Abstract
Men with testicular failure, either primary or secondary, have been shown to be interested in fertility preservation. Spermatogonial stem cell (SSC) transplantation is currently being investigated as a treatment for this. Currently this experimental technique consists of cryopreservation of a testicular biopsy prior to cancer treatment, followed by optional in vitro expansion of SSCs and auto transplantation after cancer treatment. This technique may restore the pool of SSCs resulting in restoration of spermatogenesis. While this technique has not been applied to humans due to its highly experimental nature and concerns of malignant contamination, animal studies have been successful. While the offspring obtained from SSCs appear to be healthy in rodent models, there is relatively little data on any genetic and epigenetic changes that occur in either the transplanted SSCs or offspring. In humans, male germ cells undergo unique and extensive chromatin and epigenetic remodeling soon after their destiny as a spermatocyte has been secured. Errors in this remodeling may cause altered genetic information to be transmitted to offspring, resulting in abnormalities. This is particularly pertinent for cancer patients as SSCs obtained from these men may have a predisposition for genetic instability even prior to starting gonadotoxic therapies. In this article, landmarks in the evolution of SSC transplantation are reviewed, along with presently known genetic, epigenetic, and imprinting abnormalities that may occur after in vitro propagation and transplantation.
Keywords: Genetic, Epigenetic, Fertility preservation, Spermatogonial, Stem cel
Background
With the use of contemporary oncologic treatment protocols, survival is oftentimes a realistic outcome and the importance of fertility preservation has become more prominent as the majority of such men have been shown to desire children in the future1. Treatment modalities such as chemotherapy and radiotherapy can have a profound and irreversible effect on fertility2, and most patients will have transient or permanent loss of sperm production following therapy, with only 20-50% recovering spermatogenesis after therapy 3. While cryopreservation of sperm is a well-established option for post-pubertal men, options are limited for pre-pubertal boys in whom spermatogenesis has not yet started. Similarly, men with conditions resulting in primary testicular failure are in need of novel options for fertility preservation or restoration. Men with severe cases of sickle cell disease or beta-thalassemia major, which may be treated with chemotherapy for the eradication of bone marrow cells, followed by hematopoietic stem cell transplantation, may also end in a state of testicular failure4. Clearly these men, in addition to oncologic patients, are a group for which there are currently very few fertility options, and in whom novel options are needed.
Spermatogonial stem cells provide the foundation for spermatogenesis in male. Men have a small number of spermatogonial stem cells (SSCs), also known as male germline stem cells. These cells reside at the base of the seminiferous tubules of the testes, and undergo self-renewing division, proliferation, and differentiation to produce sperm5. In mice it is estimated that they constitute approximately 0.03% of the spermatogonia in the testis6. In pre-pubertal testes, the absence of differentiating germ cells creates a relatively higher proportion of SSCs compared with adult testes7.
These SSCs are responsible for continual sperm production and the transmission of genetic information from males to their progeny8. SSCs are derived from gonocytes and divide into two populations. One is constantly active to maintain continuous spermatogenesis, while the other is quiescent under normal conditions but becomes active at the time of gonadotoxic injury9. The regenerative potential of SSCs logically leads clinicians to consider options for fertility restoration, in both oncologic and non-oncologic men with testicular failure. As a result, there has been interest in identifying novel options for SSC preservation (Figure 1).
Figure 1.
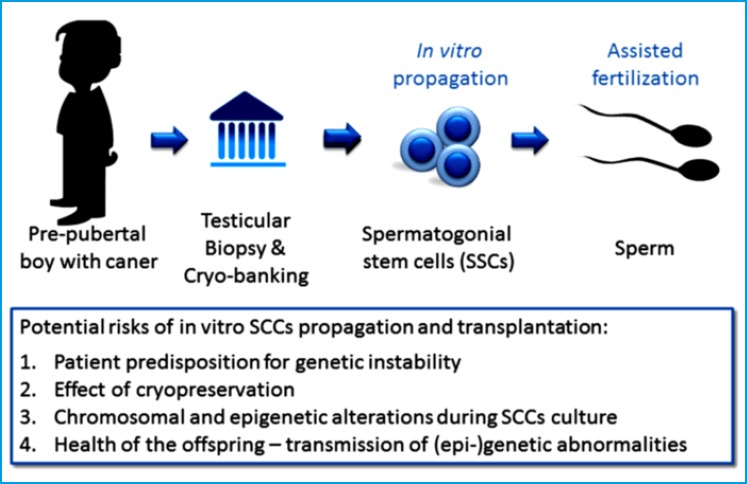
With the use of contemporary oncologic treatment protocols, survival is oftentimes a realistic outcome and the importance of fertility preservation has become more prominent. Spermatogonial stem cell (SSC) transplantation is currently being investigated as a treatment for this. Currently this experimental technique consists of cryopreservation of a testicular biopsy prior to cancer treatment, followed by optional in vitro expansion of SSCs and auto transplantation after cancer treatment. These SSCs may then be used with assisted reproductive technologies for fertility options in these patients. While the offspring obtained from SSCs appear to be healthy in rodent models, there is relatively little data on any genetic and epigenetic changes that occurs in either the transplanted SSCs or offspring.
