Abstract
Pharmacogenetic (PGx) testing has the potential to improve drug therapy in an individual by informing appropriate drug dosing or drug selection in order to maximize efficacy and safety. Although multiple studies have illustrated the potential benefits of such testing when applied to specific drugs across a broad range of therapy areas, the uptake of PGx testing in routine clinical practice has been relatively limited. Implementation appears to be hampered by the absence of sufficiently strong evidence linking the results of testing with actionable benefits in terms of clinical outcomes. Meanwhile, there are now adequate data to allow dosing recommendations as have been developed by bodies including the Dutch Pharmacogenetics Working Group (DPWG) and the Clinical Pharmacogenetics Implementation Consortium (CPIC) in several settings, including TPMT/thiopurines, CYP2C19/clopidogrel, CYP2D6/codeine, VKORC1-CYP2C9/warfarin, HLA-B*5701/abacavir, SLCO1B1/simvastatin and HLAB*5801/allopurinol. The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) have also recently initiated surveys in order to better understand the extent of, and the role played by, PGx testing in clinical practice. This should help identify where further training and education may be beneficial. To this end, in collaboration with ESPT, the IFCC Pharmacogenetic Laboratory Network has now been formed, with the aim of improving the uptake and quality of PGx testing.
Key words: pharmacogenetic testing, guidelines, survey
INTRODUCTION
Pharmacogenetics (PGx) was and is considered a potentially important tool to improve drug therapy by allowing tailored drug dosing or drug choice, based on the genetically determined metabolic potential of the individual. The fact that patients can differ significantly in their capacity to metabolize drugs is now recognized, yet implementation of this knowledge by analyzing patients prior to therapy, or having a PGx test to explain adverse drug reactions or lack of efficacy, still seems to be rather limited.
In the last 10 years, there has, been much progress in our understanding of how genetics may influence drug response and potential new markers are published at an exponentially increasing rate. The keywords ’pharmacogenetics‘, ’pharmacogenomics‘ or ’personalized medicine‘ retrieves over 28,000 in PubMed. This makes that application of PGx in the clinical setting is now the major challenge. As the costs of genotyping have dropped substantially in the last decade and more information about interpretation is becoming available, PGx is becoming even more attractive for routine diagnostics. Convincing scientific evidence, knowledge, and awareness by the medical profession, as well as accessibility to testing facilities are important aspects to support the uptake of PGx testing. So what is the current situation on several PGx markers?
RECENT DEVELOPMENTS IN PGX TESTING FOR CLINICAL DIAGNOSTICS
Thiopurine S-methyltransferase (TPMT)
One of the early potential applications of PGx testing was the use of TPMT genotyping prior to initiation of azathioprine (used in Crohn’s disease, dermatology, rheumatology, solid organ transplantation) or 6-mercaptopurine (used in acute lymphoblastic leukemia) therapy [1]. For TPMT, three single nucleotide polymorphisms (SNPs) can predict TPMT enzyme activity with 95% accuracy. Prescription of normal doses of azathioprine or 6-mercaptopurine to TPMT-deficient patients may be lethal, and information on this has been incorporated into the drug labeling. Despite this, twenty years after the major clinically important SNPs were identified, TPMT genotyping is still not widely used.
HLA-B*5701
By contrast, the uptake of HLA-B*5701 testing addressing suitability for abacavir treatment has been quite rapid. The availability of a randomized controlled trial showing the benefit of HLA-B*5701 testing in reducing immunologically proven adverse reactions [2] facilitated this greatly.
