Introduction
The human stress system is a highly complex system that acts to protect the body by responding to internal and external stressors in order to achieve stability for the organism. Selye labeled agents or experiences that cause stress “stressors”, and believed these agents to arise from the organism’s external, internal, and psychosocial environment 1. Accordingly, organisms show a systemic response of resistance to a stressor, and in the early phase this enhances system functioning. With repeated exposures, the same stressor produces unique responses related to the adaptive capability or “conditioning factors” required for systemic regulation. Development and neural function establish the extent to which responses to stressors are efficient or dysregulated. In situations where environmental demands exceed coping regulatory capabilities, toxic stress and neurobiologic dysfunction may result 2. For the preterm infant, the neonatal intensive care unit (NICU) is harsh in comparison with intrauterine environment the infant was required to leave prematurely. Even during the current era of developmental sensitivity to NICU design 3, preterm infants are often exposed to lights, sounds, and disruption of sleep with frequent handling episodes 4. These experiences are assumed to cause distress in infants too immature to cope with such high environmental demands4. Moreover, early life experience and the impact of protracted stress activation during the critical period of early postnatal development are known to shape later neurobiological development and function in humans5, 6.
The best-evaluated methods for determining biological stress activation in humans include: cardiovascular, respiratory and hormonal responses to stressors. However, fluctuation in these measurements may be altered by multiple physiological changes, including hemodynamic changes that occur post-birth. In addition, hormonal stress responses may be confounded by exposure to steroids given antenatally to enhance lung maturation 7. In contrast to other biologic measures, electrodermal activity of the sympathetic nervous system, also known as sympathetic mediated sweating, is reflected in skin conductance response (SCR) and is not influenced by hemodynamic changes8 nor antenatal steroid administration. SCR represents changes in the palmar and plantar sweat glands during emotional arousal, which occur secondary to the secretion of acetylcholine acting on muscarinic receptors, resulting in filling and reabsorbing sweat from the sweat glands8. During sympathetic arousal, there is a quantifiable increase in the number and amplitude of electrodermal or skin conductance responses (Figure 1). SCR/sec likely represents a more accurate reflection of true biological stress/arousal because unlike blood pressure (BP) and heart rate (HR), SCR is not influenced by hemodynamic changes8. SCR measurement is a non-invasive approach to stress monitoring, requiring only the application of surface electrodes to the palmar or plantar surfaces of the skin (Figure 2). Additionally, this method of stress monitoring has been validated as an accurate measure of biological stress during emotional challenge in adults and crying in full and preterm infants9, 10.
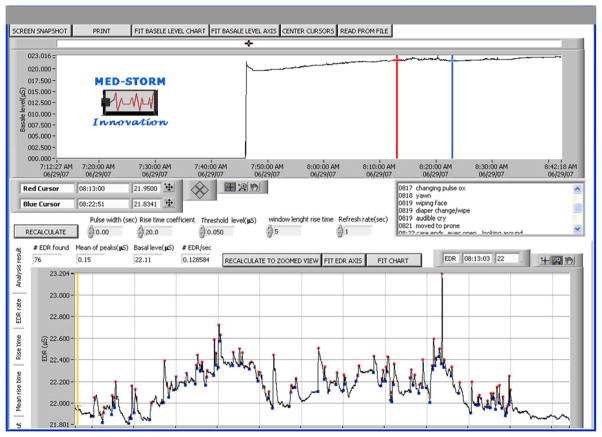
Figure 1.
Sample SCR measurement epoch during care/handling
This representation shows the analysis of a subject’s SCR data using MedStorm software. The representative window of measure between the red and blue cursor shows #SCR/sec, also known as electrodermal responses (#EDR/sec) during the 4 minutes of handling associated with nursing care. In this example, the number of waveforms (each wave height represented by a red dot) is divided by the total time of the epoch in seconds, to equal SCR (EDR)/sec. The written log shows the timing and written description of each tactile stimulus that was given by the nurse and recorded by the observer.