Historical aspects
SSC transplantation was first performed by Brinster et al. in 199410. Spermatogonia from fertile mice were transplanted into the testes of infertile mice. Donor spermatogonia were able to colonize the seminiferous tubules of the recipients and initiate spermatogenesis in >70% of recipients, and up to 80% of progeny were sired by donor-derived spermatozoa. This group then applied this technique to cryopreserved donor murine testis cells, which resulted in restoration of spermatogenesis in the recipient seminiferous tubules11. Schlatt et al. applied this technique to primates, with similar spermatogenic recovery in gonadotoxin-induced azoospermia treated with autologous testicular germ cells transplantation12. These encouraging results suggest that these methods may be successfully applied to humans.
At present, re-establishing spermatogenesis after SSC transplantation is fairly well established in murine models. In mice, transplanted males are able to spontaneously mate and produce offspring and these offspring have been shown to be fertile 13,14. However, compared with fertile controls, spermatozoa from SSC transplants have been shown to have a diminished fertilization capacity when used for in vivo conception or in vitro fertilization (IVF), but not intracytoplasmic sperm injection (ICSI), as these sperm have been shown to have a lowered motility15.
The ultimate goal of SSC transplantation is to yield healthy offspring. In this respect, studies have been conflicting but seem to overall suggest favorable outcomes. Early studies demonstrated that IVF conception (but not ICSI) with transplanted mouse SSCss resulted in reduced fertilization rates, delayed blastocyst developmental rates, and smaller litter sizes compared with controls 15. Follow-up studies from this group on fetus preimplantation development demonstrated that blastocysts obtained after IVF with sperm from transplanted male mice again showed lower fertilization and developmental rates, as well as reduced numbers of inner cell mass cells and lower inner cell mass to trophectoderm ratios, implicating lower implantation potential. These differences were not seen after ICSI conception; both fertilization and development were normal when comparing controls with ICSI conceptions16. However, these results should be interpreted with caution due to technical differences in ICSI in mice versus humans. Finally, this group evaluated post-implantation development and by mating (spontaneous pregnancy) female mice with male mice after testicular stem cell transplantation. Litter sizes after testicular stem cell transplantation were decreased compared with controls and on the 17th gestational day fetuses demonstrated developmental retardation of a quarter of a day, but no major external abnormalities were observed. The live born pups were able to produce normal litter sizes, with developmentally normal pups, until the 3rd generation 13 Live born pups were developmentally normal in this study, which has also been shown in other studies. Short-term cryopreserved immature mouse or rabbit testicular tissue transplanted into mouse testes, allowed to mature, and then used for ICSI has been shown to result in grossly normal offspring 17. Likewise, long-term (>14 years) cryopreserved testis cells from mouse used for ICSI or natural mating have been shown to result in grossly normal offspring 18.
Of note, some studies seem to demonstrate reduced litter sizes, which may be due to lower sperm concentrations and poor motility, which have been demonstrated after SSC transplantation 16. In addition, the work of Wu et al. 18 demonstrates that there is some variability in the number of pups obtained per litter, regardless of if ICSI or natural mating is used. It is likely that offspring conceived by testicular stem cell transplantation have higher rates of spontaneous abortion, a form of natural selection against developmentally abnormal animals, which has never been assessed in the literature.
