Abstract
PSA screening reduces PCa-mortality but the disadvantages overdiagnosis and overtreatment require multivariable risk-prediction tools to select appropriate treatment or active surveillance. This review explains the differences between the two largest screening trials and discusses the drawbacks of screening and its meta-analysisxs. The current American and European screening strategies are described.
Nonetheless, PSA is one of the most widely used tumor markers and strongly correlates with the risk of harboring PCa. However, while PSA has limitations for PCa detection with its low specificity there are several potential biomarkers presented in this review with utility for PCa currently being studied. There is an urgent need for new biomarkers especially to detect clinically significant and aggressive PCa. From all PSA-based markers, the FDA-approved prostate health index (phi) shows improved specificity over percent free and total PSA. Another kallikrein panel, 4K, which includes KLK2 has recently shown promise in clinical research studies but has not yet undergone formal validation studies. In urine, prostate cancer gene 3 (PCA3) has also been validated and approved by the FDA for its utility to detect PCa. The potential correlation of PCA3 with cancer aggressiveness requires more clinical studies. The detection of the fusion of androgen-regulated genes with genes of the regulatory transcription factors in tissue of ~50% of all PCa-patients is a milestone in PCa research. A combination of the urinary assays for TMPRSS2:ERG gene fusion and PCA3 shows an improved accuracy for PCa detection. Overall, the field of PCa biomarker discovery is very exciting and prospective.
Keywords: PSA, Screening, Prostate cancer, Prostate health index, PCA3, MPRSS2, Biomarkers
1. Prostate-specific antigen (PSA) and prostate cancer (PCa) screening
PSA-screening reduces PCa-specific mortality
The widespread and increasing use of PSA within the last 25 years has revealed PCa to be the most frequent malignancy in the Western world accounts for ~25% of all cancer cases in men [1, 2]. Since 2009, PSA-based screening for prostate cancer (PCa) has been heavily debated with clearly contrasting results of the two largest randomized screening studies. On one hand, the “European Randomized Study of Screening for Prostate Cancer“ (ERSPC) with data on more than 162,000 men from 7 European countries found a PCa-specific mortality reduction of 20% [3] in the PSA screened group, which increased to 21% after a median follow-up of 11 years [4]. When the data is adjusted for nonattendance and PSA-contamination, the mortality risk reduction rises to 29-31% [5].
In marked contrast, the “prostate, lung, colorectal, and ovarian (PLCO) screening trial“ with data from 76,693 American men, found no difference in the PCa-specific mortality after 7 years and also after 10 years of follow-up [6].
The reasons for these large differences and the drawbacks of general screening and several meta-analysis as well as the current screening strategies will be discussed in this review. The second key aspect in addition to screening in this review article is the evaluation of PSA and all PSA-based tumor markers and all currently available serum and urine biomarkers.
Differences between ERSPC and PLCO trial
First, the wide distribution of the PSA test in the U.S. resulted in a significant so-called PSA-contamination of the control group in the PLCO trial as at least 52% of the control group underwent at least one PSA test during the six years of screening. With a compliance rate of 85% in the screening group, the real difference in PSA testing was only 33%. However, it is more likely that within the PLCO screening trial actually only 15% of men in the control group never had a PSA test [7]. Thus, when 85% of men in the control group had a PSA test at least once in their life including 44% already before entering the study, the difference to the 85% of PSA-screened men is actual zero [8]. Thus, the PLCO trial became a comparison of frequent screening versus (somewhat) less frequent screening. Therefore, a mortality difference is very unlikely between PSA-screened and officially non-screened men in the PLCO trial. In the ERSPC study, the PSA contamination rate was much lower with 15% at the most [9]. The highest reported contamination from a single ERSPC center was 24% while other center specific contamination rates were below 10%. With 82% of all screen group participants screened at least once, the difference between screening and no screening in the ERSPC was 67% or at least 58%. This difference is ~2-fold in comparison to the PLCO (33%).
Second, for those screened men in the PLCO trial with positive tests (abnormal digital rectal examination (DRE) and/or PSA ≥4ng/ml) only 40.2% and 30.1% respectively, were biopsied [9]. The low biopsy rate indicates that two-thirds of men suspicious for PCa were not subsequently diagnosed. In the ERSPC, 85.8% of screening participants with positive tests (abnormal DRE and/or PSA ≥4ng/ml, changed 1996–1997 to PSA cutoff ≥3ng/ml without DRE) were in fact biopsied [3]. This rate is 2 to 3-fold higher as compared with the PLCO trial.
Third, there was no difference in stage distribution for all organ confined stages I and II between the screening arm (95.9%) and the control arm (94.4%) in the PLCO trial [6]. Also, the Gleason scores of ≤6 were not different between the screening (65.7%) and the control arm (60.3%). Since a PCa in such early stages normally does not show any symptoms, it is possible that the PSA-contamination in the control group was much higher than 52%. The stage distribution in the screening group in the ERSPC was 80.9% for stages I and II, while the control group had a significantly lower rate of the early stages with 58.9% [3]. Further, the proportions of men who had a less aggressive PCa with Gleason scores of ≤6 were 72.2% in the screening group and only 54.8% in the control group while a Gleason score ≥7 was detected in 27.8% in the screening group and in 45.2% in the control group [3]. These differences were predicted. Additionally, there was a relative reduction of 30% of detected metastatic PCa in the screening group [10].
Beside these above mentioned three important differences between both trials, the shorter follow-up of the PLCO trial as compared with the ERSPC [9] and the insufficient statistical power of the PLCO with its high PSA-contamination do also influence the PCa-specific mortality [8]. A reduced overall mortality should not be expected because the overall risk for men to die of PCa is reduced from 3% to 2.4% with PSA screening as shown from the ERSPC data [11].
All these characteristics of the PLCO trial with a narrow window of only 33% difference in screening between the two arms [9], the low biopsy rate, and the resulting identical stage distribution of the detected PCa in the screen and control arms [9], results in features that make the occurrence of a difference in PCa-mortality unlikely even with a longer follow-up [9].
Drawbacks of screening
On the other hand, the 21% reduced PCa-specific mortality in the ERSPC that increased in single centers to 32% [12], 44% [13] or 51% with correction for nonattendance and contamination [14] has drawbacks with a significant overdiagnosis and detection of insignificant cancers. Overdiagnosis is a major problem for regular PSA screening and the risk to suffer from any PCa is 1.5-fold. The risk of a stage I PCa is almost 2-fold when summarizing data from 6 screening trials with almost 400,000 men [15]. While overdiagnosis itself may harm the patient in the way of a negative psychological effect, subsequent overtreatment can lead to incontinence, impotence and other clinical side effects. Data from the ERSPC show that 32-43% of low risk PCa may have avoided treatment [16]. In those cases, the active surveillance strategy is accepted as a non-treatment option for all low risk PCa-patients (reviewed in [17, 18]). Data from 439 patients initially screened and positively identified with PCa, showed no tumor progression in 86% of individuals after a 10 year follow-up [19].
While active surveillance becomes an increasingly popular management option it should be mentioned that the definition of those early disease stages only relies on biopsy results. An insignificant tumor on biopsy may become clinically significant in the final pathology of the prostatectomy specimen. Two studies on more than 12,000 men treated with radical prostatectomy showed that only 1/4 to 1/3 of tumors were still defined as insignificant on the final prostate pathology [20, 21]. Additionally, 1/5 to 1/3 also showed an upgrading from the biopsy to the final pathological result and ~10% had already extracapsular extension of the disease [20, 21]. This demonstrates that the proportion of men with an apparently insignificant PCa who actually have a clinical relevant tumor is not negligible [20, 21]. The topic of insignificant tumors has been already discussed elsewhere [22]. Finally, at long term follow-up after radical prostatectomy a biochemical recurrence occurs in up to 40% [23], indicating that these tumors were not insignificant but already in an advanced stage.
Problems of meta-analysis on PSA-screening
The differences between these two screening studies are substantial and it is questionable if data from the PLCO can be compared with the ERSPC data or used in meta-analysis [11]. Thus, it is not surprising that most meta-analysis incorporating the PLCO study and other screening studies with different clinical designs (reviewed in [24]) could not prove a lower PCa-specific mortality with PSA screening. Since 2010, at least 5 meta-analysis (including updates) have been published [15, 25-27], with almost all concluding no evidence of a PCa-specific mortality reduction. Only one meta-analysis found a 24% PCa-specific mortality reduction with PSA-screening using as exclusion criteria insufficient follow-up length, unacceptably high PSA-contamination in the control group or insufficient participation in the screening group [27]. All other meta-analysis did not consider those important aspects. Exemplarily, the meta-analysis of Djulbegovic et al. [15] showed an inconsistency grade of 55% and should be therefore valued as questionable [24, 28]. It should be emphasized that a meta-analysis mostly based on studies with severe limitations cannot correctly answer the question of PSA-screening utility [29].
