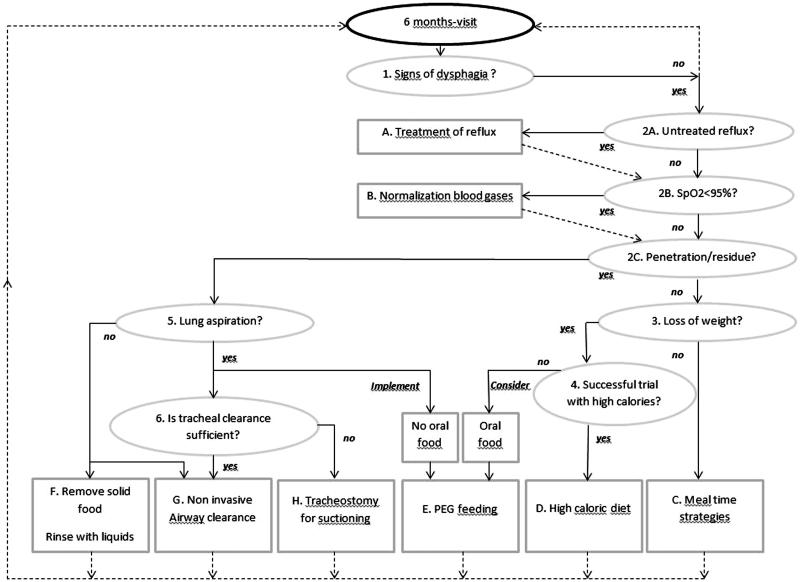
Proposed management strategies for dysphagia may vary considerably as they should be tailored according to the symptoms identified. Accordingly, we have developed an algorithm with a step-by-step set of operations leading to a well-defined decision to include the factors triggering dysphagia and its management. A decision-making-tree algorithm was considered by our team to fit with the complex problem of dysphagia in patients affected by DMD. This algorithm is presented in Figure 2. It is especially designed for late non-ambulatory patients. It consists of two phases. The first phase includes factors triggering or perpetuating dysphagia (item 1–6). Each item from 1 to 6 requires a single answer – yes or no. In the second phase, each of the six items from phase 1 is followed until a specific treatment (A to H) is proposed. Once the treatment is implemented, reading the algorithm will recommence from item 1 for ongoing evaluation.
Figure 2.
Clinical algorithm to guide management of dysphagia in the DMD population. See explanations in the text.
Factors triggering dysphagia
The first phase of algorithm presented in Figure 2 follows three steps: the suspicion of dysphagia (item 1), the suspicion of extrinsic factors triggering or perpetuating dysphagia (items 2A and B) and finally the evaluation of its severity (item 2C–item 6).
Item 1. Is there dysphagia?
An assessment of late non-ambulatory patients with DMD is desirable every 6 months. On this occasion patients will be questioned by the speech therapist or the dietician on signs of possible dysphagia and the SSQ will be completed.[7] The SSQ threshold value of 224.50/1700 points suggested by Archer et al. [7] discriminates between patients with dysphagia from those without. If dysphagia is present, the algorithm recommends going to item 2A, B and C.
Item 2A. Is there untreated gastroesophageal reflux?
When dysphagia is identified, it is necessary to exclude the presence of gastroesophageal reflux (GER). GER is frequent in wheelchair-bound patients with NMDs. Prevalence of reflux in large series of patients with DMD is not available. In one study, clinical signs of GER requiring treatment were found in 4% of DMD, mainly after 18 years of age and slightly increasing with age.[15] The main cause is the long term sitting position that induces a permanent pressure gradient between the abdomen content and the thoracic one, combined with the progressive degradation of the diaphragmatic muscular tone. Gastric hypomobility and delayed gastric emptying may also contribute to GER or exacerbate it.[20–22] Consequences can be purely oesophageal leading to disease (painful erosions, bleeding, peptic ulcer, Barrett's disease), or be painful or painless full-channel reflux, triggering laryngeal food floods with possible risks for aspiration. GER may be evident through subtle signs such as early satiety or progressive lack of appetite and decreased intake of food. The diagnosis is made via 24 h pH monitoring. The choice for this technique was made according to the preference in our centre for minimally invasive techniques for our patients. It is recommended to treat GER if present (Figure 2, treatment A) and to reach item 2B and 2C that are investigated during the same visit at hospital.
Item 2B. Is there any oxygen desaturation over 24 h?