Because small testicular biopsies do not contain sufficient SSCs to fully repopulate the testis after transplantation, in vitro propagation of human spermatogonial stem cells will likely be necessary to obtain an adequate amount of cells for successful transplantation. In 2009, Sadri-Ardekani et al. reported their impressive results on human SSC culture and xenografting 19. SSCs were cultured and propagated from testicular tissue from men undergoing orchidectomy as part of prostate cancer treatment, and then transplanted into the testes of immunodeficient mice. SSC numbers increased 53-fold within 19 days in the testicular cell culture and increased 18,450-fold within 64 days in the germline stem cell subculture. In 4 of 6 men, xenotransplantation demonstrated the presence of functional SSCs, even after prolonged in vitro culture. Similar experiments were then performed using testis tissue from 2 pre-pubertal boys being treated for Hodgkin’s lymphoma 20. Xenotransplantation of cultured cells from these patients showed a 9.6-fold increase in the number of SSCs after 11 days of culture. Eight weeks after xenotransplantation, human SSCs were detected on the basal membrane of seminiferous tubules of recipient mouse testes. As it has been estimated that a 1300-fold increase in SSC number would be adequate to repopulate the adult human testis, based on these results, a 1 month period of culture will likely be sufficient. However, the effect of short versus long-term culture is not currently known.
The effects of in vitro culture and transplantation on the genetic and epigenetic characteristics of SSCs are still under investigation. Studies have demonstrated that cells with a high replicative potential often exhibit many abnormalities when they are maintained in vitro, in part due to chromosomal abnormalities and also from degenerative cellular changes that culminate in apoptosis21. While stem cells are considered to have special machinery to maintain their replicative potential without accumulating genetic abnormalities 22, embryonic stem cells are sensitive to stresses and often exhibit abnormalities in chromosome structure and genomic imprinting patterns after culture 23. It is possible that SSCs grown in vitro will have a higher risk of being genetically modified by exposure to growth factors and the maturation processes 24. Embryonic stem cells have been shown to have a spontaneous mutation frequency that is approximately 100 fold below that of somatic cells 25. However, in vitro culture of SSCs may induce genetic and epigenetic changes. Therefore, special attention will need to be paid to the genetic and epigenetic status of cells cultured or matured in vitro and after transplantation.
While the offspring obtained from SSCs appear to be grossly normal in rodent models 17,18, there is relatively little data on any genetic and epigenetic changes that occurs in humans (Figure 1). In humans, male germ cells undergo unique and extensive chromatin and epigenetic remodeling soon after their destiny as a spermatocyte has been secured and during the differentiation process to become a mature spermatozoon 26. Errors in this remodeling may cause altered genetic information to be transmitted to offspring, causing abnormalities.
Interestingly, the composition of the culture medium can influence the epigenetic imprinting and gene expression pattern on stem cells. However, there are limited studies of the effects of culture medium on SSCs. One group found that after culture of 2-cell mouse embryos to blastocysts, the imprinted H19 gene exhibited biallelic expression after embryo culture, and this loss of imprinting correlated with the loss of DNA methylation in the differentially methylated region implicated in H19 expression 27. Another group looking at the effect of different culture media on the behavior of offspring from 2-cell mouse embryos found behavioral differences (anxiety, locomotor activity, and spatial memory) in culture-derived mice, which could not be ascribed to differences in genotype 28. Other studies have found that the culture of mouse embryonic stem cells may give rise to fetal and offspring abnormalities, which may be linked to alterations in imprinted genes 29. However, all of these studies were on either 2-cell embryos or embryonic stem cells, not SSCs, and it is possible that embryonic stem cells are more sensitive than SSCs. One of the rare studies on SSCs found that SSCs can change their phenotype according to their microenvironment. Specifically, SSCs cultured on laminin demonstrated increased c-kit tyrosine kinase expression, which correlated with a distinct phenotype and increased renewal pattern 30. However, even in this study, the genetic and epigenetic fingerprint after culture was not examined. The susceptibilities of embryonic stem cells are likely reflective of their innate susceptibility to subtle changes in the maternal environment 21. However, it does seem that these stem cells have adapted advanced repair mechanisms, as well as the ability to proceed down an apoptotic route, to prevent the transmission of genetic or epigenetic damage to progenitors, which are generally similar to those found in postnatal stem cells in other self-renewing tissues 22,31.