However, other screening studies without randomization (Tyrol study), with low numbers of patients and no PSA in the first two screening rounds (Norköping study), with a too short follow-up (French ERSPC) or with several methodological limitations (Quebec study) have been extensively reviewed elsewhere [15, 30] and will not be discussed here.
Current screening strategies
Despite strong discrepancies, the authors of the ERSPC and PLCO trial found shared conclusions for the future use of PSA in PCa screening [31]. PSA is able to predict PCa up to 30 years in advance [31]. Based on an initial PSA test (without age specification, but 40-45 years seems useful, at least before 60 years [32]) the frequency of follow-up PSA tests should be estimated depending on the individual PCa risk considering age, comorbidities, prostate volume, race and PCa family history [31]. With known PSA values, risk calculators can be used for biopsy indications [31].
Here, the current recommendation of the American Urological Association (AUA) and the European Urological Association (EAU) on PCa screening from 2013 should be mentioned [33, 34]. According to the American guidelines, PSA is not recommended for individuals below the age of 40 years or higher than 70 years. Regular biannual screening after careful counseling should be performed in men aged 55-69 years [33]. In Europe, a baseline PSA is recommended for men 40-45 years to initiate a risk-adapted follow-up approach with the purpose of reducing PCa-mortality and the incidence of advanced and metastatic PCa [34]. To prevent overdiagnosis and overtreatment, multivariable risk-prediction tools will be necessary [34]. This strategy seems to be a reasonably balanced approach so far.
The economically emphasized and widely distributed recommendation of the “US Preventive Services Task Force” completely abandoned PSA as screening tool [35] and has already been critically discussed elsewhere [11, 36].
Considering the above-mentioned points, we view the ERSPC results as reliable. A reduced PCa-specific mortality by more than 20% can be achieved. However, the likelihood of overdiagnosis is about 2-fold. PSA needs to be used in a more rational, strategic way and active surveillance should be included as a serious management option in appropriate patients.
2. Biology of PSA and its correlation with PCa
After the development of the first immunoassay for the PSA antigen in serum, the PSA test replaced the PAP test and revolutionized the management of PCa. Biologically, PSA is responsible for semen liquefaction and secreted into the seminal plasma but a retrograde release of PSA into the bloodstream is a rare event in healthy men (reviewed in [37]). An excessive escape of PSA into the blood circulation only occurs in cases of destruction of the basement membrane of prostate epithelial cells. Although an increased PSA can also be caused by benign prostate diseases, such as benign prostate hyperplasia (BPH) or prostatitis, there is a strong correlation of serum PSA with the incidence of PCa [37]. Thus, increased PSA levels indicate pathologies of the prostate gland including PCa, but PSA is not cancer-specific. In addition to the relationship of an elevated PSA with a higher PCa risk, PSA can predict the occurrence of PCa several years in advance as already mentioned [37]. Furthermore, PSA can predict death from PCa with up to 25 years in advance [38, 39]. The risk to die from metastatic PCa is as high as 44% for men aged 45 to 55 years when their PSA is within the 10th percentile as compared with those men with a PSA below the median with a risk of <0.3% [39].
3. Efforts to overcome PSA limitations
While PSA is the key parameter for the management of a known PCa, there are decisive limitations for diagnosing PCa. As mentioned, benign prostate diseases as well as prostate manipulations such as bicycling, digital rectal exam (DRE), biopsy, catheterization or ejaculation can also cause at least temporary elevated PSA serum concentrations [40]. This leads to low specificity if a single PSA measurement is used to predict PCa, especially in the PSA “grey zone” of 2-10ng/ml [40]. Avoiding factors such as bicycling, DRE or ejaculation a few days before a PSA blood draw may facilitate interpretation of results. In addition, a biological variation of the PSA value up to 20-30% [41] should be considered. A simple repeat measurement of PSA can significantly reduce the number of prostate biopsies [42] but 60-80% of all biopsies are still unnecessary. The traditional PSA cutoff of 4ng/ml is no longer valid because the PCa detection rate at the 2-4ng/ml range [43] is comparable to the 4-10ng/ml range in the PSA screening environment today [44]. Further, differences between PSA assays additionally with or without WHO calibration may complicate the interpretation of results [45, 46].
To increase the specificity of PSA, different parameters have been developed like PSA density (ratio of PSA to prostate volume), PSA velocity (change of PSA over a time period) or age-/race-specific reference ranges [40]. All these PSA based parameters have been only partially successful. PSA density is perhaps the single most specific parameter but requires an ultrasound procedure to obtain an accurate assessment of prostate size.
4. PSA based serum markers
PSA complexes with proteinase inhibitors
In the early 1990s two independent groups found PSA to exist in different molecular forms [47, 48]. Approximately 65-95% of PSA is bound to alpha-1-antichymotrypsin (PSA-ACT) while the remaining PSA circulates as free PSA (fPSA). PSA-ACT is higher in PCa-patients compared with non-PCa-patients [48]. The enzymatically-active form of PSA is rapidly and irreversibly complexed with prostate inhibitors while inactive PSA (free PSA) is not complexed [49]. PSA also complexes with alpha2-macroglobulin (A2M), which is not measurable with the current assays. The measurement of PSA-A2M needs rather complicated methods [50]. A very small amount of PSA is also complexed with the protease inhibitor alpha1-protease inhibitor (API). The very small amounts of API to total PSA (tPSA) are analytically challenging [51] so that both the A2M-PSA complex and API-PSA complex assays have never become commercially available. Current PSA immunoassays measure free and complexed PSA which is sometimes referred to as total PSA.
Using a blocking antibody against fPSA, all complexed PSA (cPSA) can be also measured. The cPSA only reaches comparable results to the ratio of fPSA to tPSA (f/tPSA ratio or percent free PSA, %fPSA) when also used as ratio to tPSA, but not as a single parameter [52]. Since the tPSA is the sum of ACT-PSA and fPSA the ratios of cPSA to tPSA should be equal to fPSA to tPSA in a clinical correlation. The fPSA to tPSA ratio was used earlier than cPSA to tPSA ratio. Therefore the vast majority of clinical utility studies on molecular forms of PSA have been published on %fPSA.
Clinical relevance of %fPSA
Since the middle of the 1990s the %fPSA has become a clinically relevant parameter to improve specificity of PSA alone [53]. This has been confirmed (reviewed in [54]). A meta-analysis on %fPSA found an area under the ROC curve (AUC) of 0.68 for more than 2800 patients within the tPSA “grey zone” of 4-10ng/ml [55]. But the authors concluded that %fPSA can only be a useful adjunct to PSA-based screening when reaching extreme values such as <7% [55]. When using high %fPSA cut-offs, the number of unnecessary biopsies could be reduced by ~10-20%. However, for a more accurate interpretation, factors such as prostate volume, prostatitis, or prostatic intraepithelial neoplasia should be considered (reviewed in [52]). Currently, %fPSA is used within multivariable models such as artificial neural networks (ANN) or logistic regression (LR) based nomograms to predict the PCa risk in a subsequent prostate biopsy (reviewed in [56]). Table 1 (modified from ref. [56]) shows the improvement of %fPSA and ANN or LR models compared with tPSA. Regardless of the different assays for tPSA and fPSA [57] and the different PSA ranges investigated, enhanced specificities by using %fPSA within ANN or LR models were observed. However, only some models are freely online available [57, 58] as illustrated in Figures 1 and 2.
Table 1.