After treating GER or in the absence of reflux, it is also necessary to ensure that blood oxygenation measured by pulse oxymetry (SpO2) is normal over 24 h. Any O2 desaturation (SpO2 < 95%), especially after meals, may trigger or exacerbate dysphagia when already present sub-clinically. Indeed, prolonged meals cause fatigue of oropharyngeal muscles involved in swallowing resulting in increased risk for aspiration. Terzi et al. showed that patients with low lung capacity reduce the time of expiratory apnoea after swallowing. Reduced expiratory apnoea triggers early inspiration which, in turn, increases the risk for aspiration and O2 desaturation.[12] This risk is further increased in the presence of pharyngeal residue.[23] In patients with normal SpO2 but who are complaining about dyspnoea and respiratory fatigue during the daytime,[24] treatment B should be considered to rest respiratory and oropharyngeal muscles. In the absence of diurnal dyspnoea and abnormal SpO2, the algorithm suggests assessing the severity of dysphagia (item 2C).
Item 2C. Is there a glottis penetration or accumulation of residue?
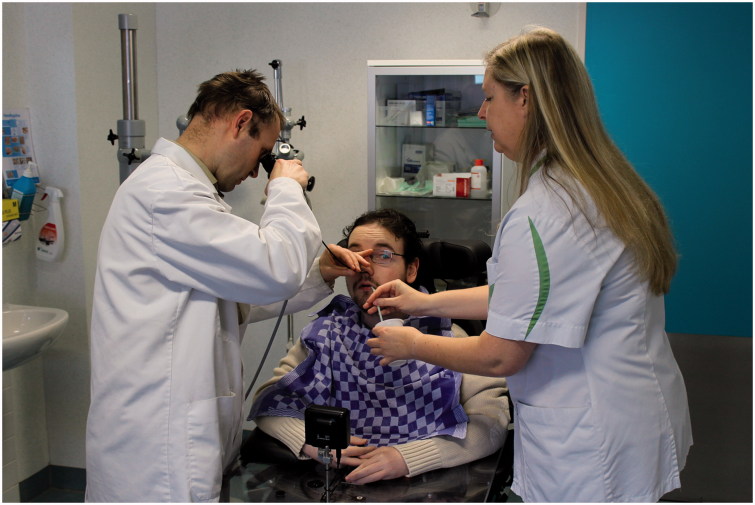
The degree of severity of dysphagia is related to the path taken by the food during and after swallowing. Penetration, accumulation of residue or aspiration may occur (Figure 1). Symptoms of penetration and accumulation of residue may be scarce. However, a positive SSQ (>224.50 points), and symptoms of coughing during and after meals and/or sensation of food blocked in the throat should result in a review by a speech therapist and a FEES by an Ear Nose Throat specialist (Figure 3). Regarding the use of VFSS, Aloysius et al. [10] suggest that this examination may not be of conclusive benefit in the observation of patients with feeding/swallowing difficulties. Accordingly, we do not systematically propose a VFSS. However, when facing uncertain diagnosis of dysphagia, VFSS may contribute to a better understanding of reported swallowing difficulties. Nevertheless, when symptoms are clear and evident, VFSS will only confirm a well-established diagnosis.
Figure 3.
Duchenne patient during FEES by an Ear Nose Throat specialist and speech therapist giving different mixtures of food and liquids.
The bolus may enter the glottis and reach the vestibule above the vocal cords (penetration of the vestibule). Penetration can be transient (termed ‘flash penetration’) or chronic. When the sensitivity of the vocal cords is preserved, penetration of food will trigger coughing. Food residue may also accumulate in the piriform sinuses [8] which are small pockets located at both sides of the supraglottic area. Both supraglottic penetration and accumulation of residue are risk factors for random episodes of silent aspiration with neither choking nor coughing. Silent aspiration may occur hours after meals, often during sleep. It is common and dramatically underdiagnosed in late stage patients with DMD and likely represents an important cause of sudden death and chest infection in the adult population. If symptoms indicate penetration and/or accumulation of residue, the algorithm suggests assessment of potential aspiration (item 5). If penetration does not appear to be occurring or if penetration is already treated and solved, go to item 3 to review body weight history.
Item 3. Is there unintentional loss of weight?