The Synactive Theory of Infant Development, proposed by Als 11, describes the preterm infant’s development as involving interacting subsystems (autonomic, motor, state and interactional) outwardly characterized by specific behavioral responses to stimuli. According to Als, the initial extra-uterine environment should: (a) be made highly similar to the intrauterine environment the infant had to prematurely leave, and (b) then slowly and progressively be elaborated at a rate appropriate to the infant’s capabilities. The NICU setting for this study uses a developmentally sensitive approach to caregiving as the framework for care delivery of infants by promoting self-regulation, while simultaneously providing environmental modifications such as dim lighting and noise control to reduce stressful stimuli. An “in-tune” caregiver provides supportive developmental positioning to promote fetal posture along with supportive handling and individualized pacing of caregiving to prevent additional stress to the already compromised infant 12. Gentle touch, non-nutritive sucking, flexed positioning, hands-on containment (supportively cradling with the hands), and nesting with boundaries are interventions that help the preterm infant to maintain neurobehavioral stability and thermoregulatory control. When procedures must be done, gently assisting the preterm infant to an awake state by soft voice and gentle touch followed by containment and positioning, help the infant to manage stress and behavioral disorganization 13. In addition, periods of undisturbed rest and facilitation of natural sleep-wake rhythms are essential for growth and tissue healing.
The purpose of this observational cohort study was to investigate biological and behavioral responses to developmentally sensitive nurse handling, the tactile stimulation during clustered morning care in the NICU, and to test the hypothesis that stress/arousal responses would be associated with the severity of illness of the infants.
2. Patients and methods
This is a prospective observational study based on a cohort of preterm neonates with mild severity of illness scores, using the Score for Neonatal Acute Physiology (SNAP < 10) 14. Subjects who were inborn and admitted to the Neonatal Intensive Care Unit (NICU) at Penn State Children’s Hospital/The Penn State Milton S. Hershey Medical Center were included after informed consent was obtained by their parents or legal representatives. The inclusion criteria were infants born preterm at 28–35 weeks postmenstrual age (PMA) and no longer requiring assisted ventilation by postnatal day 3 of life. Relatively ‘healthy’ preterm, low birthweight (LBW) infants were selected for study as the biological and behavioral indicators of stress are known to be confounded by extreme prematurity and/or critical illness. Exclusion criteria were: congenital anomalies or conditions known to affect neurodevelopmental outcomes (including: intrauterine growth restriction, grade II+ periventricular-intraventricular hemorrhage, or cord blood gas pH less than 7.2), admission to the NICU after 12 hours of birth, maternal illness preventing the ability to obtain informed consent, administration of narcotics and or sedatives to the infant prior to postnatal day 3 of life, or reported maternal use of illicit substances, since these conditions or medications are known to impact biological and behavioral stress measures.
Data Collection- Biological & Behavioral Data
Postnatal day 4–5 of life was selected as the ideal window of stress measurement as infants had made the successful transition ex-utero, recovered from the stress of birth and the impact of antenatal medications; which are all factors known to impact biological and behavioral responsiveness. Infants were monitored continuously via cardio-respiratory monitors with trend recording capabilities. During standard morning nursing care (axillary temperature, assessment, diaper change, repositioning) continuous recordings of infant biological parameters including: HR, RR, and SCR were obtained during pre-care (thirty minutes prior to the scheduled initiation of handling) and during the intra-care phase. Using these data, mean HR and RR, and change in HR and RR from baseline was determined from trend recording data for each infant. SCR parameters were measured with three surface electrodes: the measurement electrode was placed on the plantar surface of the foot of the infant, and the two others were placed on each side of the ankle. These were connected to the biomedical SCR device, Med-Storm® as described previously 9 and SCR data were collected using Skin Conductance Analysis On-line™ software, Med-Storm®. An observer manually entered each tactile stimulus that was given to the infant using the software observation log (Figure 1).
