Abstract
The goal of this study was to evaluate routine flow cytometric (FC) immunophenotypic markers in differentiating between Burkitt lymphoma (BL) and CD10+ diffuse large B-cell lymphoma (DLBCL). We performed retrospective analysis of FC data from 55 patients. We evaluated 9 FC parameters: forward and side scatter (FSC and SSC); mean fluorescent intensity (MFI) for CD20, CD10, CD38, CD79b, CD43, and CD71; and the percentage of neoplastic cells positive for CD71 (%CD71). The FSC; MFIs of CD10, CD43, CD79b, and CD71; and %CD71 cells were significantly different between BL and CD10+ DLBCL (P < .05; Student t test). A 5-point scoring system (FSC, %CD71, and MFIs of CD43, CD79b, and CD71) was devised, and 6 (60%) of 10 BLs scored 3 or greater and 1 (10%) of 10 CD10+ DLBCLs scored 3 (P = .04; χ2). Our findings indicate that routine FC parameters can aid in differentiating BL from CD10+ DLBCL.
Keywords: Flow cytometry, Burkitt lymphoma, CD10+ diffuse large B-cell lymphoma
Mature B-cell lymphomas that commonly express CD10 include follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), and B-cell lymphoma, unclassifiable (BCLU) with features intermediate between DLBCL and BL. Most FLs, particularly grade I or II, can be easily distinguished from the other CD10+ groups given an adequate specimen. However, differentiation among DLBCL, BL, and BCLU can present diagnostic difficulty as they sometimes share common morphologic and immunophenotypic characteristics.
DLBCL is the most common adult B-cell lymphoma in the Western world and accounts for 25% to 30% of non-Hodgkin lymphomas. DLBCL was initially defined by the World Health Organization (WHO) in 20011 as a heterogeneous group of lymphomas. The group has since been separated into a variety of clinically and biologically distinct entities in the current WHO classification of lymphoid malignancies.2 These subgroups have been defined using clinical, morphologic, immunophenotypic, and molecular features. One such subgroup is BCLU, which is considered to reside somewhere on the B-cell lymphoma spectrum between DLBCL and BL. The morphologic features of BCLU often resemble BL, with the exception of more nuclear irregularity and heterogeneity and the presence of some cells with prominent single nucleoli. BCLU often, but not always, has a high proliferation rate (>90% by Mindbomb homolog-1 [MIB-1] staining) and an immunophenotype that is similar to BL except for strong BCL2 positivity in some instances. Certain cases of BCLU and DLBCL can also harbor v-myc myelocytomatosis viral oncogene homolog (c-MYC) rearrangements, making the distinction between these groups and BL even more difficult. A proportion of DLBCLs and BCLUs are double-hit lymphomas harboring both c-MYC and BCL2 and/ or BCL6 rearrangements.2–4
In the most recent WHO classification, BL has been given more strict diagnostic criteria as it requires distinctly different treatment than DLBCL. BL is defined by a combination of several features, including the following: (1) monotonous, round, medium-sized cellular morphology; (2) typical Burkitt phenotype (CD10+, BCL6+, BCL2−, negative for terminal deoxynucleotidyl transferase, and positive for monotypic surface immunoglobulin); (3) an MIB-1 proliferation rate of virtually 100%; and (4) with or without evidence of Epstein-Barr virus infection.2 In addition, the vast majority of cases carry the c-MYC rearrangement.
Proper classification of these CD10+ B-cell lymphomas, particularly identifying BL, has become increasingly complex and significant for treatment decision and prognostic stratification.5,6 Molecular genetic studies, particularly fluorescent in situ hybridization (FISH) studies for documenting c-MYC, BCL2, and/or BCL6 rearrangements as mentioned, have a crucial role in supporting proper categorization of CD10+ B-cell lymphomas. However, these tests are relatively expensive and not routinely available at many clinical laboratories, requiring submission to a reference laboratory, which can delay accurate diagnosis. Additional methods to distinguish between BL and other CD10+ lymphomas in a timely and cost-effective manner are needed. The goal of this study was to evaluate the usefulness of routinely used immunophenotypic markers by flow cytometry to assist in this important determination.