There are likely several causes of unintentional weight loss in late non-ambulatory patients with DMD of which dysphagia is one. Older patients may in fact be hypercatabolic with increased energy requirements.[25] This effect may be negated by the presence of respiratory support; reduced energy expenditure has been documented in patients with DMD receiving home mechanical ventilation.[26] Previous weight history should be considered: boys who are normal or underweight at 13 years of age are more likely to be underweight later in life.[27] Surgery may also impact on nutritional status; however, weight loss after surgery has been associated with loss of ability to self-feed.[28] The impact of muscle wasting and changes in energy needs on weight loss is clearly accentuated by increased difficulties with eating and swallowing.
It is essential to weigh patients during each visit under the same conditions (similar clothing, possibly in the wheelchair). If a patient is weighed in his chair, remove all additional items and ensure that no major modifications have been made to the chair since the patient’s last weight. If weight has remained stable, the algorithm suggests treatment C. If unintentional weight loss has occurred, move to item 4. Body mass index can also be monitored using weight and height with height estimated from measurement of ulnar length using the equation by Gauld et al.[29] However, body mass index cut-offs likely do not apply due to unique changes in body composition associated with DMD.[30] Instead, serial measures of weight or body mass index will be most informative.
Item 4. Is a trial with high calories desirable and effective?
An unintentional loss of weight can be a sign of dysphagia. In our experience, unintentional loss of weight greater than 10% in a year is considered as clinically significant. However, depending on the initial morphology of patients, this amount of weight loss can be significant or not. Overweight or obese patients may have adequate fat reserves and a diet with high calorie fluids may be tried especially if the patient still has a good appetite. Underweight patients will have limited fat reserves and may require more aggressive nutrition therapy. Regardless of initial morphology, any patient presenting with unintentional weight loss should undergo a thorough nutrition assessment by a dietitian. The dietitian should consider weight history, current oral intake and recent changes to intake, appetite, length of meals, gastrointestinal symptoms and other markers of nutritional status. This assessment will then provide advice on whether a high caloric diet may be implemented or if the Percutaneous Endoscopic Gastrostomy (PEG) catheter should be considered as a priority (treatment E). If the trial is conclusive, treatment D can be implemented. If the trial is ineffective and weight loss continues, the placement of a PEG can be considered (treatment E). Importantly in both cases, oral feeding and drinking is still allowed but appropriate texture modification of food should be utilised.
Item 5. Is there lung aspiration?
Aspiration reflects the passage of a bolus through the vocal cords into the subglottic floor (Figure 2C). Once the bolus has progressed to this point, no anatomical structure can prevent its progression to the lungs. Whatever the origin of airway intrusion, i.e. food, saliva or stomach contents from GER, the risk for bacterial/chemical pneumonia is high. If the presence of aspiration is unclear, a VFSS can be conducted to distinguish true aspiration from penetration and accumulation of residues. When aspiration is evident, the algorithm suggests moving to item 6 to investigate the use of effective cough assistance techniques. In the absence of aspiration, treatment F and G should be utilised. In this case, DMD patients may eat normally but carefully. Nevertheless, according to Aloysius [10] and Van den Engel,[14] it is advised to stop solid food, to promote pureed meals and to rinse the throat regularly during and after meals with an appropriate amount and type of fluid to avoid exacerbation of reflux and decrease in nutrient intake. For example, patients who are underweight would benefit from a calorie-dense liquid whilst water is appropriate for overweight patients.
Item 6. Is tracheal clearance sufficient?
The effectiveness of instrumental and non-instrumental non-invasive tracheal clearance techniques should be evaluated. When aspiration is evident, the choice of the fastest and most effective technique must be made: manual chest compression and air-stacking in combination or mechanical insufflation-exsufflation (MI-E) technique type Cough-Assist®,[31] particularly in the weakest patients or in those patients who cannot cooperate enough to obtain cough effectiveness with simple techniques.[13] Figure 2 suggests testing all non-invasive cough augmentation techniques (treatment G). Nevertheless, when techniques become ineffective and inadequate, it is essential to consider a permanent tracheal opening (tracheostomy) to ensure adequate clearance in any circumstances in the future (treatment H).
Practical recommendations to treat dysphagia
The second phase of algorithm presented in Figure 2 suggests available management strategies according to the severity of the dysphagia as previously described in the different items of the algorithm. These are described in detail below. After each management strategy is investigated, the clinician should return to item 1 to evaluate its effectiveness and to consider possible persisting dysphagia.