Simultaneous real-time behavioral data were observed continuously and scored at cribside by a single Newborn individualized developmental care and assessment program (NIDCAP®) certified specialist using the Naturalistic Observation Record (Als, 1982). These behaviors included infant sleep/wake cycles, facial movements (i.e. grimace, eye wince), motor stress signals (i.e. finger splay, startle), visceral responses (i.e. hiccup, gag), attentional signals (vocalizations) and self-consoling behaviors (sucking, hand or foot-bracing, hands-to-mouth, hands-to-face). The observations were conducted around the scheduled morning care between 7:30–11:00 am during a twenty-minute baseline, during the standard morning nursing care (axillary temperature, assessment, diaper change, repositioning), and up to thirty minutes post-interaction. The standard morning care was done by an expert nurse who received education and training in developmental care principles. The duration of cluster care was mean (SD) 8.5 (2.7) minutes. Behavioral data were recorded while simultaneously measuring biological parameters (HR, RR, and SCR). Using the behavioral data sheets, the newborn’s behavior was later quantified for stress behaviors of total frequency per domain for each of three subsystems: autonomic behaviors, motor behaviors, and state behaviors 15. A total behavioral arousal/stress score was computed by adding the three domains as previously described16.
Severity of Illness Score and days before enteral feeding
The Score for Neonatal Acute Physiology (SNAP)14, a measure of severity of illness was used to rate subjects at 48 hours of age. A mild severity of illness index for SNAP is a rating of 0 – 9. The authors of the index have reported a predicted mortality (PM) of SNAP as follows; SNAP 0 – 19 (PM = 0 – 42%) and SNAP > 19 (PM = 52 – 68%). Maternal and infant demographics were recorded from the medical record.
Ethics
The study was conducted in the level IV neonatal intensive care unit (NICU) of Penn State Children’s Hospital/The Penn State Milton S. Hershey Medical Center. Human study protocols were approved by Penn State Hershey College of Medicine Institutional Review Board. Written parental consent was obtained for each infant prior to study enrollment.
Statistics
Demographic and clinical data were collected prospectively, removed of all identifiers and entered into IBM-SPSS 21.0 for Windows® (SPSS, Chicago, IL). Descriptive statistics (frequencies, means, medians and scatterplots) were done and all variables were checked for outliers and normality before analysis. Non-parametric (Wilcoxon Signed Rank) test was used to assess differences in biological and behavioral measures from before to during care. Pearson correlation coefficients were used to assess association of measurements to the severity of illness. For all analyses, alpha was set at 0.05 and two-tailed tests were used.
Results
Thirty preterm infants with mean (SE) postmenstrual age of 32.7 (0.27) weeks and birth weight 1880 (74.8) grams were studied on postnatal day 4–5 of life. Demographic characteristics of the sample are presented in Table 1.
Table 1.
Sample Characteristics
| No. of patients included (N=30) | Mean | SE | Min | Max |
|---|---|---|---|---|
| Male gender (%) | 50 | - | - | - |
| PMA at birth (weeks) | 32.7 | 0.27 | 29.3 | 35.2 |
| Birth weight (grams) | 1877 | 74.8 | 1256 | 2803 |
| SNAP | 4.1 | 0.44 | 0 | 9 |
| Days to Enteral Feeds | 8.3 | 1.50 | 1 | 37 |
PMA= postmenstrual age; SNAP= Score for Neonatal Acute Physiology
There were statistical increases for biological (SCR/sec (fig. 1), HR, RR) and behavioral (NIDCAP®) measures from before to during the care (P <0.01), see Table 2. This increase in overall stress score was found to be statistically significant according to the NIDCAP® observation (P <0.01). Further analyses of the individual variables within the NIDCAP®, showed significant increases in motor and attentional cues and the ability to self-console (P ≤ 0.01) from before to during the care. However, facial and visceral stress signals did not increase between the two periods of observation (Table 2). In addition, total NIDCAP® stress behaviors during nurse care showed a significant negative association to SNAP (morbidity index); r= −0.45, P= 0.01. Conversely, the SCR/sec did not correlate with SNAP or gestational age. Birth weight and adjusted age at the time of measurement did not confound with these correlations.