Examples for multivariate models using %fPSA for diagnosis of PCa (1998-2004)
| First Author [Ref.] (n of pts.; % of PCa) |
Year | Screening | Model (ranking) | PSA assays (company) | tPSA range (ng/ml) | contributing factors (if numbered, by value) | AUC | Specificity at 95% sensitivity |
|---|---|---|---|---|---|---|---|---|
|
Carlson (n=3773; 33% PCa) |
1998 1999 | no yes | LR | Tosoh (Dianon) | 4-20 | l.%fPSA, 2.age 3.tPSA | n.a. | 34 (LR) 23(%fPSA) |
|
Virtanen (n=212; 25% PCa) |
1999 | yes | l.LR 2. ANN |
ProStatus (Wallac) | 3-10 (3-45) |
l.%fPSA 2.DRE 3.heredity |
0.81 (LR for tPSA3-45) %fPSAn.a. | n.a. |
|
Finne [58] (n=656; 23% PCa) |
2000 | yes | 1.ANN 2.LR |
ProStatus (Wallac) | 4-10 | l.%fPSA 2.volume 3.DRE4.tPSA |
n.a. | 33 (ANN) 24 (LR) 19 (%f PSA) |
|
Babaian (n=151; 25% PCa) |
2000 | yes | ANN | Tandem R (Beckman Coulter) |
2.5-4 | %fPSA, tPSA, age, PAP, CK | 0.74 ANN (0.64%fPSA) |
51 (ANN) 39 (PSAD) 10(%fPSA) |
|
Horninger (n=3474; n.a.) |
2001 | yes | ANN LR |
Abbot IMX (Abbott) |
n.a. PSA>4 or DRE+ |
age, tPSA, %f PSA, DRE, volume, PSAD, PSAD-TZ, TZ-volume | n.a. |
~27 (ANN) ~13 (%f PSA) ~13(tPSA) |
|
Stephan (n=1188; 61% PCa) |
2002 | no | ANN LR |
IMMULITE (Bayer) |
2-20 | 1.DRE 2.%f PSA 3.volume 4.tPSA5.age |
0.86 (ANN) 0.75 (%f PSA) |
43 (ANN) 26 (%f PSA) |
|
Remzi (n=820; 10% PCa) |
2003 | no | ANN, LR | AxSYM (Abbott) |
4-10 | tPSA,%f PSA, volume, PSAD, PSAD-TZ, TZ-volume | 0.83 (ANN) 0.79 (LR) 0.745 (%f PSA) |
68 (ANN) 54 (LR) 33.5 (%f PSA) |
|
Finne (n=1775; 22% PCa) |
2004 | yes | 1. LR 2.ANN |
ProStatus (Wallac) | 4-10 | 1.DRE 2.%f PSA 3.volume 4.tPSA | 0.764 (LR) 0.760 (ANN) 0.718 (%f PSA) |
22 (LR) 19 (ANN) 17(%f PSA) |
|
Sokoll [70] (n=566; 43% PCa) |
2010 | not available | 0.79 (LR model) | 80 | 45 |
Abbreviations: AUC: area under the (ROC) curve; n.a.: not available; LR: logistic regression; ANN: artificial neural network, PAP: prostate alkaline phosphatase, CK: creatinkinase; PSAD: PSA density, PSAD-TZ: transition zone density; DRE: digital rectal examination
Fig. 1.
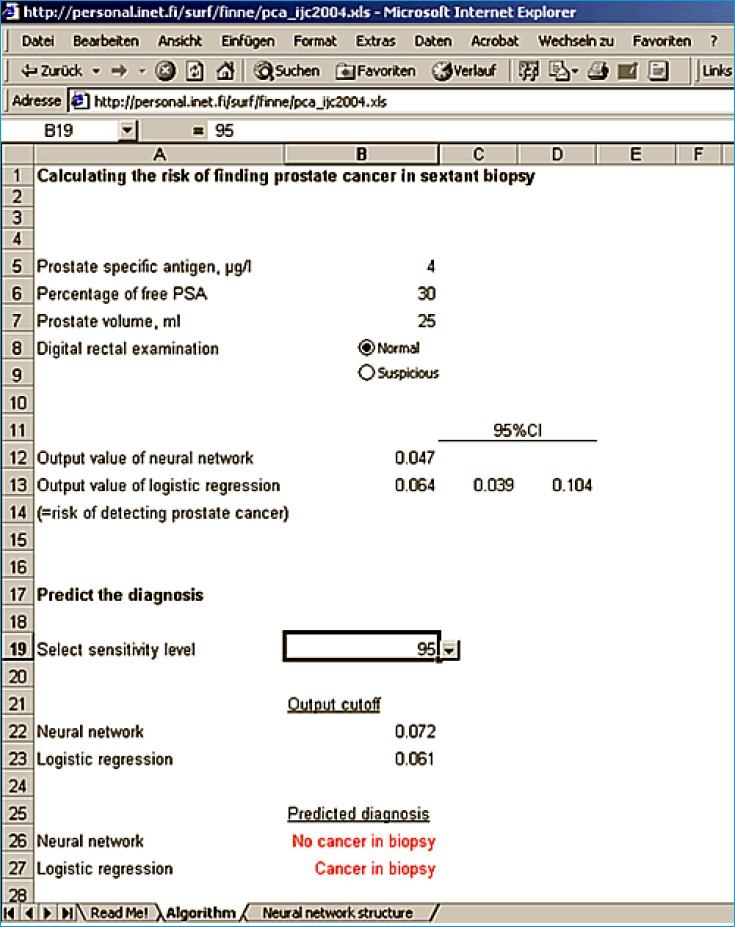
1. The program at www.finne.info to estimate the risk of PCa based on ANN and LR at the 95% sensitivity level.
Fig. 2.
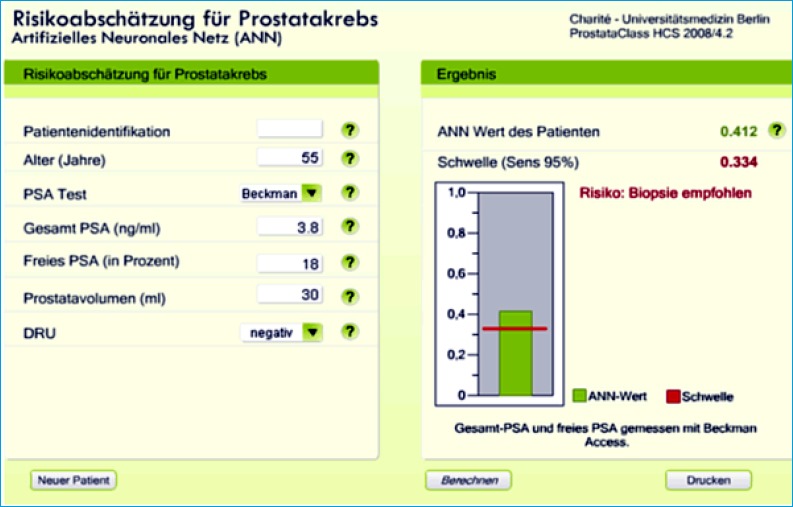
Program “ProstataClass” version 2008 for 5 different PSA assays at http://urologie.charite.de and the link: “ProstataClass”. Provided example of the ANN output (only available in German) indicating “Risiko” (risk)” at the 95% sensitivity level.
Subforms of free PSA
Beginning in 2000, researchers focused to define subforms of fPSA in search for ways to further enhance the specificity of %fPSA. The free PSA became more complex [59]. Figure 3 indicates the different molecular forms of PSA.
Fig. 3.
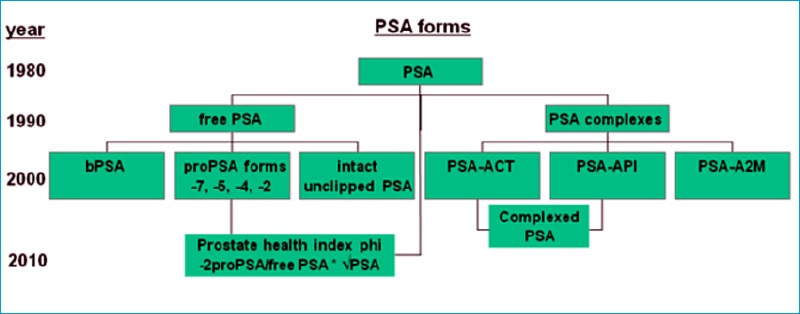
Molecular forms of PSA and the prostate health index phi including the respective times of detection.
The so called “benign”PSA (bPSA) is a clipped subform of free PSA that is highly associated with the transition zone of the prostate, containing BPH nodules. The bPSA could potentially be used as marker for BPH, but was unable to distinguish between BPH and PCa [60, 61]. But within a multivariable model, bPSA improved specificity of %fPSA by ~15% [61].
Another fPSA subform was detected by using anti-PSA antibodies that do not recognize internally cleaved PSA at Lys145-Lys146. This special PSA subform was termed “intact”, unclipped PSA (iPSA) [62]. Although iPSA could distinguish between PCa and BPH, its further use has been limited since a commercial assay is lacking. A lab-based test may now be available as a panel termed 4K. This panel combines tPSA, fPSA, iPSA and the human glandular kallikrein 2 (KLK2) and showed a high predictive accuracy [63, 64].