Treatment of gastro-oesophageal reflux
When GER is present and untreated (item 2A: yes), the treatment is threefold. The first point is positioning, i.e. a reverse Trendelenburg position in bed and, during the daytime, a sitting position in the wheelchair that avoids epigastric pressures during meals. The second point is medication. Tensioactive drugs such as sodium alginate (E401) may be proposed during meals, limiting any bolus reflux into the oesophagus. The third point is the administration of additional oral proton pump inhibitors (PPIs, type omeprazole) to reduce gastric acidity. The use of anti-reflux surgery (e.g. Nissen fundoplication) is rarely necessary in patients with NMDs. When needed, the laparoscopic approach has greatly improved the feasibility of this technique in the weaker patients. Small frequent meals and limiting large volumes of fluid around meal ties can be encouraged.
Blood gas normalization
When O2 desaturation defined by SpO2 < 95% is present (item 2B: yes), an adequate interpretation requires additional carbon dioxide tension (pCO2) monitoring. Any O2 desaturation with normal pCO2 (pCO2 < 50 mmHg) generally reflects airway encumbrance and requires appropriate airway clearance techniques. Percussive ventilation may be offered for distal airway clearance [13] while cough augmentation techniques are useful to provide upper airway clearance.[32] High CO2 tension (pCO2 > 50 mmHg) accompanying O2 desaturations reflects alveolar hypoventilation requiring implementation, improvement or extension of non-invasive ventilation (NIV). The improvement of NIV at night assists in minimising leakage and optimising the synchronisation between patient and ventilator. Adaptation of the titration of NIV with time may be helpful by increasing the pressure, the inspiratory time and the rate of the ventilator.
At a late stage of disease progression, the extension of nocturnal NIV into the daytime reduces dyspnoea, restores respiratory muscle endurance [24] and improves eating.[33] Physiologically, normal swallows are accompanied by a brief apnoea and followed by expiration and delay of the next breath. At the time of extension of nocturnal ventilation into the daytime, very weak dyspneic patients with DMD often complain of difficult swallow. The structure of swallow is disturbed and post-swallow apnoea is suppressed in 50% of the cases.[12] This is accompanied by a loss of appetite and weight. Interestingly, after some months of daytime ventilation, patients recover appetite and weight.[33,34] During the daytime, patients may use mechanical ventilation with tracheostomy, nasal mask or mouthpiece. Terzi et al. have shown that swallowing parameters are better in tracheostomized patients when they are under mechanical ventilation than in spontaneous breathing. Swallow is less fragmented and is again followed by apnoea before next breath, leading to a better swallow/nutrition and to a lower risk for aspiration. When using NIV via nasal mask, patients also better swallow [35] on the condition that any autotriggering of the ventilator can be suppressed. Patients using nasal NIV during chewing improved fatigue during meals. The authors conclude that dyspnoea and swallow are both improved with NIV via nasal mask. As opposed to the previous interface, no data is available regarding swallowing during ventilation via a mouthpiece. Our experience with a large population of adult patients with DMD shows that patients use mouthpiece during meals, especially during chewing to avoid fatigue. However, patients must pay attention to avoid aspiration during deglutition. We have observed that patients are able to manage perfectly. They are able to actively make leaks around their lips to avoid the positive pressures from the ventilators pushing the bolus down into the pharynx.[34]
Meal time strategies
In patients with difficulty swallowing but without either patent penetration (item 2C: no) or loss of weight (item 3: no), the diet may remain nutritionally balanced. However, the presentation of the meals may be modified to allow for easier swallowing by reducing the efforts of chewing and transporting the bolus. The speech pathologist or dietitian should advise on appropriate texture modifications for each patient (e.g. minced, mashed or pureed consistency). When recommending texture modifications, health professionals should also consider the presence of malocclusion and/or macroglossia which can be more common in patients with DMD.[36,37] These dentofacial characteristics can reduce tongue movements and impact on the transport of the bolus to the pharynx.[38] In patients with DMD, diet texture has been associated with maximum tongue pressure, whereby the lower the tongue pressure the less solid the diet.[23] In addition, small, calorie rich and frequent meals (e.g. six small meals per day) can be recommended to reduce meal time and patient effort. Rinsing the throat with water should be suggested after each meal.[14]
High-caloric diet
For patients with unintentional weight loss <10%/year (item 4: no), a high-caloric diet is proposed by the dietitian aiming at providing maximal calories in a minimal volume of food and drinks. A full nutrition assessment should be completed at this stage by a dietitian. High protein and energy food fortification strategies should be utilised. Also, commercial products may be convenient dietary additions to increase caloric intake. There are many products available on the market such as ready to drink or powdered milk- or juice-based supplements, as well as high energy dessert products and meal additives. The dietitian should advise on suitable products available locally for each patient. In addition to a high caloric diet, meal time strategies described in treatment C should also be considered to promote increased intake.