Table 2.
Biological & Behavioral Measures Before and During Nurse Care (N=30)
| Before Care | During Care | ||||
|---|---|---|---|---|---|
| Variable | Mean | SE | Mean | SE | * P value |
| Biological Responses | |||||
| HR (bpm) | 151.8 | 1.84 | 173.9 | 2.06 | <0.0001 |
| RR (bpm) | 49.8 | 2.72 | 75.7 | 2.38 | <0.0001 |
| SCR (#SCR/sec) | 0.06 | .009 | 0.13 | .014 | <0.0001 |
|
| |||||
| Behavioral Responses | |||||
| Total NICAP® Cues | 24.08 | 3.65 | 50.63 | 3.93 | 0.002 |
| Facial Cues | 1.0 | .444 | 2.13 | .639 | 0.086 |
| Visceral Cues | 1.7 | .562 | 3.27 | .823 | 0.357 |
| Motor Cues | 19.0 | 3.38 | 34.97 | 2.67 | 0.002 |
| Attentional Cues | 2.92 | 1.04 | 10.27 | 1.70 | 0.007 |
| Self-consoling | 3.17 | 1.11 | 12.30 | 1.81 | 0.01 |
HR = heart rate, beats per minute; RR = respiratory rate, breaths per minute; SCR/sec = #Skin conductance responses per second
Non-parametric, Wilcoxon Signed Rank Test was used to determine the difference between pre and post- measures
Discussion
There were significant increases for both biological and behavioral stress responses from before to during the care including: HR, RR, SCR/sec, and NIDCAP® behaviors. Interestingly, of the NIDCAP® behaviors the facial and visceral stress signals did not show the same significant increases with tactile stimulation during care as the motor, attentional and self-consoling cues. The findings of fewer visceral stress signals in preterm infants and higher whole motor and extremity stress signals are characteristically documented by NIDCAP® certified observers16.
Previous research has demonstrated that preterm infants display stress responses both during noxious and anoxious non-invasive procedures17, 18. Lagercrantz found that the catecholamine levels increased more during the nursing procedure than during heel lance 18, while Hellerud found that the SCR/sec increased more during the nursing procedure than during heel lance 19. Our findings suggest that preterm infants do react with a similar stress response to handling as the stress response that occurs during heel lance. This is similar to findings from Holsti et al 17, who reported that preterm infants react similarly to diaper changes as to lance/squeeze, both in regards to physiological and behavioral responses. Thus, non-invasive stressful procedures should carefully be monitored in this patient population. Certain subpopulations, particularly preterm, low birth weight infants, are at particular risk because preterm infants have less ability to display their discomfort through behavioral cues20.