Materials and Methods
Patient Cohort
We performed a retrospective analysis of flow cytometric (FC) data obtained at the time of diagnosis from 55 patients with lymph node or soft tissue specimens at The Methodist Hospital (Houston, TX), its affiliate hospitals, and Texas Children’s Hospital (Houston) between 2003 and 2010. The cases were selected on the basis of having undergone FC evaluation. Patient diagnoses included 19 BLs (median age, 7.5 years; 17/19 males) and 12 CD10+ DLBCLs, including 1 case of BCLU (median age, 63 years; 8/12 males). These were compared with 24 control cases including the following: 8 CD10− DLBCLs (median age, 68 years; 6/8 males); 12 cases of FL, grade I or II (median age, 72 years; 6/12 males); and 4 lymph nodes with follicular hyperplasia (FHyp). The diagnoses of all cases were reviewed and established based on 2008 WHO classification and derived from combined clinical, morphologic, and FC data in all cases and FISH and immunohistochemical results in a subset of cases. All BL cases had classical morphologic features and were confirmed by FISH studies documenting the presence of c-MYC/immunoglobulin translocations. The single BCLU case harbored “double hits” with rearrangements of c-MYC and BCL2 by FISH. This study was approved by the institutional review boards of the participating institutions.
FC Parameters and Data Analysis
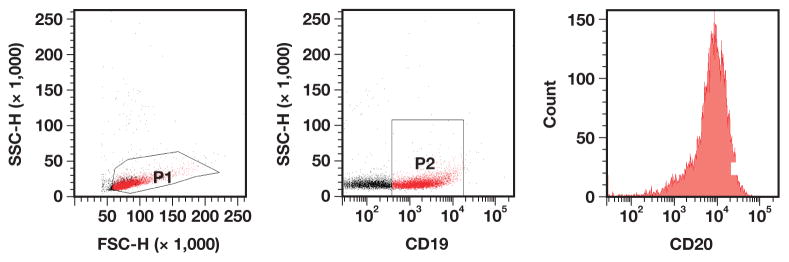
The sample preparation and 4-color FC evaluation was performed as previously described.7 The antibody combinations and their respective fluorochromes are listed in Table 1. A population-based gating strategy on FACsDiva software (BD Biosciences, San Jose, CA) was used to analyze the archived list mode data. Nine FC parameters were obtained for the neoplastic populations by selectively gating on the CD19+ B-cell population in each case Image 1. The forward and side scatter characteristics (FSC and SSC, respectively) and the mean fluorescent intensities (MFIs) for CD20, CD10, CD38, CD79b, CD43, and CD71 were then determined and recorded. The percentage of the neoplastic population that was positive for CD71 (%CD71) was the ninth parameter and was determined by using the upper border of CD71 intensity of the nonneoplastic, CD3+ T-cell population in each case as the negative cutoff point. CD10− DLBCL and FL cases were gated in the same manner. Of note, the FHyp cases were analyzed similarly but were specifically gated on the CD19+ and CD10+ B cells.
Table 1.
Fluorochrome/CD Marker Combinations for the Three Four-Color Tubes Used for Flow Cytometric Analysis
| Marker | Fluorochrome | Clone Name |
|---|---|---|
| Tube 1 | ||
| CD19 | PerCP | SJ25C1 |
| CD10 | PE | HI10a |
| CD20 | FITC | L27 |
| CD38 | APC | HB7 |
| Tube 2 | ||
| CD19 | PerCP | SJ25C1 |
| CD79b | PE | SN8 |
| CD43 | FITC | DF-T1 |
| CD10 | APC | HI10a |
| Tube 3 | ||
| CD3 | PerCP | SK7 |
| CD19 | PE | SJ25C1 |
| CD71 | FITC | L01.1 |
| CD10 | APC | HI10a |
APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin-chlorophyll protein.
Image 1.
Flow cytometric gating strategy. FSC-H, forward scatter height; SSC-H, side scatter height.
The mean and SD for each parameter were calculated. The Student t test was used to determine if the differences in parameters between BL and CD10+ DLBCL were statistically significant (P < .05). The numbers of cases in each group were too small and SDs too high to allow for multivariate analysis; however, parameters that showed differences between BL and CD10+ DLBCL cases were further studied.