Gastrostomy
The timing of PEG placement should be informed by the success of the trial of a high caloric diet for 6 months, where success is defined as either a stabilisation of or gain in body weight. The degree of respiratory impairment and cardiac dysfunction should also be considered. In accordance with Birnkrant et al.,[39] the provision of early and proactive information about PEG placement to patients and their family is important and useful, just as information regarding electronic powered wheelchair or ventilatory and cardiac support is provided.
Ultimately, PEG may be considered when a high caloric diet trial is unsuccessful (item 4: no). The trial is considered as unsuccessful when the weight still decreases with high calories. At this time, oral food and drinks are allowed with the exception of solid (including minced and mashed) food. The catheter of gastrostomy, provided with its protection button, can be inserted by external surgery for which general anaesthesia is required. Fortunately, PEG placement at the bedside is possible and desirable. PEG placement requires only local anaesthesia of the pharynx, a very mild sedation and NIV during the procedure.[39] Initially assisted by Domperidone® which increases the transit of food through the stomach, feeding with PEG is ideally nocturnal which allows low flow filling of the stomach (1 L/8 h). The type of formula is the same as the formula given via nasogastric tube. An adult DMD uses a mean 1–1.5 L/24 h but this can be titrated with the volume of food and drink taken orally. When the decision to place the PEG tube is evident, nasogastric tube feeding, such as ultra-thin mini-type probe, is proposed (e.g. Nutricia, Flow Care, No. 35219, 6-8-10 c). After a few weeks, a PEG is placed with the advantage of its discretion and hygiene. In addition, oral feeding can be maintained but without the social pressure or the need for performance. PEG is reported as an effective intervention to improve weight status and to decrease the rate of chest infections [40,41] and is generally well tolerated although complications such as peritonitis are reported in many patients.[40] Nevertheless no mortality is reported.[41,42] When aspiration is evident (item 5: yes) and PEG feeding is dictated by the critical need to protect the lungs from inappropriate intrusion of food, normal feeding is prohibited and the whole volume of food-drinks is given via PEG catheter. In patients with tracheostomy presenting with severe aspiration, lungs can be protected by inflating the cuff.
Suggest liquid rather than solid food
When penetration and accumulation of residues are frequent (item 2C: yes) but without aspiration (item 5: no), the speech therapist and the dietitian should suggest patients permanently stop solid food including food of minced and mashed consistency. Based on the experience of managing dysphagia in those patients affected by cerebral palsy, thickening fluids has also been used to manage dysphagia in DMD for many years. However, recent studies clearly suggest that, in contrast with previous recommendations,[43] thickening fluids is probably not appropriate in DMD or in other NMDS.[5] This is because thick liquid and solid foodstuffs may actually enhance oral phase problems and accumulation of pharyngeal residue. In patients with DMD who experience penetration/accumulation of residue but without aspiration providing food of pureed consistency and drinking water during and after meals should improve swallowing and clear oropharyngeal residues.[14] These new recommendations should decrease a great number of chest infections due to invasion of debris from the piriform sinus and the pharynx during and after meals.
It must be noted that non-surgically aligned scoliosis may lead to reclining sitting positions during meals. This may contribute to worsening dysphagia which requires food texture modifications as previously mentioned. In our experience, a systematic screening of scoliosis led to early spine surgery in our patients with DMD for more than 30 years so that reclining positions during the daytime are avoid.[44] A recent study also suggests that the long-term use of the glucocorticoid in patients with DMD results in a substantial decrease in the prevalence of scoliosis.[45]
Non-invasive tracheal clearance techniques
When aspiration is evident (item 5; yes) and non-invasive tracheal clearance techniques are available and effective (item 6: yes), they should be used.[13] Inspiratory aids such as air-stacking and expiratory aids such as manual chest compression alone or in combination are often effective.[32] Mechanical insufflation–exsufflation (MI–E) is the first option in the weakest DMD patients. MI–E may be implemented within the home environment and taught to the family and caregivers to ensure effective airway clearance at all times.