Another subform, proPSA is termed [-7]proPSA and contains a seven amino acid N-terminal pro-leader peptide in this native form, which is rapidly truncated by proteolytic cleavage to [-4]proPSA, or [-2] proPSA. The proPSA derivative [-2]proPSA cannot be cleaved to form enzymatically-active PSA and accumulates in the prostate cancer regions of the prostate. A research assay measuring the [-7, -5] proPSA was of limited usefulness and has subsequently not been commercialized (reviewed in [52]).
Only the [-2]proPSA [65] and especially the commercial and FDA-approved [-2]proPSA [66, 67] showed the expected further improvement in specificity over %fPSA. Since 2010 the Prostate health index (phi) (calculated as: [-2]proPSA / fPSA * √PSA) has been used to discriminate between PCa and non-PCa [68]. These data on phi have been confirmed in large multicenter cohorts and it further seems that phi may preferentially detect aggressive PCa [66, 67, 69, 70].
Clinical importance of prostate health index phi
In 2012, [-2]proPSA was approved by the FDA to be used for initial biopsy decision in men with PSA in the range of 4-10ng/ml and negative DRE. A comprehensive review summarizes all aspects on different proPSA forms as well as the cost-effectiveness of phi [71]. The addition of phi to the common screening strategy with PSA alone slightly increases the costs of the blood tests but could reduce the number of required office visits, laboratory tests and biopsies [71].
A recent meta-analysis for phi and the percentage of [-2]proPSA to fPSA (%[-2]proPSA) analyzed data from more than 5000 biopsied men within the tPSA range of 2-10ng/ml [72]. At 90% sensitivity a pooled specificity of ~32% for phi and %[-2]proPSA was found; both parameters were superior to tPSA and %fPSA. Table 2 provides data on all available studies using phi with at least 200 biopsy proven patients.
Table 2.
Selected studies with more than 200 subjects on Phi (2010-2013)
| First author [Ref.] (n of pts.;% of PCa) |
Year | Phi cutoff | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
|
Sokoll [70] (n=566; 43% PCa) |
2010 | not available | 0.79 (LR model) | 80 | 45 |
|
Jansen [68] (n=756; 50% PCa) |
2010 2 cohorts |
not available | 0.75(0.71) | 90 | 31 |
|
Liang (n=250+250; 50% PCa, matched) |
2011 | 36.45 (at 90% spec.) | 0.73 | 42 | 90 |
|
Guazzori (n=268; 40% PCa) |
2011 | 48.5 (at 90% spec.) Hybr. calibr. | 0.76 | 43 | 90 |
|
Catalona [66] (n=721;17% PCa) |
2011 | 21.3(24.1) Hybr.calibr. |
0.70 | 95(90) | 16(26) |
|
Loeb(see also[66]) (n=721;17%PCa) |
2013 | 24.3(27.9) WHO calibr. |
0.70 | 95(90) | 16(27) |
|
Lazzeri (n=222; 32% PCa) |
2012 | 28.8 Hybr. calibr. |
0.67 | 90 | 25 |
|
Stephan [67] (n=1362; 49% PCa) |
2013 | 31(24) | 0.74 | 95 (90) | 15(35) |
|
*Stephan [98] (n=246; 45% PCa) |
2013 | 27.5 | 0.68 | 90 | 21 |
|
*Ferro (n=300;36% PCa) |
2013 | 31.6 | 0.77 | 90 | 40 |
|
Ito (n=239;22 % PCa) |
2013 | 23.9(24.9) Hybr. calibr. |
0.72 | 95(90) | 28(33) |
|
Lazzeri [69] (n=646;40%PCa) |
2013 | 27.6 41.5 61.7 |
0.67 | 90 63 25 |
19 62 90 |
|
*Scattoni (n=211; 33 % PCa) |
2013 | 28.3(30.6) 24.1(35.5) |
0.70 all 0.69 1st bx 0.72 2nd bx |
90(80) 90(80) |
16-34 7-47 |
|
Ng (n=230;9 % PCa) |
2013 | 26.5 Hybr.calibr. |
0.78 | 90 | 50 |
*alsoPCA3 values available
Abbreviations: AUC: area under the (ROC) curve; bx: biopsy; Hybr. calibr.: Hybritech calibration (for PSA & fPSA); n.a.: not available; WHO calibr, calculated (not measured) as WHO calibrated
Increasing phi values were associated with an increased probability of detecting Gleason ≥7 PCa [66, 67]. Two studies with more than 2,200 men independently found that the relative risk of any PCa is 3.6-fold [67] to 4.7-fold [66] higher in those men with phi values in the highest as compared with the lowest quartile. The risk of a Gleason ≥7 PCa increases 1.6-fold with phi values the highest quartile [66]. Phi had also significantly higher median values in aggressive PCa and the proportion of Gleason ≥7 PCa increased with the phi score [67].
However, when using phi within multivariable models, the AUC-gain was very modest or not visible [67, 73]. As reviewed [74], the inclusion of new biomarkers such as urinary prostate cancer antigen 3 (PCA3) and [-2]proPSA in risk calculators amounted only to a marginal improvement in the accuracy of these prediction tools. Despite this, phi shows overall promising data, especially when focused to detect aggressive PCa.
5. Other prostate cancer serum marker
The kallikreins
Beside the pancreatic/renal kallikrein KLK1, KLK2 and KLK3 which is widely known as PSA, 12 new members of the human kallikrein family have been characterized [75]. The human kallikrein genes are named KLK1 to KLK15 and they encode for the proteins KLK1 to KLK15.
KLK2 can convert proPSA to active PSA (reviewed in [52]) and has been investigated extensively [75]. However, early promising data could not be confirmed (reviewed in [52] and [54]) and KLK2 has not been transferred into a commercial assay.
Beside KLK2 and PSA, at least 6 other kallikreins (KLK4, KLK10-13 and KLK15) are also expressed in relatively high amounts in prostate tissue [75] but again, no commercial immunoassay is available. Only KLK11 showed promising values but data have not been confirmed independently (reviewed in [52] and [54]). Reviews on kallikreins have been published elsewhere [75, 76].
Other serum markers
Details on several markers like caveolin, IGF, PSP94, macrophage inhibitory cytokine 1, cytokine macrophage migration inhibitory factor, the calcium-binding proteins S100A8 and S100A9 that have never reached clinical significance or at least assay commercialization have been already reviewed [77].
The extracellular matrix protein Spondin-2 [78], and Galectin-3, a tumor-associated protein [79], have been published in 2013. Spondin-2 showed an extremely high AUC of 0.95 as compared with %fPSA (0.81), sarcosine (0.67) and tPSA (0.56) [78]. The galectin-3 levels were in contrast only compared in the sera of metastatic PCa-patients with non-cancer patients [79].
Sarcosine in serum
In the above mentioned study on Spondin-2 [78], sarcosine showed limited success. Others found an increased PCa risk and a further increased risk for aggressive PCa (odds ratio 1.44) with increasing sarcosine levels [80]. In contrast, another large study found high sarcosine and glycine concentrations to be associated with a reduced PCa risk of borderline significance (odds ratio 0.86) [81]. Other studies on sarcosine in serum and plasma with smaller numbers of patients also showed inherent data [82-84]. Interestingly, first data on sarcosine have been published in urine.
6. Urine markers
Sarcosine
Sreekumar et al. [85] found sarcosine to be significantly higher in urine sediments and supernatants in PCa as compared with men without PCa [85]. In 53 men, the AUC for sarcosine (0.69) was significantly higher than the AUC of PSA (0.53) at PSA levels of 2-10ng/ml [85]. In contrast, another study in 139 men found significantly lower sarcosine values in PCa-patients compared with non-PCa-patients and no difference between healthy men and PCa-patients [86]. Also, %fPSA (AUC: 0.81) had a significantly larger AUC than sarcosine (0.63), and PSA (0.64) was equal to sarcosine [86]. Sarcosine was measured with a commercial amino acid assay and values were normalized to urine creatinine [86]. There was a strong correlation (rs=0.86) between the sarcosine and creatinine showing that the occurrence of sarcosine in urine is due to renal excretion [86]. Sarcosine is not specific to prostate tissue nor is it related to tumor aggressiveness or recurrence, which is in contrast to PCA3, which is prostate-specific and not found elsewhere. Therefore it is unlikely that sarcosine is suitable as a marker for PCa detection. Further details on sarcosine have been reviewed recently [87].
