Abstract
Prostate cancer (PCa) is one of the most commonly diagnosed cancers among men but has limited prognostic biomarkers available for follow up. MicroRNAs (miRNAs) are small non-coding RNAs that regulate expression of their target genes. Accumulating experimental evidence reports differential miRNA expression in PCa, and that miRNAs are actively involved in the pathogenesis and progression of PCa. miRNA and androgen receptor signaling cross-talk is an established factor in PCa pathogenesis. Differential miRNA expression was found between patients with high versus low Gleason scores, and was also observed in patients with biochemical failure, hormone-resistant cancer and in metastasis. Metastasis requires epithelial-mesenchymal transition which shares many cancer stem cell biological characteristics and both are associated with miRNA dysregulation. In the era of personalized medicine, there is a broad spectrum of potential clinical applications of miRNAs. These applications can significantly improve PCa management including their use as diagnostic and/or prognostic markers, or as predictive markers for treatment efficiency. Preliminary evidence demonstrates that miRNAs can also be used for risk stratification. Circulatory miRNAs can serve as non-invasive biomarkers in urine and/or serum of PCa patients. More recently, analysis of miRNAs and circulating tumor cells are gaining significant attention. Moreover, miRNAs represent an attractive new class of therapeutic targets for PCa. Here, we summarize the current knowledge and the future prospects of miRNAs in PCa, their advantages, and potential challenges as tissue and circulating biomarkers.
Prostate cancer (PCa) is the most commonly diagnosed cancer among men in western populations. The American Cancer Society estimated 239, 590 new cases and 29, 720 expected deaths in the USA in 2013. One in every six men are at risk of developing PCa during their lifetime (1).
Currently, the standard biomarker for PCa diagnosis is prostate-specific antigen (PSA), which has its limitations, leading to the risks of PCa over diagnosis and harmful overtreatment. The prognostic value of PSA is also questionable (2). Stepping into the new epoch of personalized medicine, molecular markers are urgently needed to improve the different aspects of PCa management (3). miRNAs represent an attractive class of emerging biomarkers that can help in this regard (4;5).
Keywords: prostate cancer, miRNA, tumor markers, circulating tumor cells, prognosis, personalized medicine, cancer stem cells, SNPs
1. Regulation of the biogenesis and function of miRNAs
miRNAs are small single stranded RNA sequences which do not encode for proteins but rather function by controlling the expression of their target genes. The biogenesis of miRNAs starts in the nucleus by a primary transcript (pri-miRNA) which is then processed by an RNase enzyme, Drosha, with the help of a microprocessor complex, to a 60 to 90 nucleotide precursor miRNA (pre-miRNA) that is then exported by Exporin-5 to the cytoplasm. In the cytoplasm, RNase III enzyme, Dicer, cleaves the double-stranded RNA (dsRNA) hairpin structure to form short double stranded 20-25 nucleotide fragments, which are then unwound into two single-stranded (ss) RNAs, namely the passenger strand and the guide strand. The passenger strand is degraded, and the guide strand is incorporated into the RNA-induced silencing complex (RISC) (6). A miRNA identifies its RNA target mainly through a 6-8 nucleotide seeding sequence. Annealing of mature miRNA to its target mRNA 3’-UTR, and the formation of RISC inhibits protein translation in the case of partial sequence complementarity, or triggers target mRNA degradation if their sequence is perfectly complementary (6;7).
miRNA production and processing is under various regulators at the different levels of biogenesis which have been elaborately reviewed (8). For example, the oncogene epidermal growth factor receptor (EGFR) suppresses miRNA maturation in response to hypoxia (9). miRNAs can function as a cross-talk between epigenetic machinery and modulators (10). Recent evidence has demonstrated complex interaction between the expression of tumor suppressor miR-31 and AR signaling, and that miR-31 and AR could mutually repress each other (11). miR-31 up-regulation was found to suppress AR expression through epigenetic modulation, and inhibit tumour growth in vivo (11). This epigenetic-miRNA interaction is new paradigm in cancer biogenesis gene regulation.
miRNAs are best described as fine tuning modulators of gene expression (6). They have essential roles in many vital processes like cell cycle, survival, differentiation, growth and apoptosis (12). miRNA function can be also tissue-specific. For example, in PCa, miR-125b acts as an oncomiR (a tumor promoter) but as a tumor suppressor in ovarian and breast cancers (7).