Tracheostomy
When aspiration is evident, recurrent and substantial (item 5: yes), and when non-invasive cough augmentation techniques no longer effectively remove food, secretions, saliva and other debris from the lungs (item 6: no), protecting the lungs becomes essential. Placement of a tracheostomy can be considered to provide easy suctioning of material that has passed through the vocal cords. Effective suctioning, also possibly through mechanical insufflation/exsufflation via tracheostomy,[46] should prevent further penetration of debris into the lungs. Tracheostomy that includes definitive suture of the edges of the stoma is preferable to tracheotomy to prevent accidental reclosing of the stoma during decannulation. Furthermore, uncuffed tubes of tracheostomy are preferred to cuffed tubes to allow easy speaking that can be improved with the addition of a speaking valve.[47] Use of tracheostomies varies internationally. Current best practise guidelines for management of patients with DMD [19] support the use of tracheostomy when there is ‘failure of non-invasive methods of cough assistance to prevent aspiration of secretions into the lung and drops in oxygen saturation below 95% or the patient’s baseline, necessitating frequent direct tracheal suctioning via tracheostomy’. There are substantial risks and comorbidity associated with a tracheostomy so it should be considered a last alternative.[48] In addition, social integration appears worse when the tracheostomy is present. Non-invasive techniques of ventilation and airway clearance should always be used when possible. In our experience, few patients required the placement of a tracheostomy during the last decade.
Conclusions
Difficulty swallowing can worsen the condition of ageing patients with DMD. Symptoms of dysphagia must be actively sought and investigated. Food can penetrate the vestibule, accumulate as residue in the piriform sinus or cause subglottic aspiration. In the case of penetration and accumulation of residue, solid, minced and mashed food should be prohibited and fluids and purees should be promoted. In the case of aspiration, food should be prohibited and PEG placement considered. When cough augmentation techniques are ineffective, tracheostomy may be considered to provide direct suctioning in the trachea. In the management of dysphagia in DMD, it may be challenging to identify the appropriate therapeutic approach to provide optimal management given the underlying disease. Suitable treatments should be carefully chosen in order to advantageously improve the quality of life of adults with Duchenne.
Acknowledgments
Declaration of interest
There is no conflict of interest for the authors of this manuscript. The current authors did not receive any funding for this work.
References
- Rideau Y, Jankowski LW, Grellet J. Respiratory function in the muscular dystrophies. Muscle Nerve. 1981;4:155–164. doi: 10.1002/mus.880040213. [DOI] [PubMed] [Google Scholar]
- Kohler M, Clarenbach CF, Bahler C, et al. Disability and survival in Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2009;80:320–325. doi: 10.1136/jnnp.2007.141721. [DOI] [PubMed] [Google Scholar]
- Eagle M, Bourke J, Bullock R, et al. Managing Duchenne muscular dystrophy – the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord. 2007;17:470–475. doi: 10.1016/j.nmd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Baiardi P, Khirani S, et al. Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. Am J Phys Med Rehabil. 2012;91:783–788. doi: 10.1097/PHM.0b013e3182556701. [DOI] [PubMed] [Google Scholar]
- Engel-Hoek LV, Erasmus CE, Hulst KC, et al. Children with central and peripheral neurologic disorders have distinguishable patterns of dysphagia on videofluoroscopic swallow study. J Child Neurol. 2014;29:643–653. doi: 10.1177/0883073813501871. [DOI] [PubMed] [Google Scholar]
- Erasmus CE, van Hulst K, Rotteveel JJ, et al. Clinical practice: swallowing problems in cerebral palsy. Eur J Pediatr. 2012;171:409–414. doi: 10.1007/s00431-011-1570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SK, Garrod R, Hart N, et al. Dysphagia in Duchenne muscular dystrophy assessed by validated questionnaire. Int J Lang Commun Disord. 2013;48:240–246. doi: 10.1111/j.1460-6984.2012.00197.x. [DOI] [PubMed] [Google Scholar]
- Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14:118–127. doi: 10.1002/ddrr.17. [DOI] [PubMed] [Google Scholar]