PCA3
PCA3 is a noncoding messenger RNA (mRNA) and is 66-fold overexpressed in PCa tissue. A molecular assay for PCA3 was introduced in 2006 [88]. In 2012, this assay was FDA-approved to aid in the decision for repeat biopsy in men ≥50 years (reviewed in [89]).
Two independent multicenter studies found excellent clinical value of the PCA3 assay in men with previous negative biopsies [90] and with first and repeat biopsies [91]. Haese et al. [90] found PCA3 to be better than %fPSA and that PCA3 was independent of prostate volume, age and tPSA. The PCa likelihood increased with the PCA3 score. However, the PCa detection rate was only 47% in those patients with PCA3 scores >100 [90]. This problem has been reported in several other studies (reviewed in [89]) proving a low sensitivity with a PCA3 cutoff of 100.
Nonetheless, several studies have proven the clinical value of PCA3 to improve specificity over PSA and %fPSA (reviewed in [89]). Table 3 provides data on studies with at least 200 patients. With exception of two studies (AUC 0.59 and 0.83), the AUCs for PCA3 are ~0.7.
Table 3.
Selected studies with more than 200 subjects on PCA3 (2007-2013)
| First author [Ref.](n of pts.; % of PCa) | Year | PCA3 cutoff | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
|
Marks (n=226; 27 % PCa) |
2007 | 35 | 0.68 | 58 | 72 |
|
Haese [90](n=463; 28% PCa) |
2008 | 35 | 0.66 | 47 | 72 |
|
Deras [91](n=570; 36% PCa) |
2008 | 35 | 0.69 | 54 | 74 |
|
Ankerst (n=443; 28% PCa) |
2008 | 25 | 0.665 | 63 | 60 |
|
Chun [92] (n=809; 39% PCa) |
2009 | 17 | 0.68 | 81 | 45 |
|
Hessels (n=336; 40% PCa) |
2010 | 35 | 0.72 | 61 | 74 |
|
Auprich (n=621; 41% PCa) |
2010 | 17 (24, 35) | 0.73-0.75 | 88 | 45 |
|
Roobol (n=721; 17% PCa) |
2010 | 35 | 0.635 | 68 | 56 |
|
Ploussard (n=301; 24% PCa) |
2010 | 35, (25, 30) | 0.69 | 44-59 | 67-79 |
|
Aubin (n=1072; 18% PCa) |
2010 | 35 | 0.69 | 48 | 79 |
|
De la Taille (n=516; 40% PCa) |
2011 | 35 | 0.76 | 64 | 76 |
|
Perdona (n=218; 33.5% PCa) |
2011 | 51 | 0.83 | 70 | 81 |
|
Bollito n=1237; 26% PCa) |
2012 | 35 (39, 50) | 0.68 | 73 (n=949 at PSA 4-10) |
49 (n=949 at PSA 4-10) |
|
Crawford (n=1913;42%PCa) |
2012 | 10 (25,35) | 0.71 | 86.5 | 37 |
|
Stephan [98](n=246; 45% PCa) |
2013 | 28 | 0.74 | 73 | 64 |
|
Hansen [93](n=692; 46% PCa) |
2013 | 21 | 0.74 | 79 | 59 |
|
Scattoni (n=211; 33% PCa) |
2013 | 16.5(13.5, 23.5) | 0.59 | 80 (90) | 16-34 |
|
Tombal (n=1024; 18% PCa) |
2013 | 20 | n.a. | 87 | 55 |
|
Gittelman (n=466; 22% PCa) |
2013 | 25 | 0.71 | 77.5 | 57 |
|
Ferro (n=300; 36% PCa) |
2013 | 22 | 0.73 | 90 | 40 |
|
Goode (n=456;19%PCa) |
2013 | 35 | 0.73 | 62 | 75 |
| Ruffion(n=601; 46% PCa) | 2013 | 35 | 0.74 | 63 | 72 |
However, PCA3 is not capable to replace PSA as a first-line test in clinical practice due to the lack of an appropriate cut-off level with acceptable performance characteristics. But addition of PCA3 to risk assessment tools leads to an increase of 4.5-7.1% in predictive capability [92, 93].
In contrast to PSA, PCA3 is not influenced by prostate volume, prostatitis or medication with 5-alpha-reductase inhibitors (reviewed in [89]). Regardless of its complicated measurement procedure, relative high costs (~300 Euros), and lower sensitivity than PSA PCA3 has clearly shown its clinical value. The potential correlation of PCA3 with tumor volume and cancer aggressiveness has shown conflicting results in several studies and needs to be clarified.
TMPRSS-2
The detection of gene fusions involving the androgen regulated TMPRSS2 and ETS transcription factor genes in PCa was a research-milestone [94]. Approximately 50% of all PCa-patients do have the TMPRSS2 fusion with the ETS family member that is regarded as a key PCa oncogene [94, 95]. Based on these important findings in PCa tissue, a urinary assay using the same format as PCA3 has been developed [96]. TMPRSS2:ERG in PCa tissue and in urine showed a strong correlation demonstrating a high tumor specificity of this marker. In 2011 a high AUC of 0.77 was reported for the TMPRSS2:ERG urinary assay in a small cohort with only 15 PCa-patients, which was higher than the AUC for PCA3 with 0.65 [97]. So far, only one study reported separate data on the TMPRSS2:ERG urine assay in a larger (n=246) and more balanced cohort [98]. ROC data showed a significant lower AUC for TMPRSS2:ERG (0.63) than for PCA3 (0.74) but both had no difference to phi (AUC 0.68) [98]. All other studies only reported AUCs on PCA3 and TMPRSS2:ERG together without separate evaluation of TMPRSS2:ERG [99, 100].
The use of TMPRSS2:ERG and PCA3 in PCa risk calculators has been published [99, 100]. The TMPRSS2:ERG had independent additional predictive value to PCA3 and to the ERSPC risk calculator parameters for predicting PCa [99]. TMPRSS2:ERG had prognostic value, whereas PCA3 did not [99]. Urinary TMPRSS2:ERG was further associated with clinically significant PCa at biopsy and prostatectomy [100].
It was postulated, that there is a rational basis for the need to combine PCA3 and TMPRSS2:ERG gene fusion for PCa diagnosis [101]. Based on tissue expression, it was visible that most false-negative results of PCA3 were corrected by TMPRSS2:ERG and that the combination of both markers would be capable to improve sensitivity [101].
Conclusion on urine markers
Detecting PCa in urine is technically feasible, as demonstrated by numerous studies, but few markers have been validated in multiple large sample sets [102]. There are several new markers like zinc alpha2-glycoprotein, thiosulfate or combinations of markers measured in multiplex models or gene panels that are only reported by one group so far. However, preanalytical conditions in urine are more difficult than in serum and the process of urine collection is subject to variability, which may result in conflicting clinical results [102].
Details on further urine marker have been published elsewhere [102, 103]. However, advanced clinical studies have identified only PCA3 and TMPRSS2:ERG fusion transcripts as promising RNA markers for cancer detection and possibly prognosis [102].
Summary
PSA screening reduces PCa-mortality as shown by the largest screening trial so far, the ERSPC. Other screening trials and meta-analysis from these trials with severe drawbacks should be interpreted cautiously. However, disadvantages of regular screening, namely overdiagnosis and overtreatment can be diminished with selective strategies including active surveillance. The most balanced screening guideline, the European EAU screening guideline, recommends a baseline PSA for men with 40-45 years to initiate a risk-adapted follow-up approach to reduce PCa-mortality and the incidence of advanced and metastatic PCa.
PSA as one of the most widely used tumor markers strongly correlates with the risk of harboring from PCa. This risk is already visible up to 20-30 years in advance but PSA has severe limitations for PCa detection with its low specificity. The FDA-approved and currently best serum parameter phi shows improved specificity over %fPSA and PSA. The best parameter in urine, the FDA-approved PCA3 has also been proven its utility in the PCa detection but correlation with aggressiveness and low sensitivity at high values have to be re-examined. While the detection of TMPRSS2:ERG gene fusion was one research milestone, the urinary assay for TMPRSS2:ERG only shows the expected improved accuracy for PCa detection in combination with PCA3.