Genetic Changes
The first group to look at genetic abnormalities after SSC transplantation was Goossens et al.32. In 2010, this group examined the karyotype of donor-derived spermatozoa using an array comparative genomic hybridization analysis. Numerical chromosomal aberrations could not be detected in spermatozoa from transplanted males. The karyotypes of first- and second-generation offspring were then evaluated, and all of these karyotypes demonstrated normal chromosome number. The few amplifications or deletions observed in chromosomes 1, 3, 4, 7, 12, 14 and 17 however, were also detected in the mother and therefore confirmed to be polymorphisms. While only 3 primary grafts were examined, the absence of abnormalities in the offspring is reassuring. Although this study is limited by the testing methodology, in that it fails to identify structural chromosome aberrations such as balanced reciprocal translocations of inversions (as these are not genomic losses or gains) and also ploidy variation, these results are nonetheless suggestive of genetic stability after SSC transplantation. In addition, in this study culture of the SSCs was not performed, and as such, the effect of culture on the genetic fingerprint could not be evaluated. Further studies are required to confirm these initial findings.
Embryonic stem cells seem to be sensitive to genetic alterations after long-term culture. Longo et al. showed that more than 70% of embryonic stem cells became aneuploid after only 25 cell passages, and these cells could no longer contribute to the germline by blastocyst injection 33. Interestingly, these abnormalities seemed to occur at specific chromosomal loci, which differ between species, with human embryonic stem cells susceptible to developing trisomy 17q and 12 23. However, long-term cultures (greater than 2 years, 139 passages) have demonstrated that SSCs maintain a euploid karyotype and androgenic imprint, even after ~1085-fold expansion. After long-term culture, these SSCs were transplanted and the resultant spermatozoa used for ICSI to produce fertile offspring. The only genetic difference identified during culture was a gradual shortening of the telomeres, suggesting that these cells are not truly immortal 21. This shortening occurred despite the presence of telomerase activity, suggesting that SSCs may have different mechanisms for the regulation of telomere length as compared with embryonic stem cells 21. Nevertheless they do demonstrate remarkable stability, suggesting that SSCs have unique mechanisms to prevent the transmission of genetic alterations to offspring 21. In addition, the fact that even after prolonged culture they can develop into functionally intact spermatozoa with a relatively normal fertilization potential, with normal appearing offspring, would seem to suggest that no gross genetic alterations are happening. These findings suggest that SSCs seem to be slightly more stable than other mammalian somatic cells, which eventually undergo senescence after a limited number of cell divisions 34.
Epigenetic Changes
We now know that execution of the genetic code is not simply limited to the nucleotide base sequence of DNA but also includes epigenetic programming, heritable changes that affect gene expression 35. The sperm epigenome is unique because of the requirements for successful fertilization. Notably, there is the need for chromatin to be tightly packaged into the sperm head to facilitate motility and protect the sperm from the hostile environment of the female reproductive tract. During this process, most of the histones are replaced with protamines, and the remaining histones can have a unique pattern of chemical modifications to either facilitate or repress gene transcription. This unique “fingerprint” maintains the sperm in a state in which the key genes are “poised” for possible activation in embryogenesis. Sperm epigenetic abnormalities have been linked with multiple diseases including male factor infertility and poor embryogenesis 35.
Embryonic stem cells have been shown to have widespread variability their epigenetic state, and after nuclear transfer, variation in imprinted gene expression is observed in most cloned mice, even those derived from the same subclone 36. This suggests that the variability of gene expression reflects epigenetic changes that occurred during in vitro culture among sister cells derived from a single cell, demonstrating the instability of the epigenetic state of embryonic stem cells. However, in spite of this epigenetic instability, many of these cloned animals survive to adulthood, and appear normal 36. However, whether the same can be applied to SSCs is still being determined.
In the study by Kanatsu-Shinohara, where SSCs maintained a euploid karyotype and androgenic imprint, even after ~1085-fold expansion, altered methylation patterns were found 21. Specifically, the methylation patterns of the differentially methylated regions of three paternally methylated regions (H19, Meg3 IG and Rasgrf1) and two maternally methylated regions (Igf2r and Peg10) were examined in SSCs after 3, 12, 18 and 24 months of continuous culture. The authors found that the androgenetic pattern was not altered in the two cultures at 24 months, indicating that cells were epigenetically stable. By contrast, a study of multipotent germline cells by the same group after 3 months of culture after a freeze-thaw treatment had a different methylation pattern, with the Meg3 IG region being slightly undermethylated compared with those in the SSCs 37. These results seem to indicate that, similar to multipotent germline cells and embryonic stem cells, methylation patterns are somewhat variable in SSCs after culture and transplantation.