- Willig TN, Paulus J, Lacau Saint Guily J, et al. Swallowing problems in neuromuscular disorders. Arch Phys Med Rehabil. 1994;75:1175–1181. doi: 10.1016/0003-9993(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Aloysius A, Born P, Kinali M, et al. Swallowing difficulties in Duchenne muscular dystrophy: indications for feeding assessment and outcome of videofluroscopic swallow studies. Eur J Paediatr Neurol. 2008;12:239–245. doi: 10.1016/j.ejpn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Chen YS, Shih HH, Chen TH, et al. Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types II and III. J Pediatr. 2012;160:447–451. doi: 10.1016/j.jpeds.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Terzi N, Orlikowski D, Aegerter P, et al. Breathing-swallowing interaction in neuromuscular patients: a physiological evaluation. Am J Respir Crit Care Med. 2007;175:269–276. doi: 10.1164/rccm.200608-1067OC. [DOI] [PubMed] [Google Scholar]
- Hull J, Aniapravan R, Chan E, et al. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax. 2012;67(Suppl 1):i1–i40. doi: 10.1136/thoraxjnl-2012-201964. [DOI] [PubMed] [Google Scholar]
- van den Engel-Hoek L, Erasmus CE, Hendriks JC, et al. Oral muscles are progressively affected in Duchenne muscular dystrophy: implications for dysphagia treatment. J Neurol. 2013;260:1295–1303. doi: 10.1007/s00415-012-6793-y. [DOI] [PubMed] [Google Scholar]
- Pane M, Vasta I, Messina S, et al. Feeding problems and weight gain in Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2006;10:231–236. doi: 10.1016/j.ejpn.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Messina S, Pane M, De Rose P, et al. Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord. 2008;18:389–393. doi: 10.1016/j.nmd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- van den Engel-Hoek L, Erasmus CE, van Bruggen HW, et al. Dysphagia in spinal muscular atrophy type II: more than a bulbar problem? Neurology. 2009;73:1787–1791. doi: 10.1212/WNL.0b013e3181c34aa6. [DOI] [PubMed] [Google Scholar]
- Hanayama K, Liu M, Higuchi Y, et al. Dysphagia in patients with Duchenne muscular dystrophy evaluated with a questionnaire and videofluorography. Disabil Rehabil. 2008;30:517–522. doi: 10.1080/09638280701355595. [DOI] [PubMed] [Google Scholar]
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, Part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- Barohn RJ, Levine EJ, Olson JO, et al. Gastric hypomotility in Duchenne's muscular dystrophy. N Engl J Med. 1988;319:15–18. doi: 10.1056/NEJM198807073190103. [DOI] [PubMed] [Google Scholar]
- Borrelli O, Salvia G, Mancini V, et al. Evolution of gastric electrical features and gastric emptying in children with Duchenne and Becker muscular dystrophy. Am J Gastroenterol. 2005;100:695–702. doi: 10.1111/j.1572-0241.2005.41303.x. [DOI] [PubMed] [Google Scholar]
- Jaffe KM, McDonald CM, Ingman E, et al. Symptoms of upper gastrointestinal dysfunction in Duchenne muscular dystrophy: case-control study. Arch Phys Med Rehabil. 1990;71:742–744. [PubMed] [Google Scholar]
- Umemoto G, Furuya H, Kitashima A, et al. Dysphagia in Duchenne muscular dystrophy versus myotonic dystrophy type 1. Muscle Nerve. 2012;46:490–495. doi: 10.1002/mus.23364. [DOI] [PubMed] [Google Scholar]
- Toussaint M, Soudon P, Kinnear W. Effect of non-invasive ventilation on respiratory muscle loading and endurance in patients with Duchenne muscular dystrophy. Thorax. 2008;63:430–434. doi: 10.1136/thx.2007.084574. [DOI] [PubMed] [Google Scholar]
- Okada K, Manabe S, Sakamoto S, et al. Protein and energy metabolism in patients with progressive muscular dystrophy. J Nutr Sci Vitaminol. 1992;38:141–154. doi: 10.3177/jnsv.38.141. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bermejo J, Lofaso F, Falaize L, et al. Resting energy expenditure in Duchenne patients using home mechanical ventilation. Eur Respir J. 2005;25:682–687. doi: 10.1183/09031936.05.00031304. [DOI] [PubMed] [Google Scholar]
- Martigne L, Salleron J, Mayer M, et al. Natural evolution of weight status in Duchenne muscular dystrophy: a retrospective audit. Br J Nutr. 2011;105:1486–1491. doi: 10.1017/S0007114510005180. [DOI] [PubMed] [Google Scholar]
- Iannaccone ST, Owens H, Scott J, et al. Postoperative malnutrition in Duchenne muscular dystrophy. J Child Neurol. 2003;18:17–20. doi: 10.1177/08830738030180011201. [DOI] [PubMed] [Google Scholar]