Taken together, risk-adapted PSA screening and diagnosing as well as appropriate use of the FDA-approved biomarkers is the most likely scenario in the near future. New techniques such as genomics, proteomics or metabolomics as well as improved imaging devices (multiparametric-MRI) and the simultaneous use of all parameters preferentially within multivariable models may further enhance the accuracy of PCa diagnosis within the next years.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-1403. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der KTBlijenberg BG, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-1328. [DOI] [PubMed] [Google Scholar]
- 4.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Paez A, Maattanen L, Bangma CH, Aus G, Carlsson S, Villers A, Rebillard X, van der Kwast T, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366:981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roobol MJ, Kerkhof M, Schroder FH, Cuzick J, Sasieni P, Hakama M, Stenman UH, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis L, Recker F, Berenguer A, Ruutu M, Kujala P, Bangma CH, Aus G, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2009;56:584-591. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL, III, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinsky PF, Blacka A, Kramer BS, Miller A, Prorok PC, Berg C. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Clin Trials 2010;7:303-311. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV. Prostate-cancer mortality after PSA screening. N Engl J Med 2012;366:2229. [DOI] [PubMed] [Google Scholar]
- 9.Schroder FH, Roobol MJ. ERSPC and PLCO prostate cancer screening studies: what are the differences? Eur Urol 2010;58:46-52. [DOI] [PubMed] [Google Scholar]
- 10.Schroder FH, Hugosson J, Carlsson S, Tammela T, Maattanen L, Auvinen A, Kwiatkowski M, Recker F, Roobol MJ. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012;62:745-752. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson S, Vickers AJ, Roobol M, Eastham J, Scardino P, Lilja H, Hugosson J. Prostate cancer screening: facts, statistics, and interpretation in response to the US Preventive Services Task Force Review. J Clin Oncol 2012;30:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roobol MJ, Kranse R, Bangma CH, van Leenders AG, Blijenberg BG, van Schaik RH, Kirkels WJ, Otto SJ, van der Kwast TH, De Koning HJ, Schroder FH. Screening for prostate cancer: results of the rotterdam section of the European randomized study of screening for prostate cancer. Eur Urol 2013;64:530-539. [DOI] [PubMed] [Google Scholar]
- 13.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, Pihl CG, Stranne J, Holmberg E, Lilja H. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11:725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bokhorst LP, Bangma CH, van Leenders GJ, Lous JJ, Moss SM, Schroder FH, Roobol MJ. Prostate-specific Antigen-Based Prostate Cancer Screening: Reduction of Prostate Cancer Mortality After Correction for Nonattendance and Contamination in the Rotterdam Section of the European Randomized Study of Screening for Prostate Cancer. Eur Urol 2013;online available, doi:10.1016/j.eururo.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Djulbegovic M, Beyth RJ, Neuberger MM, Stoffs TL, Vieweg J, Djulbegovic B, Dahm P. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ 2010;341:c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postma R, Schroder FH, van Leenders GJ, Hoedemaeker RF, Vis AN, Roobol MJ, van der Kwast TH. Cancer detection and cancer characteristics in the European Randomized Study of Screening for Prostate Cancer (ERSPC)--Section Rotterdam. A comparison of two rounds of screening. Eur Urol 2007;52:89-97. [DOI] [PubMed] [Google Scholar]
- 17.Klotz L. Active surveillance: patient selection. Curr Opin Urol 2013;23:239-244. [DOI] [PubMed] [Google Scholar]
- 18.Dall’Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, Freedland SJ, Klotz LH, Parker C, Soloway MS. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012;62:976-983. [DOI] [PubMed] [Google Scholar]
- 19.Godtman RA, Holmberg E, Khatami A, Stranne J, Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol 2013;63:101-107. [DOI] [PubMed] [Google Scholar]
- 20.Mitsuzuka K, Narita S, Koie T, Kaiho Y, Tsuchiya N, Yoneyama T, Kakoi N, Kawamura S, Tochigi T, Habuchi T, Ohyama C, Arai Y. Pathological and biochemical outcomes after radical prostatectomy in men with low-risk prostate cancer meeting the Prostate Cancer International: Active Surveillance criteria. BJU Int 2013;111:914-920. [DOI] [PubMed] [Google Scholar]
- 21.Beauval JB, Ploussard G, Soulie M, Pfister C, Van AS, Vincendeau S, Larue S, Rigaud J, Gaschignard N, Roupret M, Drouin S, Peyromaure M, Long JA, Iborra F, Vallancien G, Rozet F, Salomon L. Pathologic findings in radical prostatectomy specimens from patients eligible for active surveillance with highly selective criteria: a multicenter study. Urology 2012;80:656-660. [DOI] [PubMed] [Google Scholar]
- 22.Trpkov K, Yilmaz A, Bismar TA, Montironi R. ‘Insignificant’ prostate cancer on prostatectomy and cystoprostatectomy: variation on a theme ‘low-volume/low-grade’ prostate cancer? BJU Int 2010;106:304-315. [DOI] [PubMed] [Google Scholar]
- 23.Boorjian SA, Eastham JA, Graefen M, Guillonneau B, Karnes RJ, Moul JW, Schaeffer EM, Stief C, Zorn KC. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol 2012;61:664-675. [DOI] [PubMed] [Google Scholar]
- 24.Roobol MJ, Carlsson S, Hugosson J. Meta-analysis finds screening for prostate cancer with PSA does not reduce prostate cancer-related or all-cause mortality but results likely due to heterogeneity - the two highest quality studies identified do find prostate cancer-related mortality reductions. Evid Based Med 2011;16:20-21. [DOI] [PubMed] [Google Scholar]
- 25.Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, Gleitsmann K, Koenig HC, Lam C, Maltz A, Rugge JB, Lin K. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:762-771. [DOI] [PubMed] [Google Scholar]
- 26.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev 2013;1:CD004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumen N, Fonteyne V, De MG, Ost P, Villeirs G, Mottrie A, De VP, De TB, Oosterlinck W. Population screening for prostate cancer: an overview of available studies and meta-analysis. Int J Urol 2012;19:100-108. [DOI] [PubMed] [Google Scholar]
- 28.Stephan C, Miller K, Jung K. Metaanalysis between claim and reality. BMJ 2010; online: http://www.bmj.com/content/341/bmi.c4543.abstract/replv#bmj_el_245270 [Google Scholar]
- 29.Kwiatkowski M, Klotz L, Hugosson J, Recker F. Comment on the US Preventive Services Task Force’s draft recommendation on screening for prostate cancer. Eur Urol 2012;61:851-854. [DOI] [PubMed] [Google Scholar]
- 30.Schroder FH. Landmarks in prostate cancer screening. BJU Int 2012;110 Suppl 1:3-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Albertsen PC, Andriole GL, Roobol MJ, Schroder FH, Vickers AJ. Risk-based prostate cancer screening. Eur Urol 2012;61:652-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeb S, Carter HB, Catalona WJ, Moul JW, Schroder FH. Baseline prostate-specific antigen testing at a young age. Eur Urol 2012;61:1-7. [DOI] [PubMed] [Google Scholar]
- 33.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad MH, Penson DF, Zietman AL. Early Detection of Prostate Cancer: AUA Guideline. J Urol 2013;190:419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidenreich A, Abrahamsson PA, Artibani W, Catto J, Montorsi F, van PH, Wirth M, Mottet N. Early detection of prostate cancer: European Association of Urology recommendation. Eur Urol 2013;64:347-354. [DOI] [PubMed] [Google Scholar]
- 35.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120-134. [DOI] [PubMed] [Google Scholar]
- 36.Catalona WJ, D’Amico AV, Fitzgibbons WF, Kosoko-Lasaki O, Leslie SW, Lynch HT, Moul JW, Rendell MS, Walsh PC. What the U.S. Preventive Services Task Force missed in its prostate cancer screening recommendation. Ann Intern Med 2012;157:137-138. [DOI] [PubMed] [Google Scholar]