2. miRNAs are involved in prostate cancer pathogenesis
The link between miRNA and PCa pathogenesis is well-established in the literature, and linked to more 50 miRNAs (13-16). miRNAs were shown to contribute to PCa tumorigenesis and progression, as reviewed in (5;17) (Figure 1). Some of these miRNAs e.g. miR-125, 145 and 221 were found to be dysregulated in many cancer-related processes such as cell proliferation, differentiation and progression (5). Table 1 summarizes these miRNAs expression pattern as reported in 8 studies (15;18-24). As observed, let-7c, miR-125 and miR-145 were down-regulated in tumors versus normal tissue (15;19;20). Tong et al, found down-regulation of miR-23, miR-100, miR-145, miR-221 and miR-22, tumors versus normal tissues (21), which is keeping consistency with Schaefer et al who found down-regulation of miR-125b, miR-145, miR-221 and miR-222 (22). Obviously, variation among study results could be attributed to sampling issues, tumor heterogeneity or technical variability (25). However, this differential miRNAs expression could be used as diagnostic biomarkers.
Figure 1.
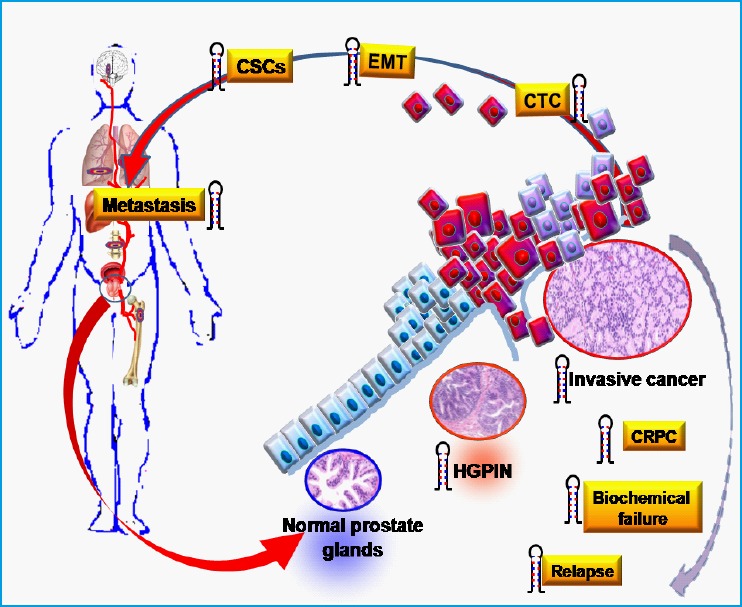
miRNAs involvement in various steps of prostate cancer pathogenesis.
miRNAs ( ) show dysregulation upon transformation of normal glands to high grade prostate intraepithelial neoplasia (HGPIN), and then to invasive PCa. They are also involved in the acquisition of an aggressive behavior including castration-resistant prostate cancer (CRPC), biochemical failure and disease relapse. Tumor spread and metastasis is associated with a number of changes including epithelial to mesenchymal transition (EMT) and gaining cancer stem cell (CSC) characteristics that results in cell detachment and metastasis to distal organs, possibly by circulating tumor cells (CTC). Recent literature showed that miRNA deregulation is associated with many of these processes, as described in detail in the text.
Table 1.