In a study by Goossens et al. SSCs in testicular cell suspensions from 5-7 day old mice were transplanted into the testes of genetically similar recipients and then allowed to mature for 4 months 38. Immunohistochemistry was used to look at a specific panel of epigenetic modifications known to be important for the fertilization potential of spermatozoa. The authors found that, in general, the epigenetic modifications were not different after grafting compared with data from adult control mice. Specifically, DNMT1 and DNMT3A expression (the enzymes catalyzing DNA methylation), the general methylation status and the stage-specific histone modifications H3K4me3, H3K9ac, H4K12ac and H4K16ac were not different from fertile adult controls. The only difference identified was in the stage-dependent expression of H4K5ac and H4K8ac in elongated spermatids, which was altered after SSC transplantation. This difference may be a true difference in expression, but may also be due to an inability to detect these marks due to the highly condensed chromatin in these relatively mature gametes. However, the full implications of this difference are still unclear as the specific function of these histone modifications is yet unknown.
Genomic imprinting is a unique epigenetic process by which certain genes can be expressed in a parent-of-origin specific manner. The effect of SSC transplantation on imprinting is still being determined, with some studies suggesting that there transplantation does result in imprinting differences13, and others suggesting that it does not 39,40. One study has demonstrated findings suggestive of altered imprinting after SSC transplantation in rodents. First generation fetuses obtained after SSC transplantation lower in size and weight compared with controls, and demonstrated developmental retardation 13. However, these pups were able produce normal litter sizes and weight offspring for the next two subsequent generations 13. Since subsequent generations did not demonstrate these abnormalities, the authors postulated that the developmental retardation was due to an imprinting disorder, however it is noteworthy that no genetic testing on these pups was performed and this speculation is based on the gross findings of litter size, weight, and development.
Studies on imprinting status after SSC transplantation are limited, but would suggest that imprinting is not altered. In 2009 Goossens et al. examined the DNA methylation pattern in a paternally methylated gene (Insulin-like Growth Factor-2 (Igf2)), a maternally methylated gene (Paternally Expressed Gene-1 (Peg1)) and a non-imprinted gene (α-Actin) 39. For the three genes studied, no alterations in the DNA methylation patterns of spermatozoa obtained after SSC transplantation, nor in first and second generation offspring were observed. Likewise, first and second generation offspring developed normally, having similar length and weights as compared with controls. While this group only looked at 3 genes, it is impossible to know how generalizable these results are.
In a study in which embryonic male germ cells were expanded into SSCs, the resultant cells repopulated seminiferous tubules and produced spermatozoa 40. However, the offspring showed growth abnormalities and were defective in genomic imprinting. The imprinting defect persisted in both the male and female germlines for at least four generations. Moreover, germ cells in the offspring showed abnormal histone modifications and DNA methylation patterns, suggesting that fetal germ cells expanded into SSCs lose the ability to undergo epigenetic reprogramming by in vitro culture.
Interestingly, it does seem that that the genetic background of the donor cells may have an influence on the incidence of methylation errors 39. This is of concern when contemplating the use of SSC transplantation in human cancer survivors; SSCs obtained from cancer patients, even prior to starting gonadotoxic therapies, may have a predisposition for genetic instability. These patients are, by definition, more genetically unstable than non-cancer patients, and there is evidence to suggest that relaxation or loss of imprinting could represent a new epigenetic mutational mechanism in carcinogenesis 41. This instability may translate into more genetic and epigenetic abnormalities after transplantation, which may, in turn, be passed on to offspring. This is particularly concerning given some evidence that alteration of SSCs to induce self-renewal machinery can induce the development of seminomatous tumors 42. While currently there is no evidence to either support or negate this, it is nonetheless an important consideration.
In addition, the effect of cryopreservation on genetic and epigenetic alterations will need to be elucidated. It is possible that the freezing process may alter the functional epigenetic machinery, and studies should be undertaken to investigate this. One recent study in zebrafish found that cryopreservation produced a decrease in most of the studied transcripts (cxcr4b, pou5f1, vasa and sox2) and upregulation of heat shock proteins (hsp70, hsp90), results which were corroborated in human spermatozoa. These data suggest that genetic alterations caused by cryopreservation should be studied in detail in order to ensure the total safety of the technique 43.