Studies have demonstrated that the biological measures of respiration and heart rate increase in infants as a response to noxious stimuli21. However, fluctuations in these parameters may also be influenced by other factors such as alterations in blood circulation, breathing patterns, environmental temperature, or sedatives8. Increased skin conductance on palms and plantar surfaces has been identified as a biological measure that can validate pain perception from noxious stimuli in infants, due to increased sympathetic activity, which is more specifically associated to emotional distress than RR and HR21. Gjerstad et al 22 found a significant correlation between SCR/sec and the COMFORT sedation score that increased from before to during suctioning from the trachea (r= 0.78, p<0.001), while other physiologic measures did not correlate with the COMFORT sedation score. Similarly, in a study by Roeggen et al 23 infants asleep had a range in the heart rate from 110–165 beats per min and the SCR/sec was lower than 0.07. In addition, Storm et al. found that during anesthesia, the SCR/sec correlated with a surgical stress score, r =0.53, P<0.001 24, and the SCR/sec also directly correlated with the epinephrine levels, P=0.0001 9, while the surgical stress score did not correlate with HR. Thus, SCR proves to be a better physiologic measure of stress reactivity than HR or BP in infants. Moreover, in newborn infants during heel lance for blood gas analyses, a significantly positive correlation was found between the Neonatal Infant Pain Scale (NIPS) and the SCR/sec (R=0.554, p=0.008)25. Findings from our study demonstrate that NIDCAP® behavioral stress responses increase significantly when preterm infants are handled, similar to the biological measures of HR, RR, and SCR/sec. In the population of LBW infants in our study, certain behaviors in NIDCAP®, such as motor and attentional cues as well as ability to self-console, were significantly increased during the stress response. These findings corroborate with findings by Holsti et al 17, where tactile stimuli resulted in less intense facial responses, but greater motor whole body responses in this population. This suggests that certain motoric cues (i.e. finger splay, startle, motor whole body responses) may be more important when assessing the state of distress in LBW, preterm infants. It also is well known that certain stress cues (facial cues, i.e. grimace) are less robust in infants who are severely ill or very preterm 26. Thus, nurses trained to read these behavioral cues should use them as an adjunct with biological measures for determining the distressed state of preterm infants. Our findings demonstrate NIDCAP® stress behaviors were negatively correlated with SNAP, suggesting that when infants had a higher severity of illness score they were less able to demonstrate stress behaviors. This suggests that the behavioral response data uniquely represent key information on which infants may be more vulnerable to the impacts of stress, yet unable to demonstrate robust distress signals.
Slater et al. 27 showed that oral sucrose has a sedative effect without influencing pain perception. This is in accordance with the findings that oral sucrose decreases the response from heel lance when studying the behavioral response and crying time, but not the biological responses such as HR and SCR/sec 28. It was also found that when sound stimuli in the ward increase above 65dB, HR and SCR/sec increase but the behavioral stress score did not increase 29. Thus, the absence of stress behaviors is not a validation of non-stress in preterm infants, but suggests these infants may not be able to outwardly display distress behaviors. This is particularly important in infants with higher severity of illness or more extreme prematurity where the behavioral repertoire may show less robust patterns of responsiveness. In this situation, biological parameters of distress (SCR/sec) may be more reliable and should be used in conjunction with behavioral assessments.
The guiding principle of developmental care is to facilitate infant interaction based on astute biobehavioral observation and to proceed with the interaction at a rate appropriate to decrease disorganization, conserve energy, and support self-regulation and stability within a nurturing environment that includes the family30. Combining biological and behavioral observations are critical to best evaluate the complexity of the preterm infant’s stress response system. In addition, the use of individualized developmentally supportive approaches and the appropriate use of sedation and analgesia for necessary procedures have been demonstrated to promote auto-regulation, protect the infant from stress and assist the infant to maintain stability and control.
Conclusion
Heart rate, respiration rate, skin conductance frequency, and NIDCAP® stress behaviors all significantly increased in LBW preterm infants during handling associated with standard nurse caregiving during the infants’ first days of life. Further analyses of the NIDCAP® subgroups identified motor and attentional cues, and ability to self-console as significant. Also, NIDCAP® behaviors were influenced by the severity of illness of the infant, while SC responses were not influenced by severity of illness. In preterm, LBW infants the complexity of the stress response systems may be best understood by using a multi-system approach to the assessment and monitoring of infant-caregiver interactions.
Acknowledgments
The authors acknowledge the medical/nursing staff of Penn State Hershey NICU for assistance with subject recruitment and the parents who gave consent for their infants to participate in the study.
Funding: Supported by The Children’s Miracle Network and Johnson & Johnson Health Behaviors and Quality of Life (KKD). Dr. Doheny receives salary support for research by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under award number 1R01DK099350. None of the funding sources had any role in the design of the study, in the analysis and interpretation of the data, in the decision to submit the manuscript, or in the preparation, review or approval of the manuscript.