- 37.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 2008;8:268-278. [DOI] [PubMed] [Google Scholar]
- 38.Kuller LH, Thomas A, Grandits G, Neaton JD. Elevated prostate-specific antigen levels up to 25 years prior to death from prostate cancer. Cancer Epidemiol Biomarkers Prev 2004;13:373-377. [PubMed] [Google Scholar]
- 39.Vickers AJ, Ulmert D, Sjoberg DD, Bennette CJ, Bjork T, Gerdtsson A, Manjer J, Nilsson PM, Dahlin A, Bjartell A, Scardino PT, Lilja H. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: case-control study. BMJ 2013;346:f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery-what we have learned and where we are going. J Urol 1999;162:293-306. [DOI] [PubMed] [Google Scholar]
- 41.Soletormos G, Semjonow A, Sibley PE, Lamerz R, Petersen PH, Albrecht W, Bialk P, Gion M, Junker F, Schmid HP, Van Poppel H. Biological variation of total prostate-specific antigen: a survey of published estimates and consequences for clinical practice. Clin Chem 2005;51:1342-1351. [DOI] [PubMed] [Google Scholar]
- 42.Singh R, Cahill D, Popert R, O’Brien TS. Repeating the measurement of prostate-specific antigen in symptomatic men can avoid unnecessary prostatic biopsy. BJU Int 2003;92:932-935. [DOI] [PubMed] [Google Scholar]
- 43.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med 2004;350:2239-2246. [DOI] [PubMed] [Google Scholar]
- 44.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, deKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol 1994;151:1283-1290. [DOI] [PubMed] [Google Scholar]
- 45.Stephan C, Klaas M, Muller C, Schnorr D, Loening SA, Jung K. Interchangeability of measurements of total and free prostate-specific antigen in serum with 5 frequently used assay combinations: an update. Clin Chem 2006;52:59-64. [DOI] [PubMed] [Google Scholar]
- 46.Stephan C, Bangma C, Vignati G, Bartsch G, Lein M, Jung K, Philippe M, Semjonow A, Catalona WJ. 20-25% lower concentrations of total and free prostate-specific antigen (PSA) after calibration of PSA assays to the WHO reference materials--analysis of 1098 patients in four centers. Int J Biol Markers 2009;24:65-69. [DOI] [PubMed] [Google Scholar]
- 47.Lilja H, Christensson A, Dahlen U, Matikainen MT, Nilsson O, Pettersson K, Lovgren T. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem 1991;37:1618-1625. [PubMed] [Google Scholar]
- 48.Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res 1991;51:222-226. [PubMed] [Google Scholar]
- 49.Stenman UH, Leinonen J, Zhang WM, Finne P. Prostate-specific antigen. Semin Cancer Biol 1999;9:83-93. [DOI] [PubMed] [Google Scholar]
- 50.Zhang WM, Finne P, Leinonen J, Vesalainen S, Nordling S, Rannikko S, Stenman UH. Characterization and immunological determination of the complex between prostate-specific antigen and alpha2-macroglobulin. Clin Chem 1998;44:2471-2479. [PubMed] [Google Scholar]
- 51.Zhang WM, Finne P, Leinonen J, Vesalainen S, Nordling S, Stenman UH. Measurement of the complex between prostate-specific antigen and alphal-protease inhibitor in serum. Clin Chem 1999;45:814-821. [PubMed] [Google Scholar]
- 52.Stephan C, Jung K, Lein M, Diamandis EP. PSA and other tissue kallikreins for prostate cancer detection. Eur J Cancer 2007;43:1918-1926. [DOI] [PubMed] [Google Scholar]
- 53.Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, Richie JP, deKernion JB, Walsh PC, Scardino PT, Lange PH, Subong EN, Parson RE, Gasior GH, Loveland KG, Southwick PC. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 1998;279:1542-1547. [DOI] [PubMed] [Google Scholar]
- 54.Stephan C, Jung K, Lein M, Sinha P, Schnorr D, Loening SA. Molecular forms of prostate-specific antigen and human kallikrein 2 as promising tools for early diagnosis of prostate cancer. Cancer Epidemiol Biomarkers Prev 2000;9:1133-1147. [PubMed] [Google Scholar]
- 55.Lee R, Localio AR, Armstrong K, Malkowicz SB, Schwartz JS. A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology 2006;67:762-768. [DOI] [PubMed] [Google Scholar]
- 56.Stephan C, Cammann H, Meyer HA, Lein M, Jung K. PSA and new biomarkers within multivariate models to improve early detection of prostate cancer. Cancer Lett 2007;249:18-29. [DOI] [PubMed] [Google Scholar]
- 57.Stephan C, Cammann H, Meyer HA, Muller C, Deger S, Lein M, Jung K. An artificial neural network for five different assay systems of prostate-specific antigen in prostate cancer diagnostics. BJU Int 2008;102:799-805. [DOI] [PubMed] [Google Scholar]
- 58.Finne P, Finne R, Auvinen A, Juusela H, Aro J, Maattanen L, Hakama M, Rannikko S, Tammela TL, Stenman U. Predicting the outcome of prostate biopsy in screen-positive men by a multilayer perceptron network. Urology 2000;56:418-422. [DOI] [PubMed] [Google Scholar]
- 59.Mikolajczyk SD, Marks LS, Partin AW, Rittenhouse HG. Free prostate-specific antigen in serum is becoming more complex. Urology 2002;59:797-802. [DOI] [PubMed] [Google Scholar]
- 60.Linton HJ, Marks LS, Millar LS, Knott CL, Rittenhouse HG, Mikolajczyk SD. Benign prostate-specific antigen (BPSA) in serum is increased in benign prostate disease. Clin Chem 2003;49:253-259. [DOI] [PubMed] [Google Scholar]
- 61.Stephan C, Cammann H, Deger S, Schrader M, Meyer HA, Miller K, Lein M, Jung K. Benign prostatic hyperplasia-associated free prostate-specific antigen improves detection of prostate cancer in an artificial neural network. Urology 2009;74:873-877. [DOI] [PubMed] [Google Scholar]
- 62.Nurmikko P, Vaisanen V, Piironen T, Lindgren S, Lilja H, Pettersson K. Production and characterization of novel anti-prostate-specific antigen (PSA) monoclonal antibodies that do not detect internally cleaved Lys145-Lys146 inactive PSA. Clin Chem 2000;46:1610-1618. [PubMed] [Google Scholar]
- 63.Vickers AJ, Cronin AM, Aus G, Pihl CG, Becker C, Pettersson K, Scardino PT, Hugosson J, Lilja H. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med 2008;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vickers AJ, Cronin AM, Roobol MJ, Savage CJ, Peltola M, Pettersson K, Scardino PT, Schroder FH, Lilja H. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin Cancer Res 2010;16:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mikolajczyk SD, Catalona WJ, Evans CL, Linton HJ, Millar LS, Marker KM, Katir D, Amirkhan A, Rittenhouse HG. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin Chem 2004;50:1017-1025. [DOI] [PubMed] [Google Scholar]
- 66.Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, Slawin KM, Marks LS, Loeb S, Broyles DL, Shin SS, Cruz AB, Chan DW, Sokoll LJ, Roberts WL, van Schaik RH, Mizrahi IA. A multicenter study of [-2] pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol 2011;185:1650-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephan C, Vincendeau S, Houlgatte A, Cammann H, Jung K, Semjonow A. Multicenter evaluation of [-2]proprostate-specific antigen and the prostate health index for detecting prostate cancer. Clin Chem 2013;59:306-314. [DOI] [PubMed] [Google Scholar]
- 68.Jansen FH, van Schaik RH, Kurstjens J, Horninger W, Klocker H, Bektic J, Wildhagen MF, Roobol MJ, Bangma CH, Bartsch G. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol 2010;57:921-927. [DOI] [PubMed] [Google Scholar]
- 69.Lazzeri M, Haese A, de la Taille A, Palou RJ, McNicholas T, Lughezzani G, Scattoni V, Bini V, Freschi M, Sussman A, Ghaleh B, Le CP, Alberola BJ, Esquena FS, Graefen M, Guazzoni G. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2-10 ng/ml: a multicentric European study. Eur Urol 2013;63:986-994. [DOI] [PubMed] [Google Scholar]
- 70.Sokoll LJ, Sanda MG, Feng Z, Kagan J, Mizrahi IA, Broyles DL, Partin AW, Srivastava S, Thompson IM, Wei JT, Zhang Z, Chan DW. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev 2010;19:1193-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hori S, Blanchet JS, McLoughlin J. From prostate-specific antigen (PSA) to precursor PSA (proPSA) isoforms: a review of the emerging role of proPSAs in the detection and management of early prostate cancer. BJU Int 2012;online available, doi:10.1111/j.1464-410X.2012.11329.x. [DOI] [PubMed] [Google Scholar]