Controlled slow-freezing of testicular tissue is currently offered to pre-pubertal boys when fertility is threatened by gonadotoxic therapies 44, as it has been shown to allow for survival of spermatogonia 45,46. Cryopreservation conserves tissues by suspending the metabolic activity of the cell, but during cooling and warming, cells are exposed to different forces (thermal, chemical and mechanical), which may interfere with their normal functioning 47. At present, controlled slow-freezing with the cryoprotectant, dimethyl sulfoxide (DMSO) is the method most commonly used to cryopreserve immature testicular tissue 48. The major advantages to controlled slow-freezing are that protocols are well-established, and relatively large samples can be frozen, up to 2 × 4 × 12 mm3 49. However, it does result in ice crystal formation, which may be damaging to SSCs, and it requires expensive computerized equipment and the process is time-consuming 48.
Vitrification has recently been explored as an alternative cryopreservation option. Samples are cooled at ultrafast rates in liquid nitrogen using high concentrations of cryoprotectants in order to remove a high proportion of cellular water and avoid ice crystal formation, minimizing cellular damage 49. Early studies have shown that vitrification is faster and less expensive than slow-freezing (only a relatively inexpensive -80°C freezer is required), as well has the potential to preserve the integrity of seminiferous tubules and maintain the long-term organotypic survival and proliferation of SSCs due to the absence of ice crystal formation 50. Vitrification has been applied to testicular tissue in immature mice 51, immature non-human primates 52, and immature humans 51,53. One potential disadvantage is that currently vitrification can only be performed successfully on very small sample sizes, up to 5 × 1 × 1 mm3 49. One of the primary concerns regarding vitrification is biological safety and sterilization, as samples are placed directly in contact with liquid nitrogen, which may mediate the transfer of pathogenic agents 54.
So far, data comparing slow-freezing cryopreservation with vitrification are few, and most seem to suggest that the methods have quite similar outcomes with respect to maintaining pre-pubertal testicular tissue cell ultrastructure, tubular morphology, and tissue function 51,53,55-57. However, at this point, controlled slow-freezing with the cryoprotectant DMSO should still be considered the standard, with vitrification considered a promising technology.
With respect to alterations in genetic and epigenetic fingerprints after cryopreservation, there is little available data. Cryopreservation has been shown to cause DNA fragmentation in spermatozoa 58,59, an effect which seems to be more pronounced in infertile men as compared with fertile men 58,60. This may be because oligozoospermia, teratospermia, and asthenospermia have all been associated with abnormal methylation of several imprinted genes 61-63. Recent data looking at the short- and mid-term impact of cryopreservation on DNA methylation of different spermatozoal genes showed that 3 maternally imprinted genes (LIT1, SNRPN, MEST), 2 paternally imprinted genes (MEG3, H19), 2 repetitive elements (ALU, LINE1), 1 spermatogenesis-specific gene (VASA) and 1 gene associated with male infertility (MTHFR) in semen samples demonstrated no alteration in methylation pattern regardless of duration of cryopreservation 64. To our knowledge there are no studies of the effect of vitrification on human sperm, likely due to its somewhat experimental nature at this point.
Finally, the choice of intratesticular tissue grafting versus in vitro culture and the resultant genetic and epigenetic effects, warrants investigation. While in vitro culture is obviously more convenient, intratesticular tissue grafting might be the better choice for fertility restoration because restoration of the stem cell niche might influence epigenetic patterns.
Conclusions
While other germline cells often acquire genetic and epigenetic changes in vitro, SSCs appear to maintain a state of relative genetic stability. These cells have been shown to retain a constant and stable growth rate after 2 years in culture 21, and subsequently maintain functional stability and were able to produce fertile offspring and these offspring displayed normal karyotypes and unmodified methylation levels in three investigated genes 32,39. Of note, some studies have demonstrated that offspring obtained from grafted SSCs have been shown to result in reduced litter size 15, altered preimplantation development 16, be smaller in size and lower in weight compared with control fetuses, and also have developmental retardation 13. However, others have demonstrated offspring that are grossly normal 17,18. In our opinion it seems likely that fetuses obtained from SSCs will have a slightly higher rate of spontaneous abortion, but that live birth progeny will likely be developmentally normal and have a normal reproductive potential. Current animal studies are limited by the small number of animals and offspring studied. In addition, it should be noted that analysis of gene expression and DNA methylation patterns is currently limited to only a selection of imprinted genes and comparative genomic hybridization is not able to detect small genetic changes. In addition, DNA methylation has not been investigated in human cultured SSCs. More research on the epigenetic level is certainly warranted before these techniques are safe for human application. In addition the optimal testicular tissue cryopreservation conditions need to be further investigated, as the technique itself may induce genetic and epigenetic changes. This is particularly true for cancer patients as SSCs obtained from cancer patients, even prior to starting gonadotoxic therapies, may have a predisposition for genetic instability, which may translate into more genetic and epigenetic abnormalities after transplantation, which may in turn be passed on to offspring.