Abbreviations
- HR
Heart rate
- NICU
Neonatal Intensive Care Unit
- NIDCAP®
Newborn Individualized Developmental Care and Assessment Program
- RR
Respiratory rate
- SNAP
Score for Neonatal Acute Physiology
- SCR/sec
Skin conductance responses per second
Footnotes
Declarations of Interest Statement:
VZ and KKD attest to not having any potential conflict-of-interests to disclose.
Dr. Hanne Storm discloses that she is the founder and co-owner of Med-Storm Innovation that owns the patents for the skin conductance technology used to assess stress responses of the infants in this study.
References
- 1.Selye H. The stress of life. New York, NY: McGraw-Hill Book Company; 1956. [Google Scholar]
- 2.McEwen BS, Gray J, Nasca C. Recognizing Resilience: Learning from the Effects of Stress on the Brain. Neurobiol Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RD, Smith JA, Shepley MM. Recommended standards for newborn ICU design, eighth edition. J Perinatol. 2013;33(Suppl 1):S2–16. doi: 10.1038/jp.2013.10. [DOI] [PubMed] [Google Scholar]
- 4.Browne JV, White RD. Foundations of developmental care. Clin Perinatol. 2011;38:xv–xvii. doi: 10.1016/j.clp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Vinall J, Grunau RE, Brant R, et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med. 2013;5:168ra8. doi: 10.1126/scitranslmed.3004666. [DOI] [PubMed] [Google Scholar]
- 6.McAnulty GB, Duffy FH, Butler SC, et al. Effects of the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) at age 8 years: preliminary data. Clin Pediatr (Phila) 2010;49:258–70. doi: 10.1177/0009922809335668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss SJ, Niemann S. Effects of Antenatal Corticosteroids on Cortisol and Heart Rate Reactivity of Preterm Infants. Biol Res Nurs. 2015 doi: 10.1177/1099800414564860. [DOI] [PubMed] [Google Scholar]
- 8.Storm H, Shafiei M, Myre K, et al. Palmar skin conductance compared to a developed stress score and to noxious and awakening stimuli on patients in anaesthesia. Acta Anaesthesiologica Scandinavica. 2005;49:798–803. doi: 10.1111/j.1399-6576.2005.00665.x. [DOI] [PubMed] [Google Scholar]
- 9.Storm H, Myre K, Rostrup M, et al. Skin conductance correlates with perioperative stress. Acta Anaesthesiol Scand. 2002;46:887–95. doi: 10.1034/j.1399-6576.2002.460721.x. [DOI] [PubMed] [Google Scholar]
- 10.Storm H. Skin conductance and the stress response from heel stick in preterm infants. Archives in Diseases of Childhood, Fetal Neonatal Education. 2000;83:F143–7. doi: 10.1136/fn.83.2.F143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Als H. Towards a synactive theory of development: Promise for the assessment and support of infant individuality. Infant Mental Health Journal. 1982;3:229–243. [Google Scholar]
- 12.Ward-Larson C, Horn RA, Gosnell F. The efficacy of facilitated tucking for relieving procedural pain of endotracheal suctioning in very low birthweight infants. MCN The American Journal of Maternal Child Nursing. 2004;29:151–158. doi: 10.1097/00005721-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Harrison LL, Roane C, Weaver M. The relationship between physiological and behavioral measures of stress in preterm infants. Journal of Obstetrical, Gynecological, & Neonatal Nursing. 2004;33:236–245. doi: 10.1177/0884217504263293. [DOI] [PubMed] [Google Scholar]
- 14.Richardson DK, Gray JE, McCormick MC, et al. Score for Neonatal Acute Physiology: A physiologic severity index for neonatal intensive care. Pediatrics. 1993;91:617–623. [PubMed] [Google Scholar]