Next, a 5-point scoring system was devised based on the approximate averaged values in BL cases for 5 specific parameters. The 5 parameters were selected for the scoring system because they were available for the majority of BL and CD10+ DLBCL cases studied and they showed significant differences (P ≤ .05) from CD10+ DLBCL cases. By using this scoring system, we reviewed BL and CD10+ DLBCL cases and gave 1 point each if they had the following values: FSC, less than 122,000; CD43, more than 2,500; CD79b, less than 2,000; CD71, more than 2,000; and %CD71, more than 65%. Only the cases containing all 5 parameters were scored, and the results of the scoring system were analyzed by using the χ2 test.
Results
FC Analysis Using Nine Parameters
The FL cases had the smallest cells by FSC, demonstrated markedly lower MFIs for all markers with the exception of CD10, and the %CD71 for FL was very low (3%) Table 2. Of note, the FHyp cells were more intermediate in average cell size and demonstrated slightly higher %CD71 than FL. The CD10− DLBCL cases demonstrated large cells by FSC and higher proliferation than the other 2 aforementioned control groups (CD71 MFI and %CD71). Based on our laboratory’s experience, the general patterns seen in these groups had the immunophenotypic findings expected from the literature, demonstrating that the gating strategies used in this study produced reliable results.
Table 2.
Means (SD) for the Nine Parameters in Each of the Five Groups
| BL | CD10+ DLBCL | P for BL vs CD10+ DLBCL* | CD10− DLBCL | Follicular Lymphoma | Follicular Hyperplasia | |
|---|---|---|---|---|---|---|
| FSC-H | 121,182 (12,390) | 134,204 (14,927) | .005 | 145,859 (22,917) | 89,926 (9,092) | 104,337 (7,814) |
| SSC-H | 35,945 (9,379) | 39,166 (11,533) | .367 | 40,733 (9,548) | 23,003 (6,577) | 36,139 (2,415) |
| CD20 | 19,753 (8,146) | 29,844 (23,042) | .145 | 13,225 (12,149) | 15,677 (15,423) | 19,805 (2,056) |
| CD10 | 9,269 (3,314) | 5,162 (3,202) | .029 | 532 (252) | 7,744 (6,984) | 1,631 (192) |
| CD38 | 26,671 (7,469) | 20,689 (15,818) | .282 | 3,155 (1,874) | 4,373 (2,532) | 33,275 (3,229) |
| CD43 | 2,356 (1,162) | 1,657 (1,178) | .029 | 1,639 (1,267) | 414 (234) | 383 (144) |
| CD79b | 1,603 (1,212) | 3,273 (2,585) | .016 | 1,838 (1,716) | 1,332 (1,213) | 4,973 (1,051) |
| CD71 | 4,094 (4,936) | 2,333 (2,706) | .054 | 6,953 (4,195) | 154 (67) | 1,770 (658) |
| %CD71 | 69 (11) | 53 (17) | .020 | 57 (22) | 3 (5) | 29 (4) |
BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; FSC-H, forward scatter height; SSC-H, side scatter height.
Student t test.
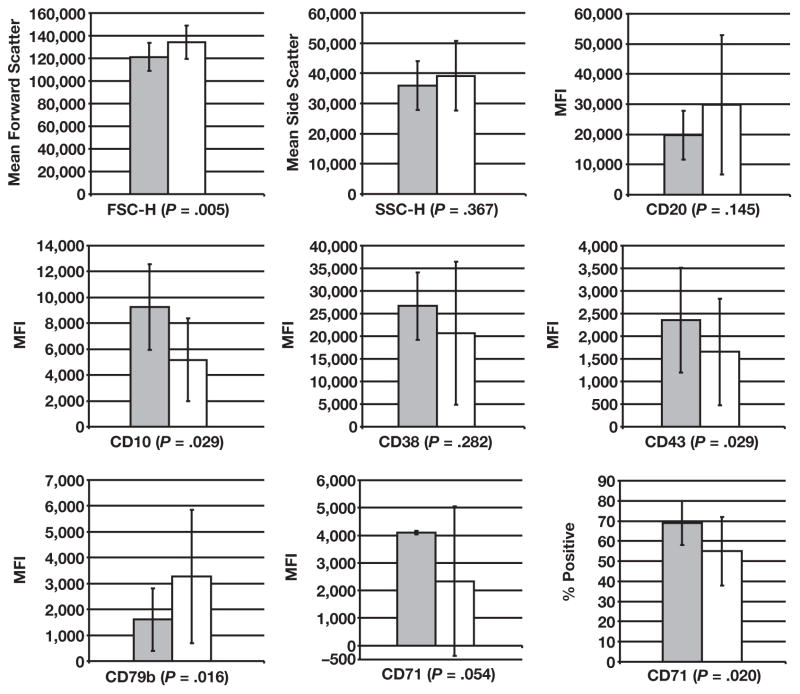
The 9 parameters measured showed significant variability (as evidenced by the high SDs) within several groups, particularly CD10+ DLBCL and CD10− DLBCL, demonstrating that these are indeed heterogeneous entities. The FL, FHyp, and BL groups demonstrated lower SDs, suggesting less intercase variability in these entities. Because of the amount of variance seen, statistically significant differences between BL and DLBCL (CD10+ and CD10− subgroups in total) were not seen for all parameters. However, several important differences between the groups of interest, BL and CD10+ DLBCL, were found Figure 1.