References
- 1.Schover L.R., Brey K., Lichtin A., Lipshultz L.I., Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 20, 1880-1889 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Lee S.J., et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 24, 2917-2931 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Carson S.A., Gentry W.L., Smith A.L., Buster J.E. Feasibility of semen collection and cryopreservation during chemotherapy. Hum Reprod 6, 992-994 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Geens M., et al. Autologous spermatogonial stem cell transplantation in man: current obstacles for a future clinical application. Hum Reprod Update 14, 121-130 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Singh S.R., Burnicka-Turek O., Chauhan C., Hou S.X. Spermatogonial stem cells, infertility and testicular cancer. Journal of cellular and molecular medicine 15, 468-483 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tegelenbosch R.A., de Rooij D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutation research 290, 193-200 (1993). [DOI] [PubMed] [Google Scholar]
- 7.Gies I., et al. Spermatogonial stem cell preservation in boys with Klinefelter syndrome: to bank or not to bank, that’s the question. Fertil Steril 98, 284-289 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Kanatsu-Shinohara M., et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod 78, 681-687(2008). [DOI] [PubMed] [Google Scholar]
- 9.Ehmcke J., Schlatt S. Identification and characterization of spermatogonial subtypes and their expansion in whole mounts and tissue sections from primate testes. Methods in molecular biology 450, 109-118 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proceedings of the National Academy of Sciences of the United States of America 91, 11298-11302 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avarbock M.R., Brinster C.J., Brinster R.L. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nature medicine 2, 693-696 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlatt S., Foppiani L., Rolf C., Weinbauer G.F., Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod 17, 55-62 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Goossens E., Frederickx V., de Block G., van Steirteghem A., Tournaye H. Evaluation of in vivo conception after testicular stem cell transplantation in a mouse model shows altered post-implantation development. Hum Reprod 21, 2057-2060 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Kanatsu-Shinohara M., et al. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Hum Reprod 18, 2660-2667 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Goossens E., Frederickx V., De Block G., Van Steirteghem A.C., Tournaye H. Reproductive capacity of sperm obtained after germ cell transplantation in a mouse model. Hum Reprod 18, 1874-1880 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Goossens E., Frederickx V., De Block G., Van Steirteghem A., Tournaye H. Blastocyst development after assisted reproduction using spermatozoa obtained after testicular stem cell transplantation in mice. Hum Reprod 21, 1759-1764 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Shinohara T., et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod 17, 3039-3045 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Wu X., et al. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Hum Reprod 27, 1249-1259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadri-Ardekani H. et al. Propagation of human spermatogonial stem cells in vitro. JAMA : the journal of the American Medical Association 302, 2127-2134 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Sadri-Ardekani H., Akhondi M.A., van der Veen F., Repping S., van Pelt A.M. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA : the journal of the American Medical Association 305, 2416-2418(2011). [DOI] [PubMed] [Google Scholar]
- 21.Kanatsu-Shinohara M., et al. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 132, 4155-4163 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Cairns J. Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America 99, 10567-10570 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draper J.S., et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nature biotechnology 22, 53-54 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Bahadur G. Ethics of testicular stem cell medicine. Hum Reprod 19, 2702-2710 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Cervantes R.B., Stringer J.R., Shao C., Tischfield J.A., Stambrook P.J. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proceedings of the National Academy of Sciences of the United States of America 99, 3586-3590 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrell D.T., Hammoud S.S. The human sperm epigenome and its potential role in embryonic development. Molecular human reproduction 16, 37-47 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Doherty A.S., Mann M.R., Tremblay K.D., Bartolomei M.S., Schultz R.M. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 62, 1526-1535 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Ecker D.J., et al. Long-term effects of culture of preimplantation mouse embryos on behavior. Proceedings of the National Academy of Sciences of the United States of America 101, 1595-1600 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean W., et al. Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development 125, 2273-2282 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Morimoto H., et al. Phenotypic plasticity of mouse spermatogonial stem cells. PloS one 4, e7909 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potten C.S., Owen G., Booth D. Intestinal stem cells protect their genome by selective segregation of template DN A strands. Journal of cell science 115, 2381-2388 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Goossens E., de Vos P., Tournaye H. Array comparative genomic hybridization analysis does not show genetic alterations in spermatozoa and offspring generated after spermatogonial stem cell transplantation in the mouse. Hum Reprod 25, 1836-1842 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Longo L., Bygrave A., Grosveld F.G., Pandolfi P.P. The chromosome make-up of mouse embryonic stem cells is predictive of somatic and germ cell chimaerism. Transgenic research 6, 321-328 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Rubin H. The disparity between human cell senescence in vitro and lifelong replication in vivo. Nature biotechnology 20, 675-681 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Gannon J.R., Emery B.R., Jenkins T.G., Carrell D.T. The sperm epigenome: implications for the embryo. Advances in experimental medicine and biology 791, 53-66 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Humpherys D., et al. Epigenetic instability in ES cells and cloned mice. Science 293, 95-97 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Kanatsu-Shinohara M., et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 69, 612-616 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Goossens E., Bilgec T., Van Saen D., Tournaye H. Mouse germ cells go through typical epigenetic modifications after intratesticular tissue grafting. Hum Reprod 26, 3388-3400 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Goossens E., De Rycke M., Haentjens P., Tournaye H. DNA methylation patterns of spermatozoa and two generations of offspring obtained after murine spermatogonial stem cell transplantation. Hum Reprod 24, 2255-2263 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Lee J., et al. Heritable imprinting defect caused by epigenetic abnormalities in mouse spermatogonial stem cells. Biol Reprod 80, 518-527 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Rainier S., et al. Relaxation of imprinted genes in human cancer. Nature 362, 747-749 (1993). [DOI] [PubMed] [Google Scholar]
- 42.Lee J., Shinohara T. Epigenetic modifications and self-renewal regulation of mouse germline stem cells. Cell research 21, 1164-1171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riesco M.F., Robles V. Cryopreservation Causes Genetic and Epigenetic Changes in Zebrafish Genital Ridges. PloS one 8, e67614 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyns C., Curaba M., Vanabelle B., Van Langendonckt A., Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update 16, 312-328 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Wyns C., Van Langendonckt A., Wese F.X., Donnez J., Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod 23, 2402-2414 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Van Saen D., Goossens E., Bourgain C., Ferster A., Tournaye H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum Reprod 26, 282-293 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Karlsson J.O., Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials 17, 243-256 (1996). [DOI] [PubMed] [Google Scholar]
- 48.Baert Y., et al. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod 28, 1816-1826 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Amorim C.A., Curaba M., Van Langendonckt A., Dolmans M.M., Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reproductive biomedicine online 23, 160-186 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Curaba M., Poels J., van Langendonckt A., Donnez J., Wyns C. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil Steril 95, 2123 e2129-2112 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Curaba M., et al. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil Steril 95, 1229-1234 el221 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Poels J., Van Langendonckt A., Dehoux J.P., Donnez J., Wyns C. Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology 77, 1008-1013 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Poels J., Van Langendonckt A., Many M.C., Wese F.X., Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod 28, 578-589 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Bielanski A., Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod 24, 2457-2467 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Baert Y., et al. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril 97, 1152-1157 e1151-1152 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Abrishami M., Anzar M., Yang Y., Honaramooz A. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology 73, 86-96 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Zeng W., et al. Preservation and transplantation of porcine testis tissue. Reproduction, fertility, and development 21, 489-497 (2009). progression and therapy for prostate cancer. Prostate, 1996. 28 (4): p. 251-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammadeh M.E., Askari A.S., Georg T., Rosenbaum P., Schmidt W. Effect of freeze-thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. International journal of andrology 22, 155-162 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Thomson L.K., Fleming S.D., Barone K., Zieschang J.A., Clark A.M. The effect of repeated freezing and thawing on human sperm DNA fragmentation. Fertil Steril 93, 1147-1156 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Donnelly E.T., Steele E.K., McClure N., Lewis S.E. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod 16, 1191-1199 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi H., et al. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Human molecular genetics 16, 2542-2551 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Marques C.J., et al. Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Molecular human reproduction 14, 67-74 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Poplinski A., Tuttelmann F., Kanber D., Horsthemke B., Gromoll J. Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. International journal of andrology 33, 642-649(2010). [DOI] [PubMed] [Google Scholar]
- 64.Klaver R., et al. Routine cryopreservation of spermatozoa is safe--evidence from the DNA methylation pattern of nine spermatozoa genes. Journal of assisted reproduction and genetics 29, 943-950 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