CYP2D6
Of the cytochrome P450 (CYP) enzymes, CYP2D6 PGx testing has been advocated as a tool to help in the appropriate dosing of antidepressants and antipsychotics. Although a good correlation between dose and CYP2D6 genotype was observed for tricyclic antidepressants, the predictive power appears to be too limited for clinicians to initiate wide-scale adoption of pre-therapy PGx screening in this field. However, specific psychiatric centers have adopted such an approach [3, 4]. The application of CYP2D6 PGx testing to guide adjuvant tamoxifen therapy for breast cancer has been subject to widespread discussion. Several studies have reported an association between CYP2D6 genotypes that lead to reduced CYP2D6 activity with a poorer outcome on tamoxifen therapy, as may to be expected from the fact that the active metabolite endoxifen is generated by CYP2D6. Indeed, endoxifen levels correspond to CYP2D6 status [5]. For outcome, one of the best studies is that of Schroth et al [6] in which 1325 individuals were analyzed for six variant CYP2D6 alleles with respect to breast cancer recurrence. However, not all studies found the association between CYP2D6 genotype and survival – those most recently discussed being the Breast International Group (BIG) (1-98) and the arimidex, tamoxifen, alone or in combination (ATAC) trials, first presented at the San Antonio Breast Cancer Symposium in 2010 [7, 8]. These controversial results mean that there is (still) an ongoing discussion regarding the need for CYP2D6 genotyping for tamoxifen therapy, which is nicely summarized in a point/counterpoint discussion in a recent issue in Clinical Pharmacology and Therapeutics [9, 10]. Recently, a meta-analysis was published, taking into account all CYP2D6/tamoxifen studies. This analysis concluded that there was indeed a correlation between CYP2D6 genotype and a poorer outcome on tamoxifen, with a hazard ratio of 1.26 [11]. It will be interesting to see if this conclusion will result in an increased uptake of CYP2D6 testing for tamoxifen therapy.
CYP2C9/VKORC1
Another PGx candidate that has not (yet) been implemented as expected is CYP2C9/VKORC1 analysis to guide coumarin therapy. Many studies have found an association between CYP2C9 and VKORC1 genotypes, and the US Food and Drug Administration (FDA) has provided dose recommendations for warfarin based on these genotypes. However, the pressure from current clinical practice, which relies on retrospective simple international normalized ratio (INR) measurements, still seems too strong in the majority of hospitals for this recommendation to be widely implemented. Recently, the results of three randomized controlled trials were published in the New England Journal of Medicine, one of which found a significant benefit associated with genotyping compared with standard of care for warfarin, in terms of reducing the time outside the INR window (by 7%) [12]. Another study on warfarin [13], comparing an algorithm without PGx testing to an algorithm including such testing, did not show a difference. The third study, addressing phenprocoumon and acenocoumarol, did indeed find a significant improvement associated with PGx testing for time within the INR window during the first 4 weeks of therapy, but this effect was no longer found after 10 weeks [14]. Interpretation of this last study is difficult: is this result in favor or not in favor of genotyping? Since one would expect the major effect of genotyping within the first few weeks, one could say that the study results support PGx testing.
However, the data on which to base the strongest conclusion – how many bleedings can be prevented? – is lacking. This can only be addressed with larger studies, with 3,000 rather than 300 participants. However, it is illustrative that in the first study [12], three bleeding events took place in the control group compared with no bleedings in the genotyped group, suggesting an advantage for the PGx screening approach.
CYP2C19
CYP2C19 testing to determine an individual’s ability to activate clopidogrel is the fifth application of PGx testing on the brink of being implemented clinically. Controversy in the literature, reflected by contradictory meta-analyses (for example [15-17]), is also hampering widespread adoption in this case. Several hospitals have implemented routine CYP2C19 genotyping for guiding clopidogrel therapy, with detected variant carriers receiving the more expensive drug prasugrel, whereas other hospitals are still continuing clopidogrel therapy without genetic testing. In several examples, hospitals reported swithching from clopidogrel to prasugrel to avoid the need for CYP2C19 genotyping.
CYP3A5
The use of genetic markers to guide tacrolimus treatment has also been the subject of many studies (see [18, 19] for recent reviews). Whereas the CYP3A5 genotype is indisputably regarded as a predictor of tacrolimus metabolism, the question is whether genotyping can compete with adequate therapeutic drug monitoring. Although it is clear that CYP3A5-based dosing will allow patients to achieve plasma levels within the therapeutic window more rapidly, an advantage in terms of a reduced risk of graft loss has not yet been demonstrated. A prospective study published in 2010 [20] showed that genotyping-based dosing improved the number of patients with plasma levels within the therapeutic window, but failed to show a reduction in biopsy-proven acute rejection. Although the relationship between CYP3A5 genotypes (and now also with CYP3A4, POR and ABCB1 genotypes) and pharmacokinetics of tacrolimus have therefore been confirmed, the fact that the relationship with biopsy-proven acute rejection has not been confirmed is an important reason why a genotype-directed strategy has not been widely adopted in the field at this moment.