Figure 1.
Graphs for each parameter for Burkitt lymphoma (gray bars) and CD10+ diffuse large B-cell lymphoma (white bars) cases demonstrate significant differences for FSC, CD10, CD79b, CD43, and %CD71. The large intergroup variance is evidenced by the large SD bars. FSC-H, forward scatter height; MFI, mean fluorescence intensity; SSC-H, side scatter height.
As expected, the FSC was lower for BL than for CD10+ DLBCL (P = .01), reflecting the typically smaller size of the BL cells. The CD43 MFIs were brighter in BL than in CD10+ DLBCL (P = .029), and the CD79b MFI was significantly lower in the BL group (P = .016). CD10 was significantly higher in BL (P = .029). With an average of 69% of cells expressing CD71, BL had the highest %CD71 of any group, and this %CD71 was significantly higher than in the CD10+ DLBCL group (P = .020). In addition, the MFIs for CD71 tended to be higher in BL than in CD10+ DLBCL, but were not significant (P = .054). Since CD71 can act as a surrogate marker of proliferation,8 these results mirror the results of the MIB-1/immunohistochemical study, which showed a nearly 100% proliferation index. No statistically significant differences between BL and CD10+ DLBCL were seen in our data set for SSC, CD20, and CD38.
Results of the Scoring System
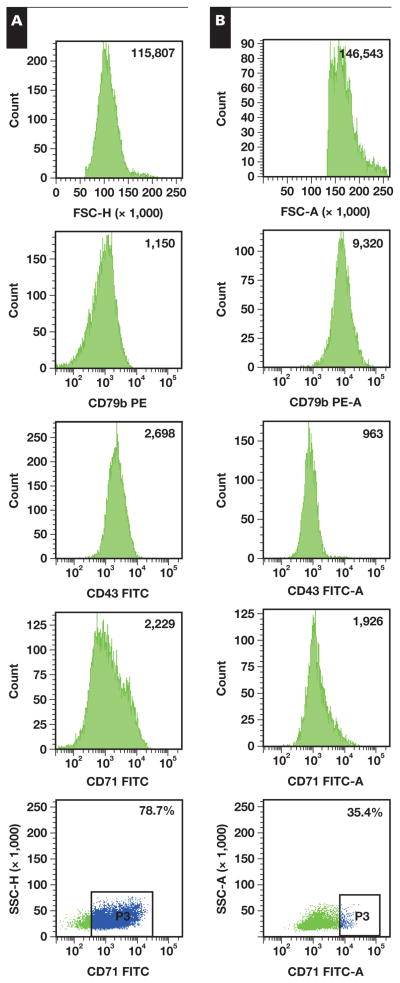
The 5-point scoring system described was applied to 10 of 19 total BL cases, 10 of 12 total CD10+ DLBCL cases, and 5 of 8 total CD10− DLBCL cases. Samples were scored only if they had all 5 parameters available for evaluation. The results of the scoring system showed that 6 (60%) of 10 BL cases had a score of 3 or greater. In contrast, only 1 (10%) of the 10 CD10+ DLBCL cases had a score of 3, and none of the 5 CD10− DLBCL cases had a score of 3 or more. The χ2 analysis of these results indicated that a score of 3 or more was significantly associated with BL (BL vs CD10+ DLBCL, 6/10 vs 1/10; P = .04; χ2 test; and BL vs all DLBCL, 6/10 vs 1/15; P = .01). Representative examples of BL and CD10+ DLBCL cases are shown in Image 2. Of note, the single case of BCLU with rearrangements of c-MYC and BCL2 had a score of 1, suggesting that the coexisting BCL2 rearrangement may have changed the overall immunophenotyping pattern of neoplastic cells.