Differentially expressed miRNAs in prostate cancer.
| Up-regulated miRNAs | Down-regulated miRNAs | Methods | Number of clinical tissue samples/PCa vs. normal | Refs |
|---|---|---|---|---|
| 202, 210, 296, 320, 370, 498, 503, 373* |
Iet7a-d, Iet7g, 16, 23a, b, 26a, 92, 99a, 103, 125a-b, 143, 145, 195, 199a, 221, 222, 497 |
|
|
(15) |
| 7d, 195, 203, 34a, 20a, 29a, 25, 95, 197, 1352, 187, 1961, 148, 191, 21, 7i, 198, 199a-2, 30c, 17-5p, 92-2, 146, 181bl, 32, 206, 184prec, 29a prec, 29b-2, 181b, 196prec, 93, 223, 16, 101, 124a, 26a, 214, 27a, 106a, 199a |
128a, let7a-2, 218-2, 29a, 149, 24-1 |
|
|
(18) |
| 32, 182, 31, 26a, 200c, 375, 196a, 370, 425, 194, 181a, 34b, 7i, 188, 25, 106b, 449, 99b, 93, 92, 125 |
520h, 494, 490, 133a, l, 218, 220, 128a, 221, 499, 329, 340, 345, 410, 126, 205, 7, 145, 34a, 487, Iet7b |
|
|
(19) |
| Let family, 34a, 29a, 16 | 145, let-7 (7b-g, 7i), 26a-b, 29a-c, 30a-e, 99a-b, 125a-b, 200a-b |
|
|
(20) |
| 141, 20a | 23b, 100, 145, 221, 222, 143 |
|
|
(21) |
| 524*, 182*, 183, 634, 96, 182, 130b, 375 |
205, 222, 221, 368, 181b, 149, 3 1, 16184.145, 125b |
|
|
(22) |
| Let7a, 17, 21, 93, 101, 141, 182, 375, 720, 1826, 12745, 106a, 106b, 200b, 200c, 20a, 20b, 768-3p |
136*, 145, 214, 221, 222, 302d*, 375* |
|
|
(23) |
| Let7, 1, 98, 126, 132, 142, 143, 144, 205, 210 |
34c, 29b, 212, 10b |
|
|
(24) |
Depending on targeted gene, miRNAs can function as tumor promoters (oncomiRs) or tumor suppressors. Tumor suppressor miR34 was down-regulated in PCa, controls tumour proliferation, apoptosis and invasiveness in PCa. miR-34 is target of tumor suppressor p53 and was reported to be frequently silenced in PCa. Overexpression of miR-34 induced G1 cell cycle arrest (26), and negatively regulated oncogenes E2F3 and BCL-2 (27) (Figure 2). Transition from high grade prostate intraepithelial neoplasia (HGPIN) to localized adenocarcinoma was associated with down-regulation of miRNAs including miR-16 and 146a which repress oncogenic genes such as anti-apoptotic BCL2 and ROCK1 which increase cell growth and invasion (14). Moreover, down-regulation of tumour suppressors miR-16 and miR-15a, in tumor epithelium and its surrounding fibroblasts, promoted tumor growth and invasion via a simultaneous effect on fibroblast growth factor 2 (FGF-2) (28). Ru et al. found down-regulation of anti-metastatic miR-29b in PCa tissues as compared with non-tumor tissues. The authors succeeded to inhibit the metastatic lesion but overexpressing miR-29b in vivo (29). Transition from localized to metastatic adenocarcinoma was associated with down-regulation of tumour suppressors miR-let7 and 143 (14), which are repressing oncogene RAS (14;15;20;27). miR-21 was also found to be down-regulated in metastatic adenocarcinoma. miR-21 induces tumour suppressor PTEN (14). Thus, tumour proliferation and invasion are correlated with loss of the suppressor miRNAs which repress certain oncogenes. Other miRNAs such as miR-221/222, 125b, miR148 and 145 promote PCa tumourgenesis via hormonal regulation and stemness. Please see Sections 2.1 and 2.4 for more information. Overall, PCa pathogenesis is under the balance between the effect of the tumour suppressor and promoter miRNAs, which could be a therapeutic potential.