Dihydropyrimidine dehydrogenase (DPYD)
Currently, in the Netherlands, there is a debate whether or not to screen for DPYD genetic polymorphisms in order to detect DPYD deficiency. This screening would be beneficial for patients receiving the chemotherapeutic agents 5-fluorouracil (5-FU) or capecitabine. The main issue here is that while toxicity is seen in only 5% of patients, 50% of this toxicity can be predicted by genetic screening. Does this warrant prospective use or not? In France, DPYD testing is used on a regular basis and is one of the most frequently performed PGx tests in that country [21].
CLINICAL IMPLEMENTATION OF PHARMACOGENETICS
The uptake of PGx testing for routine care has not been as rapid as originally anticipated. Barriers such as lack of knowledge, logistical/financial aspects and lack of evidence are being mentioned as obstacles [22]. Implementation initiatives are ongoing in the USA, for instance at the Mayo Clinic, Mount Sinai Medical Center, and at St Jude Children’s Research Hospital [23]. In Europe, the Erasmus University Medical Center in Rotterdam, The Netherlands and the Karolinska Institute in Stockholm, Sweden are providing PGx testing (ESPT congress 2013 Lisbon). These laboratories are certainly not the only ones: in The Netherlands, 12 laboratories participate in the Dutch PGx quality control (QC) scheme, 60-80 participate in the German PGx scheme (TPMT, DPYD data, resp., RfB), and in France 44 laboratories were at least active in 2012 [24], showing that PGx is indeed becoming more accessible for routine diagnostics.
At the Erasmus MC, PGx testing in routine clinical care has been implemented and available since 2005. At this center, a 30-50% yearly increase in test requests for clinical PGx has been seen, but total numbers are still modest. The most popular tests in 2013 at the Erasmus MC were TPMT (24% of total PGx test requests) and HLA-B*5701 (16% of total requests), both of which are requested prior to therapy. This was followed by CYP2D6 (16%), CYP2C19 (13%) and CYP3A4 (9%) testing (Figure 1). This picture may be totally different in other hospitals and/or countries. In a survey of six laboratories in The Netherlands in 2010, over 6,000 PGx tests were ordered, with 80% of test requests concerning CYP2D6 and CYP2C19. These requests were mainly for psychiatry (Committee for Molecular Biology Diagnostics survey of the Dutch Society for Clinical Chemistry and Laboratory Medicine [NVKC]). In France in 2011, a total of 21,037 PGx tests were ordered, with DPYD being the most requested test (4,500/year), followed by IL28B (3,300 tests/year), UGT1A1 (3,300 tests/year), and HLA-B*5701 (3,200 tests/year) [21], underlining that major differences may exist between countries (Figure 2).
Figure 1.
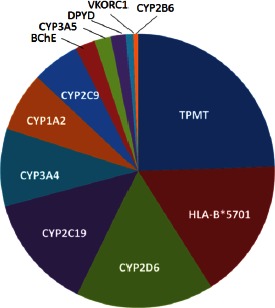
PGx test distribution 2013 for Erasmus MC Rotterdam.
Figure 2.

PGx test distribution 2011 for France [24].
GENOTYPE-BASED DOSING RECOMMENDATIONS
One of the most challenging aspects and main barriers for implementing PGx testing are clear and actionable outcomes regarding how to adjust drug therapy. In 2007, the Dutch Pharmacogenetics Working Group started to produce evidence-based dosing guidelines based on PGx markers. These recommendations, along with new recommendations, can be seen at www.pharmgkb.org [25]. The Clinical Pharmacogenetics Implementation Consortium (CPIC) has also drafted practice guidelines, including those relating to TPMT/thiopurines, CYP2C19/clopidogrel, CYP2D6/codeine, VKORC1-CYP2C9/warfarin, HLA-B*5701/abacavir, SLCO1B1/simvastatin and HLA-B*5801/allopurinol, which can be accessed through the Clinical Pharmacology and Therapeutics journal, or at www.pharmgkb.org [25].