Image 2.
Mean fluorescent intensity (MFI) for 5 parameters in representative cases of Burkitt lymphoma (A) and CD10+ diffuse large B-cell lymphoma (B). FITC, fluorescein isothiocyanate; FSC-H, forward scatter height; PE, phycoerythrin; SSC-H, side scatter height.
Discussion
Our results demonstrate that FSC and expression levels (as determined by MFI) of several commonly used surface markers, including CD10, CD43, CD79b, and CD71, and the %CD71, were different between BL and CD10+ DLBCL. In this study, we used the MFI of the neoplastic populations for each of the cell markers. While other strategies are available, such as using the difference in the MFI (MFI-diff) for the neoplastic population and MFI for an internal negative control population (such as T cells), we found our method to be more reproducible between the 2 pathologists involved in data collection (P.M. and N.N.; data not shown). Also, no improvement in variance was demonstrated when the MFI-diff was used for several pilot cases from each group. From our experience, the MFI-diff method may be superior in studies in which different fluorochrome/antibody combinations and/or various specimen types (eg, peripheral blood, bone marrow, lymph node) are evaluated. We also found our method to be of more value than simple qualitative criteria (ie, positive or negative for a marker). This allowed us see if there were more subtle differences in expression patterns of markers between groups. For example, CD79b is expressed on the majority of CD10+ DLBCL and BL cases based on qualitative analysis, which would not help to distinguish between the 2 entities.
As noted previously, there was substantial variability of MFI, particularly within DLBCL cases. Although the testing procedures (see “Materials and Methods”) were standardized, and, because the laboratory is a clinical laboratory, daily quality control and routine maintenance were performed during the entire 8-year period from which cases were collected, it should be noted that variability in day-to-day compensation/laser voltage changes would have introduced a degree of error into the measurement of MFI values.
FC methods have been previously used to distinguish CD10+ DLBCL from BL. For example, CD44 and CD54 expression was shown to be significantly different between these 2 lymphomas by Schniederjan et al.9 However, these markers are not commonly used in many clinical laboratories. Although no differences in the expression of CD18 or CD43 were observed in that study, BL showed brighter CD43 intensity than CD10+ DLBCL in our analysis (P = .029). One possible explanation for these differences could be related to our gating/mean MFI strategy compared with that of the previous study, which looked at the MFI-diff for the neoplastic populations vs the T cells. Another limitation and possible explanation for different findings in our study is the fact that all of the BL cases in our cohort were pediatric, and adult BL cases may have different expression patterns. Another study used immunohistochemical analysis of BL and other CD10+ lymphomas and showed that CD38 positivity was a better predictor of BL and c-MYC status than CD10 and BCL2.10 Our CD38 MFI results were brightest in the BL samples, consistent with their findings (P = .282).
Our data showed that CD79b is quite dim in BL compared with CD10+ DLBCLs (P = .016). To the best of our knowledge, this marker commonly used in FC laboratories has not been evaluated specifically in differentiating BL from CD10+ DLBCL. In addition, the CD10− DLBCL had a similar CD79b MFI to BL in the present study. Further studies are warranted to confirm this observation. In addition, as CD79b is involved in immunoglobulin anchoring to the cell surface, evaluation of surface immunoglobulin intensity in BL and DLBCL would be of interest in future studies. (These data were not available for retrospective review in this study.)
In our study, %CD71 (transferrin receptor) was higher in BL than in other groups (P = .020). CD71 has been used as a surrogate marker of proliferation and has been previously shown to aid in differentiating between FL and higher-grade B cell lymphomas (including BL and DLBCL).8 However, the use of MFI alone failed to differentiate between BL and DLBCL, suggesting that a multiparameter system as used in our study is needed for such distinction.