Figure 2.
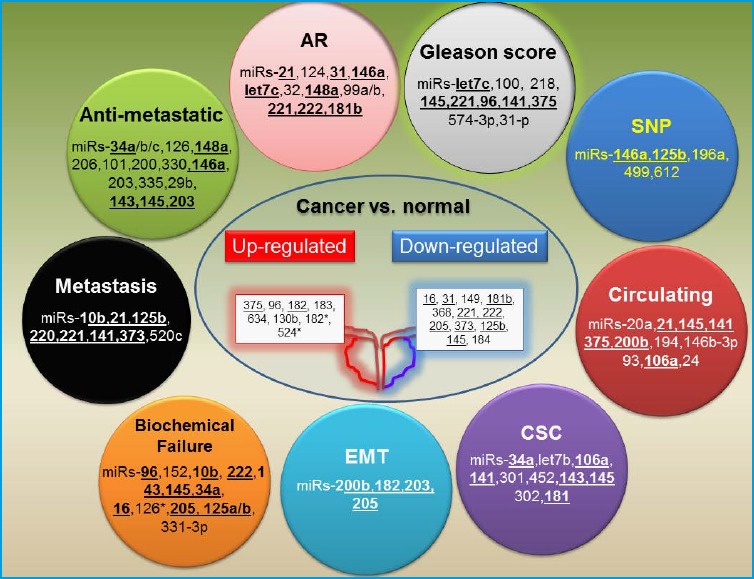
Illustration of dysregulated miRNAs in prostate cancer.
Recent literature has shown that certain miRNAs are associated with specific steps in PCa pathogenesis, including androgen receptor (AR) signaling, biochemical failure, metastasis, cancer stem cell (CSC) formation, Gleason score, epithelial to mesenchymal transition (EMT). Other miRNAs were shown to be associated with SNPs that can be useful in screening for cancer risk. miRNAs which are identified in more than two studies are shown in bold.
2.1 Androgen dependence-associated miRNAs
Several miRNAs were found to be androgen-dependent such as miR-125b, miR-101, miR-148, 221/222 and miR-146a. These miRNAs were reported to control tumour proliferation, invasion and metastasis by regulating several genes as BAK1, EZH2, CAND1 and ROCK1 (17). Jalava et al. identified miR-221 and miR-148a as androgen-regulated miRNAs that expressed in CRPC versus BPH (30). In CRPC, miR-221/-222 were also related to PCa relapse and metastasis (13;19;30;31). Table 2 summarizes examples of androgen-dependent miRNAs expression.
Table 2.
Prognostic miRNA biomarkers and their applications in prostate cancer
| Clinical condition | miRNAs expression | Methods | Patients numbers | Refs |
|---|---|---|---|---|
| Biochemical failure risk Low vs. high risk | 23a, 449a, 449b, 200a, 1233, 10b, 1825, 186, 1275, 532-5p, 193b, 886-3p, 664, 196b, 1274b, 720, 146b5p, 222, 31, 127-5p |
|
|
(13) |
| Hormone refractory tumors |
Uq-regulated 184, 198, 302c*, 345, 491, 513 |
|
|
(15) |
|
Down-reeulated 7f, 19b, 22, 26b, 27a, 27b, 29a, 29b, 30a, 30b, 30c, 100, 148a, 205 | ||||
| Extra-prostatic disease | 101, 200a, 200b, 196a, 30c, 484, 99b, 186, 195, 7f, 34c, 371, 373, 410, 491 |
|
|
(19) |
| Androgen-regulated tumors | 338, 126, 146b, 181b, c (cluster), 219, 221(cluster) | |||
| Biochemical failure |
Uq-regulated 135b, 23a, 34c, 194, 218, 96, 16 |
|
|
(21) |
|
Down-reeulated 342, 154, 140, 298, 129, 126, 122a, 213, 300 | ||||
| Gleason score High vs. low grade |
Uq-regulated 122, 335, 184, 193, 34, 138, 373, 9, 198, 144, 215 |
|
|
(24) |
|
Down-reflated 96, 222, 148, 92, 27, 125, 12627 | ||||
| PrognosticAndrogen-regulated |
Uq-regulated 21, 32, 148a, 590-5p |
|
|
(30) |
|
Down-reeulated 99a, 99b, 221 | ||||
| Biochemical failure riskLow vs. high risk | 148a, 141, 135a, 19a, 19b, 26b, 29c, 174b, 196b, 26a, 3313p, 193a, 365, 12a, 125b |
|
|
(48) |
\s
mirMASA, microRNA multianalyte suspension array
AR inhibition is important for PCa therapy. In androgen-independent tumor, AR signaling was found to be susceptible to miRNA regulation (13;17). miRNAs were identified to influence AR expression level in PCa. For example, miR-34a and miR-34c were found to target the AR 3’UTR and decrease its expression, and thus, could affect PCa progression (32). Moreover, miR-124 was found to be significantly down-regulated in malignant compared with benign prostatic cells, and directly target AR, which induces up-regulation of the p53 apoptotic activity (33). Notably, in androgen refractory, hormonal resistant PCa, AR expression and signaling could remain intact (34). Accordingly, the androgen-dependent state of PCa is regulated not only by androgen and AR, but also by miRNAs which are important for tumourgenesis and hormonal therapy.