THE INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE (IFCC) TASK FORCE ON PHARMACOGENETICS (IFCC TF-PG) SURVEY ON PGX FOR ROUTINE CARE
To get an impression of the use of PGx for routine care for two important prospective tests, the IFCC TF-PG conducted a survey beginning in 2013 on TPMT and CYP2C19 genetic testing. In total, 18 laboratories replied to the TPMT testing survey and 14 to the CYP2C19 survey. It is estimated that this is only a minor fraction of laboratories performing this test, therefore the results are to be interpreted with caution.
TPMT genotyping
For TPMT, most laboratories (95%) analyze *2/*3A/B/C alleles, whereas 15% of laboratories also analyzed *4 and/or *16 (Table 1). The use of laboratory-developed tests was 60%, compared with commercial assays used in 40% of tests. Most samples were obtained from children’s oncology units (67%), whereas dermatology, rheumatology and gastroenterology units were also mentioned as sources (30-40%). The estimated error rate is conceived to be low: 14% of laboratories report a <1% error rate, 22% <0.1%, and 50% indicated that there believed to be no errors. From our own reference laboratory experience, where we routinely run samples on two different platforms, we see an approximate 1% error rate due to technical reasons, usually the unequal amplification of alleles. The error rate reported in the Reference Institute for Bioanalytics (DGKL/RfB) molecular biology proficiency testing for TPMT (October 2013) was 13% (68/78) for a TPMT*3A/*3A sample, but this also includes administrative and interpretation errors. Quality is mostly ensured by assay validation and running positive/negative controls (66%). Surprisingly, only 28% of clinical laboratories report participation in proficiency testing. The turnaround time for TPMT genotyping ranged from 2-3 days (22%) to 2-3 weeks (33%), with the majority taking 4-7 days (44%) to produce test results. Dosing information was provided in 33% of the laboratories, was not provided by 11%, and 56% of laboratories did not provide an answer to this question.
Table 1.
Results of the IFCC TF-PG survey 2013 for TPMT testing
| TPMT genotyping (18 laboratories) | Remarks | |
|---|---|---|
| Testing for *2/*3A/B/C | 95% | 5% did not test for *2 |
| Testing for additional alleles | 15% | *4 (5%), *16 (5%), sequencing (5%) |
| Lab developed test | 60% | |
| Commercial assay | 40% | |
| Samples from children’s oncology | 67% | |
| Samples from dermatology | 33% | |
| Samples from rheumatology | 39% | |
| Samples from other departments | 45% | Gastroenterology (33%), Hematology (12%) |
| Estimated error rate <10% | 0% | |
| Estimated error rate <1% | 14% | |
| Estimated error rate <0.1% | 28% | |
| Estimated error rate 0% | 50% | |
| Do not know | 7% | |
| Quality control by proficiency testing | 28% | |
| Quality control by positive/negative controls | 61% | |
| Quality control by assay validation | 61% | |
| Quality control by run on another platform | 11% | |
| Quality control by duplicate runs (same platform) | 17% | |
| Turnaround time <24 hours | 0% | |
| Turnaround time 2-3 days | 22% | |
| Turnaround time 4-7 days | 44% | |
| Turnaround time 2-3 weeks | 33% | |
| Turnaround time >1 month | 0% | |
| Providing dosing information | 33% | IM 50%, PM 10% of standard dose (27%); IM 100% + extra monitoring, PM: another drug (5%) |
| Not providing dosing information | 11% | |
| No reply | 56% |
IFCC TF-PG, International Federation of Clinical Chemistry and Laboratory Medicine Task Force on Pharmacogenetics
CYP2C19 genotyping
For CYP2C19 genotyping, relevant for clopidogrel and antidepressant therapies, all laboratories test for CYP2C19*2, 80% test for CYP2C19*3, and 70% test for CYP2C19*17 (Table 2). For CYP2C19, there is some controversy in the literature regarding whether the CYP2C19*1/*17 patient should be interpreted as a normal or intermediate and not as a normal metabolizer. This lack of clarity is also reflected in the survey, although most (71%) laboratories report this genotype as an ultra-rapid metabolizer. Most samples are received from cardiology units (36%) followed by neurology units (22%). Interestingly, not many samples are being reported as originating from psychiatry. For CYP2C19, the error rate is also estimated to be low by the reporting laboratories: 28% report an (assumed) error rate of <0.1% and 55% report no errors. Again, quality was reported to be assessed by running positive and negative controls in a validated assay (80-90%), whereas only 56% of laboratories report participation in proficiency testing. The October 2013 RfB molecular biology survey showed one wrong report out of 47 (2%) for a CYP2C19*1/*2 sample. The turnaround time was reported as 2-3 days (22%), 4-7 days (33%), or 2-3 weeks (44%). No laboratories reported a 24-hour turnaround time, which is actually what would be needed/requested if CYP2C19 genotyping is to be used for pre-therapy screening for clopidogrel. For reporting, 66% of the laboratories report SNPs, 55% alleles, 55% test characteristics, and 44% provide dosing advice.