- Gauld LM, Kappers J, Carlin JB, et al. Height prediction from ulna length. Dev Med Child Neurol. 2004;46:475–480. doi: 10.1017/s0012162204000787. [DOI] [PubMed] [Google Scholar]
- Pessolano FA, Suarez AA, Monteiro SG, et al. Nutritional assessment of patients with neuromuscular diseases. Am J Phys Med Rehabil. 2003;82:182–185. doi: 10.1097/01.PHM.0000052588.28912.A4. [DOI] [PubMed] [Google Scholar]
- Homnick DN. Mechanical insufflation-exsufflation for airway mucus clearance. Respir Care. 2007;52:1296–1305. [PubMed] [Google Scholar]
- Toussaint M, Boitano LJ, Gathot V, et al. Limits of effective cough-augmentation techniques in patients with neuromuscular disease. Respir Care. 2009;54:359–366. [PubMed] [Google Scholar]
- Khirani S, Ramirez A, Delord V, et al. Evaluation of ventilators for mouthpiece ventilation in neuromuscular disease. Respir Care. 2014;59:1329–1337. doi: 10.4187/respcare.03031. [DOI] [PubMed] [Google Scholar]
- Toussaint M, Steens M, Wasteels G, et al. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J. 2006;28:549–555. doi: 10.1183/09031936.06.00004906. [DOI] [PubMed] [Google Scholar]
- Terzi N, Normand H, Dumanowski E, et al. Noninvasive ventilation and breathing-swallowing interplay in chronic obstructive pulmonary disease. Crit Care Med. 2014;42:565–573. doi: 10.1097/CCM.0b013e3182a66b4a. [DOI] [PubMed] [Google Scholar]
- Ghafari J, Clark RE, Shofer FS, et al. Dental and occlusal characteristics of children with neuromuscular disease. Am J Orthodont Dentofac Orthoped. 1988;93:126–132. doi: 10.1016/0889-5406(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Morel-Verdebout C, Botteron S, Kiliaridis S. Dentofacial characteristics of growing patients with Duchenne muscular dystrophy: a morphological study. Eur J Orthodont. 2007;29:500–507. doi: 10.1093/ejo/cjm045. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Umaki Y, Sugishita S, et al. Videofluorographic assessment of swallowing function in patients with Duchenne muscular dystrophy. Rinsho Shinkeigaku. 2007;47:407–412. [PubMed] [Google Scholar]
- Birnkrant DJ, Ferguson RD, Martin JE, et al. Noninvasive ventilation during gastrostomy tube placement in patients with severe Duchenne muscular dystrophy: case reports and review of the literature. Pediatric Pulmonol. 2006;41:188–193. doi: 10.1002/ppul.20356. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Komaki H, Sasaki M, et al. Efficacy and tolerance of gastrostomy feeding in Japanese muscular dystrophy patients. Brain Dev. 2012;34:756–762. doi: 10.1016/j.braindev.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Martigne L, Seguy D, Pellegrini N, et al. Efficacy and tolerance of gastrostomy feeding in Duchenne muscular dystrophy. Clin Nutr. 2010;29:60–64. doi: 10.1016/j.clnu.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Philpot J, Bagnall A, King C, et al. Feeding problems in merosin deficient congenital muscular dystrophy. Arch Dis Child. 1999;80:542–547. doi: 10.1136/adc.80.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Dowling JJ, North K, et al. Consensus statement on standard of care for congenital myopathies. J Child Neurol. 2012;27:363–382. doi: 10.1177/0883073812436605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen P, Hody JL, Clairbois J, et al. Surgical treatment of spinal deformities in Duchenne muscular dystrophy. Rev Chir Orthop Reparatrice Appar Mot. 1992;78:470–479. [PubMed] [Google Scholar]
- Lebel DE, Corston JA, McAdam LC, et al. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. J Bone Joint Surg Am. 2013;95:1057–1061. doi: 10.2106/JBJS.L.01577. [DOI] [PubMed] [Google Scholar]
- Guérin C, Bourdin G, Leray V, et al. Performance of the cough-assist insufflation-exsufflation device in the presence of an endotracheal tube or tracheostomy tube: a bench study. Respir Care. 2011;56:1108–1114. doi: 10.4187/respcare.01121. [DOI] [PubMed] [Google Scholar]
- Buckland A, Jackson L, Ilich T, et al. Drilling speaking valves to promote phonation in tracheostomy-dependent children. Laryngoscope. 2012;122:2316–2322. doi: 10.1002/lary.23436. [DOI] [PubMed] [Google Scholar]
- Soudon P, Steens M, Toussaint M. A comparison of invasive versus noninvasive full-time mechanical ventilation in Duchenne muscular dystrophy. Chron Respir Dis. 2008;5:87–93. doi: 10.1177/1479972308088715. [DOI] [PubMed] [Google Scholar]