- 72.Filella X, Gimenez N. Evaluation of [-2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med 2013;51:729-739. [DOI] [PubMed] [Google Scholar]
- 73.Ferro M, Bruzzese D, Perdona S, Mazzarella C, Marino A, Sorrentino A, Di CA, Autorino R, Di LG, Buonerba C, Altieri V, Mariano A, Macchia V, Terracciano D. Predicting prostate biopsy outcome: prostate health index (phi) and prostate cancer antigen 3 (PCA3) are useful biomarkers. Clin Chim Acta 2012;413:1274-1278. [DOI] [PubMed] [Google Scholar]
- 74.Roobol MJ, Carlsson SV. Risk stratification in prostate cancer screening. Nat Rev Urol 2013;10:38-48. [DOI] [PubMed] [Google Scholar]
- 75.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev 2001;22:184-204. [DOI] [PubMed] [Google Scholar]
- 76.Thorek DL, Evans MJ, Carlsson SV, Ulmert D, Lilja H. Prostate-specific kallikrein-related peptidases and their relation to prostate cancer biology and detection. Established relevance and emerging roles. Thromb Haemost 2013;110:484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stephan C, Rittenhouse H, Cammann H, Lein M, Schrader M, Deger S, Miller K, Jung K. New markers and multivariate models for prostate cancer detection. Anticancer Res 2009;29:2589-2600. [PubMed] [Google Scholar]
- 78.Lucarelli G, Rutigliano M, Bettocchi C, Palazzo S, Vavallo A, Galleggiante V, Trabucco S, Di CD, Selvaggi FP, Battaglia M, Ditonno P. Spondin-2, a secreted extracellular matrix protein, is a novel diagnostic biomarker for prostate cancer. J Urol 2013;190:2271-2277. [DOI] [PubMed] [Google Scholar]
- 79.Balan V, Wang Y, Nangia-Makker P, Kho D, Bajaj M, Smith D, Heilbrun L, Raz A, Heath E. Galectin-3: a possible complementary marker to the PSA blood test. Oncotarget 2013;4:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koutros S, Meyer TE, Fox SD, Issaq HJ, Veenstra TD, Huang WY, Yu K, Albanes D, Chu LW, Andriole G, Hoover RN, Hsing AW, Berndt SI. Prospective evaluation of serum sarcosine and risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Carcinogenesis 2013;34:2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Vogel S, Ulvik A, Meyer K, Ueland PM, Nygard O, Vollset SE, Tell GS, Gregory JF, III, Tretli S, Bjorge T. Sarcosine and other metabolites along the choline oxidation pathway in relation to prostate cancer--a large nested case-control study within the JANUS cohort in Norway. Int J Cancer 2014;134:197-206. [DOI] [PubMed] [Google Scholar]
- 82.Bohm L, Serafin AM, Fernandez P, Van der Watt G, Bouic PJ, Harvey J. Plasma sarcosine does not distinguish early and advanced stages of prostate cancer. S Afr Med J 2012;102:677-679. [DOI] [PubMed] [Google Scholar]
- 83.Lucarelli G, Fanelli M, Larocca AM, Germinario CA, Rutigliano M, Vavallo A, Selvaggi FP, Bettocchi C, Battaglia M, Ditonno P. Serum sarcosine increases the accuracy of prostate cancer detection in patients with total serum PSA less than 4.0 ng/ml. Prostate 2012;72:1611-1621. [DOI] [PubMed] [Google Scholar]
- 84.Struys EA, Heijboer AC, van MJ, Jakobs C, Blankenstein MA. Serum sarcosine is not a marker for prostate cancer. Ann Clin Biochem 2010;47:282. [DOI] [PubMed] [Google Scholar]
- 85.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009;457:910-914. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 86.entzmik F, Stephan C, Miller K, Schrader M, Erbersdobler A, Kristiansen G, Lein M, Jung K. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumours. Eur Urol 2010;58:12-18. [DOI] [PubMed] [Google Scholar]
- 87.Cernei N, Heger Z, Gumulec J, Zitka O, Masarik M, Babula P, Eckschlager T, Stiborova M, Kizek R, Adam V. Sarcosine as a potential prostate cancer biomarker-a review. Int J Mol Sci 2013;14:13893-13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Groskopf J, Aubin SM, Deras IL, Blase A, Bodrug S, Clark C, Brentano S, Mathis J, Pham J, Meyer T, Cass M, Hodge P, Macairan ML, Marks LS, Rittenhouse H. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem 2006;52:1089-1095. [DOI] [PubMed] [Google Scholar]
- 89.Filella X, Foj L, Mila M, Auge JM, Molina R, Jimenez W. PCA3 in the detection and management of early prostate cancer. Tumour Biol 2013;34:1337-1347. [DOI] [PubMed] [Google Scholar]
- 90.Haese A, de la Taille A, Van Poppel H, Marberger M, Stenzl A, Mulders PF, Huland H, Abbou CC, Remzi M, Tinzl M, Feyerabend S, Stillebroer AB, van Gils MP, Schalken JA. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol 2008;54:1081-1088. [DOI] [PubMed] [Google Scholar]
- 91.Deras IL, Aubin SM, Blase A, Day JR, Koo S, Partin AW, Ellis WJ, Marks LS, Fradet Y, Rittenhouse H, Groskopf J. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol 2008;179:1587-1592. [DOI] [PubMed] [Google Scholar]
- 92.Chun FK, de la Taille A, Van Poppel H, Marberger M, Stenzl A, Mulders PF, Huland H, Abbou CC, Stillebroer AB, van Gils MP, Schalken JA, Fradet Y, Marks LS, Ellis W, Partin AW, Haese A. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol 2009;56:659-668. [DOI] [PubMed] [Google Scholar]
- 93.Hansen J, Auprich M, Ahyai SA, de la Taille A, van PH, Marberger M, Stenzl A, Mulders PF, Huland H, Fisch M, Abbou CC, Schalken JA, Fradet Y, Marks LS, Ellis W, Partin AW, Pummer K, Graefen M, Haese A, Walz J, et al. Initial prostate biopsy: development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol 2013;63:201-209. [DOI] [PubMed] [Google Scholar]
- 94.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644-648. [DOI] [PubMed] [Google Scholar]
- 95.Esgueva R, Perner S, LaFargue J, Scheble V, Stephan C, Lein M, Fritzsche FR, Dietel M, Kristiansen G, Rubin MA. Prevalence of TMPRSS2-ERG and SLC45A3-ERG gene fusions in a large prostatectomy cohort. Mod Pathol 2010;23:539-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Groskopf J, Siddiqui J, Aubin SMJ, Sefton-Miller L, Day J, Blase A, Varambally S, Schalken J, Sakamoto K, Fradet Y, Rittenhouse H, Chinnaiyan A. Feasibility and clinical utility of a TMPRSS2:ERG gene fusion urine test [Abstract]. Eur Urol Suppl 2009;8:195. [Google Scholar]
- 97.Salami SS, Schmidt F, Laxman B, Regan MM, Rickman DS, Scherr D, Bueti G, Siddiqui J, Tomlins SA, Wei JT, Chinnaiyan AM, Rubin MA, Sanda MG. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol 2013;31:566-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stephan C, Jung K, Semjonow A, Schulze-Forster K, Cammann H, Hu X, Meyer HA, Bogemann M, Miller K, Friedersdorff F. Comparative Assessment of Urinary Prostate Cancer Antigen 3 and TMPRSS2:ERG Gene Fusion with the Serum [-2]Proprostate-Specific Antigen-Based Prostate Health Index for Detection of Prostate Cancer. Clin Chem 2013;59:280-288. [DOI] [PubMed] [Google Scholar]
- 99.Leyten GH, Hessels D, Jannink SA, Smit FP, de JH, Cornel EB, de Reijke TM, Vergunst H, Kil P, Knipscheer BC, van Oort IM, Mulders PF, Hulsbergen-van de Kaa CA, Schalken JA. Prospective Multicentre Evaluation of PCA3 and TMPRSS2-ERG Gene Fusions as Diagnostic and Prognostic Urinary Biomarkers for Prostate Cancer. Eur Urol 2012;in press doi:10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 100.Tomlins SA, Aubin SM, Siddiqui J, Lonigro RJ, Sefton-Miller L, Miick S, Williamsen S, Hodge P, Meinke J, Blase A, Penabella Y, Day JR, Varambally R, Han B, Wood D, Wang L, Sanda MG, Rubin MA, Rhodes DR, Hollenbeck B, et al. UrineTMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med 2011;3:94ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robert G, Jannink S, Smit F, Aalders T, Hessels D, Cremers R, Mulders PF, Schalken JA. Rational basis for the combination of PCA3 and TMPRSS2:ERG gene fusion for prostate cancer diagnosis. Prostate 2013;73:113-120. [DOI] [PubMed] [Google Scholar]
- 102.Truong M, Yang B, Jarrard DF. Toward the detection of prostate cancer in urine: a critical analysis. J Urol 2013;189:422-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hessels D, Schalken JA. Urinary biomarkers for prostate cancer: a review. Asian J Androl 2013;15:333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]