Table 2.
Results of the IFCC TF-PG survey 2013 for CYP2C19 testing.
| CYP2C19 genotyping (14 laboratories) | Remarks | |
|---|---|---|
| Testing for *2 | 100% | |
| Testing for *3 | 80% | |
| Testing for *17 | 60% | |
| Testing for additional alleles | 10% | *4 (5%), *10 (5%) |
| *1/*17 is translated as normal metabolism | 29% | |
| *1/*17 is translated as ultra-rapid metabolism | 71% | |
| Samples mostly from psychiatry | 9% | |
| Samples mostly from cardiology | 36% | |
| Samples equal from psychiatry and cardiology | 9% | |
| Samples from other departments | 45% | Neurology (22%), pharmacy (11%), external laboratories (11%) |
| Estimated error rate <10% | 0% | |
| Estimated error rate <1% | 0% | |
| Estimated error rate <0.1% | 28% | |
| Estimated error rate 0% | 58% | |
| Do not know | 14% | |
| Quality control by proficiency testing | 56% | |
| Quality control by positive/negative controls | 89% | |
| Quality control by assay validation | 78% | |
| Quality control by run on another platform | 11% | |
| Quality control by duplicate runs (same platform) | 22% | |
| Turnaround time <24 hours | 0% | |
| Turnaround time 2-3 days | 22% | |
| Turnaround time 4-7 days | 33% | |
| Turnaround time 2-3 weeks | 44% | |
| Turnaround time >1 month | 0% | |
| Reporting SNPs | 66% | |
| Reporting alleles | 55% | |
| Reporting predicted phenotype | 66% | |
| Dosing advice | 44% | |
| Reporting test characteristics and limitations | 55% |
IFCC TF-PG, International Federation of Clinical Chemistry and Laboratory Medicine Task Force on Pharmacogenetics
International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) test survey
In July 2013, the IATDMCT Pharmacogenetics Committee (Chaired by Prof. Dr. van Schaik) sent out a PGx survey to IATDMCT members (IATDMCT Compass, November 2013). Information was obtained from 35 laboratories in 19 different countries. A total of 23 genes were reported to be used, with 40-50% of responding laboratories offering TPMT, CYP2C9, CYP2C19 and CYP2D6 PGx testing. Overall, the number of tests offered for clinical use was quite extensive, ranging up to 21 genetic markers in some laboratories. As for the number of tests, most laboratories report between 100 and 1,000 tests per year; five laboratories reported 1,000-10,000 tests per year. The most-offered test was TPMT (53% of laboratories), followed by CYP2C9, CYP2C19, CYP2D6, and HLA-B*5701. Of the disciplines involved in testing, clinical chemistry was mentioned in 43% of cases, followed by genetics (21%), pharmacy (14%), and clinical pharmacology (10%). For reporting, most laboratories indicated that they used alleles and predicted phenotypes. In 50% of laboratories, the test result was accompanied by dosing advice.
IFCC PHARMACOGENETIC LABORATORY NETWORK
In order to facilitate exchange of experience and expertise between laboratories offering PGx testing for patient care, and thus improve uptake and quality of PGx testing, the IFCC Task Force on Pharmacogenetics has initiated the formation of an IFCC Pharmacogenetic Laboratory Network, coordinated by Prof. Dr. van Schaik. This Network will have regular meetings at IFCC international conferences, and is being developed together with the European Society for Pharmacogenetics (ESPT). Potential participants may indicate their interest by sending an email to r.vanschaik@erasmusmc.nl, with the header ‘IFCC Pharmacogenetic Laboratory Network’.