A scoring system was devised that counted different parameters (FSC, CD79b, CD43, CD71, and %CD71) to further aid in differentiating BL from CD10+ DLBCL. By using this scoring system, we found only 1 DLBCL case with a score (equal to 3) similar to most BL cases studied. The only case of BCLU in our series had a very low score, but because there was only 1 case, conclusions regarding BCLU cannot be made based on these data. Recent studies addressing this group of lymphomas have suggested that double-hit lymphomas, which can fall into the current BCLU or, rarely, DLBCL group, often have dim CD20 and bright CD38.11–13
Our findings indicate that there are significant differences in FC parameters between BL and CD10+ DLBCL that can be obtained by using commonly used markers. BL is highly suggested when 3 or more of the following findings are present: FSC height, less than 122,000; CD79b, less than 2,000; CD43, more than 2,500; CD71, more than 2,000; and %CD71, more than 65%. This is the first such study, to the best of our knowledge, to use this multiparameter strategy for differentiating BLs from other CD10+ DLBCLs. Further studies with larger samples are warranted to validate our findings.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al., editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon, France: IARC Press; 2008. [Google Scholar]
- 3.Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC [editorial] Haematologica. 2007;92:1297. doi: 10.3324/haematol.11263. [DOI] [PubMed] [Google Scholar]
- 4.Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34:327–340. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson MØ, Gang AO, Poulsen TS, et al. Concurrent BCL2 and MYC translocations in a prospective cohort of diffuse large B-cell lymphomas. [Accessed May 18. 2011];Blood. 2011 Abstract 319. http://ash.confex.com/ash/2010/webprogram/Paper26440.html.
- 6.Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 7.Chen HI, Akpolat I, Mody DR, et al. Restricted κ/λ light chain ratio by flow cytometry in germinal center B cells in Hashimoto thyroiditis. Am J Clin Pathol. 2006;125:42–48. [PubMed] [Google Scholar]
- 8.Wu JM, Borowitz MJ, Weir EG. The usefulness of CD71 expression by flow cytometry for differentiating indolent from aggressive CD10+ B-cell lymphomas. Am J Clin Pathol. 2006;126:39–46. doi: 10.1309/X8LB-RRBB-C43P-L9HC. [DOI] [PubMed] [Google Scholar]
- 9.Schniederjan SD, Li S, Saxe DF, et al. A novel flow cytometric antibody panel for distinguishing Burkitt lymphoma from CD10+ diffuse large B-cell lymphoma. Am J Clin Pathol. 2010;133:718–726. doi: 10.1309/AJCP0XQDGKFR0HTW. [DOI] [PubMed] [Google Scholar]
- 10.Rodig SJ, Vergilio JA, Shahsafaei A, et al. Characteristic expression patterns of TCL1, CD38, and CD44 identify aggressive lymphomas harboring a MYC translocation. Am J Surg Pathol. 2008;32:113–122. doi: 10.1097/PAS.0b013e3180959e09. [DOI] [PubMed] [Google Scholar]
- 11.Wu D, Wood BL, Dorer R, et al. “Double-hit” mature B-cell lymphomas show a common immunophenotype by flow cytometry that includes decreased CD20 expression. Am J Clin Pathol. 2010;134:258–265. doi: 10.1309/AJCP7YLDTJPLCE5F. [DOI] [PubMed] [Google Scholar]
- 12.Willis JS, Seegmiller AC, Uddin N, et al. Bright CD38 and dim CD20 by flow cytometry may predict for double hit lymphomas in MYC rearranged high-grade B-cell lymphomas. Presented as an abstract at the 2011 Annual Meeting of the United States and Canadian Academy of Pathology; March 2, 2011; San Antonio, TX. [Accessed May 11, 2011]. Abstract 1397. http://www.abstracts2view.com/uscap11/view.php?nu=USCAP11L_1397. [Google Scholar]
- 13.Harrington AM, Olteanu H, Kroft SH, et al. The unique immunophenotype of double-hit lymphomas [letter] Am J Clin Pathol. 2011;135:649–650. doi: 10.1309/AJCPL11MAHISIJBQ. [DOI] [PubMed] [Google Scholar]