2.2 miRNAs are involved in acquiring an aggressive behavior in prostate cancer
Current experimental evidence suggests that a group of miRNAs; “metastamirs”, are involved in PCa aggressive behavior and metastasis (16). miRNAs expression was significantly different among cases with early PSA recurrence after surgery, and non-aggressive tumours with long remission (>1 year but <5 years) (20). miR-145 and 125b regulate tumor cell cycle progression, apoptosis and cellular transformation (27). miRNAs expression in PCa tumour compared with benign peripheral zone tissues showed miR-125b down-regulation. miR-125b targets candidate genes such as BAK1 and EIF4EBP1 combined with the AKT/mTOR pathway, which could be responsible for the aggressive phenotype characteristics including high Gleason score, stage, and biochemical failure (20;22). miR-145 and miR-143 were found to regulate tumor progression, EMT and cancer stem cells (CSCs) through targeting Oct4, c-Myc, and Klf4 (35). They were also implicated in PCa cell acquiring invasive behavior, in addition to let-7c and miR-218 (14).
Evidence of aggressive tumor behavior, such as biochemical recurrence, as well as local and distant metastasis, was found to be associated with altered expression of the metastamir miR-32 and miR-21 (30). This miRNA targets tumor-suppressor genes including TPM1 and PDCD4 and decreases BTG2 levels which induce the acquisition of epithelial-mesenchymal transition (EMT) in PCa (16). Moreover, alteration of Dicer expression is documented to be related to tumor growth and progression (19).
2.3 miRNAs in epithelial to mesenchymal transition (EMT)
Cancer progression is linked to EMT (Figure 1&2). Cells undergoing EMT share many biological characteristics with CSCs, and literature suggest that the two processes are interrelated. A recent study showed that PCa cells with EMT phenotype displayed stem-like cell features which were associated with decreased expression of miR-200 and the let-7 family (36). Loss of epithelial markers is associated with transcription suppressors such as zinc-finger E-box binding homeobox (ZEB) 1 and 2. In PC3 cells, miR-200b-c overexpression were found to be inversely associated ZEB 1 expression, and acquiring an EMT phenotype (36).
miR-182 and miR-203 are found to be down-regulated during EMT. These miRNAs regulate SNAI2 and P-cadherin (37). Presence of miR-205 is an essential factor for the inhibitory effects of p63, a metastasis suppressor, on EMT markers, ZEB1 and vimentin in PCa cells (38).