References
- 1.Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003; 348(6):538–549. [DOI] [PubMed] [Google Scholar]
- 2.Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008; 358(6):568–579. [DOI] [PubMed] [Google Scholar]
- 3.Loovers HM, van der Weide J. Implementation of CYP2D6 genotyping in psychiatry. Expert Opin Drug Metab Toxicol. 2009; 5(9):1065–1077. [DOI] [PubMed] [Google Scholar]
- 4.Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010; 12(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011; 89(5):708–717. [DOI] [PubMed] [Google Scholar]
- 6.Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009; 302(13):1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rae JM, Regan M, Leyland-Jones B, Hayes DF, Dowsett M. CYP2D6 genotype should not be used for deciding about tamoxifen therapy in postmenopausal breast cancer. J Clin Oncol. 2013; 31(21):2753–2755. [DOI] [PubMed] [Google Scholar]
- 8.Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst. 2012; 104(6):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rae JM. CYP2D6 genotype should not be used to determine endocrine therapy in postmenopausal breast cancer patients. Clin Pharmacol Ther. 2013; 94(2):183–185. [DOI] [PubMed] [Google Scholar]
- 10.Ratain MJ, Nakamura Y, Cox NJ. CYP2D6 genotype and tamoxifen activity: understanding interstudy variability in methodological quality. Clin Pharmacol Ther. 2013; 94(2):185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Province MA, Goetz MP, Brauch H, Flockhart DA, Hebert JM, Whaley R, et al. CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin Pharmacol Ther. 2013; Sep 23. doi: 10.1038/clpt.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013; 369(24):2294–2303. [DOI] [PubMed] [Google Scholar]
- 13.Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013; 369(24):2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhoef TI, Ragia G, de Boer A, Barallon R, Kolovou G, Kolovou V, et al. A randomized trial of genotype-guided dosing of acenocoumarol and phenprocoumon. N Engl J Med. 2013; 369(24):2304–2312. [DOI] [PubMed] [Google Scholar]
- 15.Geisler T, Bigalke B, Schwab M. CYP2C19 genotype and outcomes of clopidogrel treatment. N Engl J Med. 2011; 364(5):481; author reply 2. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011; 306(24):2704–2714. [DOI] [PubMed] [Google Scholar]
- 17.Zabalza M, Subirana I, Sala J, Lluis-Ganella C, Lucas G, Tomas M, et al. Meta-analyses of the association between cytochrome CYP2C19 loss- and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012; 98(2):100–108. [DOI] [PubMed] [Google Scholar]
- 18.Elens L, Hesselink DA, van Schaik RH, van Gelder T. Pharmacogenetics in kidney transplantation: recent updates and potential clinical applications. Mol Diagn Ther. 2012; 16(6):331–345. [DOI] [PubMed] [Google Scholar]
- 19.Kurzawski M, Drozdzik M. Pharmacogenetics in solid organ transplantation: genes involved in mechanism of action and pharmacokinetics of immunosuppressive drugs. Pharmacogenomics. 2013; 14(9):1099–1118. [DOI] [PubMed] [Google Scholar]
- 20.Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87(6):721–726. [DOI] [PubMed] [Google Scholar]
- 21.National pharmacogenetic activity practiced in the context of care, reporting to the Biomedicine Agency. Available at: http://www.pharmacogenetics.fr/11.html (last accessed January 2014). [Google Scholar]
- 22.Johnson JA, Cavallari LH. Pharmacogenetics and cardiovascular disease--implications for personalized medicine. Pharmacol Rev. 2013;65(3):987–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roden DM, Tyndale RF. Genomic medicine, precision medicine, personalized medicine: what’s in a name? Clin Pharmacol Ther. 2013; 94(2):169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French Biomedicines Agency. Available at: http://www.agence-biomedecine.fr (last accessed January 2014). [Google Scholar]
- 25.The Pharmacogenomics Knowledgebase. Available at: http://www.pharmgkb.org/ (last accessed January 2014). [Google Scholar]