- 15.Pressler JL, Hepworth JT. A quantitative use of the NIDCAP® tool. The effect of gender and race on very preterm neonates’ behavior. Clinical Nursing Research. 2002;11:89–102. doi: 10.1177/105477380201100107. [DOI] [PubMed] [Google Scholar]
- 16.Pressler JL, Hepworth JT, Helm JM, et al. Behaviors of very preterm neonates as documented using NIDCAP observations. Neonatal Netw. 2001;20:15–24. doi: 10.1891/0730-0832.20.8.15. [DOI] [PubMed] [Google Scholar]
- 17.Holsti L, Grunau RE, Oberlander TF, et al. Specific newborn individualized developmental care and assessment program movements are associated with acute pain in preterm infants in the neonatal intensive care unit. Pediatrics. 2004;114:65–72. doi: 10.1542/peds.114.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagercrantz H, Nilsson E, Redham I, et al. Plasma catecholamines following nursing procedures in a neonatal ward. Early Hum Dev. 1986;14:61–5. doi: 10.1016/0378-3782(86)90170-2. [DOI] [PubMed] [Google Scholar]
- 19.Hellerud BC, Storm H. Skin conductance and behaviour during sensory stimulation of preterm and term infants. Early Hum Dev. 2002;70:35–46. doi: 10.1016/s0378-3782(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 20.Stevens B, McGrath P, Gibbins S, et al. Determining behavioural and physiological responses to pain in infants at risk for neurological impairment. Pain. 2007;127:94–102. doi: 10.1016/j.pain.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Storm H, Stoen R, Klepstad P, et al. Nociceptive stimuli responses at different levels of general anaesthesia and genetic variability. Acta Anaesthesiol Scand. 2013;57:89–99. doi: 10.1111/aas.12017. [DOI] [PubMed] [Google Scholar]
- 22.Gjerstad AC, Wagner K, Henrichsen T, et al. Skin conductance versus the modified COMFORT sedation score as a measure of discomfort in artificially ventilated children. Pediatrics. 2008;122:e848–53. doi: 10.1542/peds.2007-2545. [DOI] [PubMed] [Google Scholar]
- 23.Roeggen I, Storm H, Harrison D. Skin conductance variability between and within hospitalised infants at rest. Early Hum Dev. 2011;87:37–42. doi: 10.1016/j.earlhumdev.2010.09.373. [DOI] [PubMed] [Google Scholar]
- 24.Storm H. Changes in skin conductance as a tool to monitor nociceptive stimulation and pain. Curr Opin Anaesthesiol. 2008;21:796–804. doi: 10.1097/ACO.0b013e3283183fe4. [DOI] [PubMed] [Google Scholar]
- 25.Pereira-da-Silva L, Virella D, Monteiro I, et al. Skin conductance indices discriminate nociceptive responses to acute stimuli from different heel prick procedures in infants. J Matern Fetal Neonatal Med. 2012;25:796–801. doi: 10.3109/14767058.2011.587919. [DOI] [PubMed] [Google Scholar]
- 26.Stevens BJ, Johnston CC. Physiological responses of premature infants to a painful stimulus. Nurs Res. 1994;43:226–31. [PubMed] [Google Scholar]
- 27.Slater R, Cornelissen L, Fabrizi L, et al. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet. 2010;376:1225–32. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storm H, Fremming A. Food intake and oral sucrose in preterms prior to heel prick. Acta Paediatr. 2002;91:555–60. doi: 10.1080/080352502753711687. [DOI] [PubMed] [Google Scholar]
- 29.Salavitabar A, Haidet KK, Adkins CS, Susman EJ, Palmer C, Storm H. Preterm infants’ sympathetic arousal and associated behavioral responses to sound stimuli in the neonatal intensive care unit. Advances in Neonatal Care. 2010;10:158–166. doi: 10.1097/ANC.0b013e3181dd6dea. [DOI] [PubMed] [Google Scholar]
- 30.Gooding JS, Cooper LG, Blaine AI, et al. Family support and family-centered care in the neonatal intensive care unit: origins, advances, impact. Semin Perinatol. 2011;35:20–8. doi: 10.1053/j.semperi.2010.10.004. [DOI] [PubMed] [Google Scholar]