2.4 miRNAs and prostate cancer stem cells
CSCs are gaining considerable attention due to their involvement in tumor initiation, progression, therapy resistance, relapse and metastasis (39). The potential effects of miRNAs on cancer stem/progenitor cells are being explored in PCa. Liu et al. found that miR-34a, let-7b, miR-106a and miR-141 are down-regulated in CSCs, whereas miR-301 and miR-452 were up-regulated (26). A recent study demonstrated miR-143 and miR-145 significant role in metastasis by repressing CSC and stemness markers, and cellular viability (35).
Hypoxia regulates CSC through hypoxia-inducible factor (HIF), which activates pluripotent stem cell inducers, including miR-302. HIFs also induces glycolysis- and EMT-associated molecules, miR-181 and let7a (40). Anti-metastatic miRNAs, including miR-34a, and let-7 (27), function by inhibiting certain CSC properties. miR-34a induces G11, cell-cycle arrest and senescence, and let-7 induces G2-M phase arrest without senescence (26).
2.5 SNPs and miRNAs in prostate cancer
Single nucleotide polymorphisms (SNPs) can alter miRNA expression through varied mechanisms. Their presence in miRNA promoter sites can alter immature and mature miRNA transcription. SNPs located at the miRNA-binding sites of target genes could modify the efficiency of miRNA binding to the 3’UTR, leading to gene dysregulation. Oncogenic or tumor suppressor miRNAs function could be modified by miRNA-SNPs site resulting in alteration in protein levels (41).
Genetic mutations contributing to PCa risk groups have been recently investigated. Emerging genome wide-associations studies (GWAS) identified a number of SNPs associated with PCa risks factors such as age at diagnosis, pathological aggressiveness, and family history of cancer (42). PCa aggressiveness was found to be associated with pairs of SNP-SNP interactions. These SNP network converge on the epidermal growth factor receptor (EGFR) pathway, and could affect PCa oncogenesis and proliferation (43). Genetic variance in miRNA regions could influence PCa development. Risk of disease development is associated with increased with the SNP of miR-146a (rs2910164), miR-196a (rs11614913), miR-499(rs3746444), and miR-612 (44-47). These SNPs have potential as predictors of PCa risk in high risk groups. It needs to be investigated if these associations are of functional significance.
3. The clinical utility of miRNA as prostate cancer biomarkers
Due to their involvement in cancer pathogenesis, miRNAs have a wide range of potential applications as diagnostic, prognostic, or predictive markers, or as potential therapeutic targets and pharmacogenomic markers for both primary and metastatic cancers (7;18;24;48). miRNAs possess many properties that make them attractive biomarkers, including the ability to detect them in small volume samples, and from formalin-fixed tissues. Furthermore, they can be detected in different body fluids, such as serum and urine, using specific and sensitive quantitative real-time PCR (qRT-PCR)(7).
3.1 miRNAs as diagnostic markers in prostate cancer
Clinical stage, Gleason score and PSA level provide the current parameters for PCa diagnosis. miRNAs provide useful information beyond these parameters, and by incorporating them into these parameters, miRNAs will improve these clinicopatholigical parameters diagnostic and prognostic effectiveness (Figure 3). For example, miRNAs were found to be associated with clinicopatholigical state (22), and dysregulated in premalignant prostate lesions before progression to cancerous and then metastatic disease (14). miRNAs were also found to be dysregulated in biochemical failure high risk group (13). The ability to quantify these miRNAs expression from archival formalin-fixed tissues and body fluids makes this approach potentially useful in determining high risk patients. Oncologist can use the archival tissues with full clinical, survival and therapeutic information (16;48;49). However, further studies are needed to examine the implication of tumour heterogeneity, stages and grades on miRNA expression. Experimental studies demonstrated the potential use of miRNAs as PCa diagnostic markers (Table 1). miRNA differential expression can also be used to identify the tissue of origin in undifferentiated tumors, which is an important problem in surgical pathology practice. Tumors from the same embryonic origin were found to share the same miRNA clusters (18;50).
Figure 3.
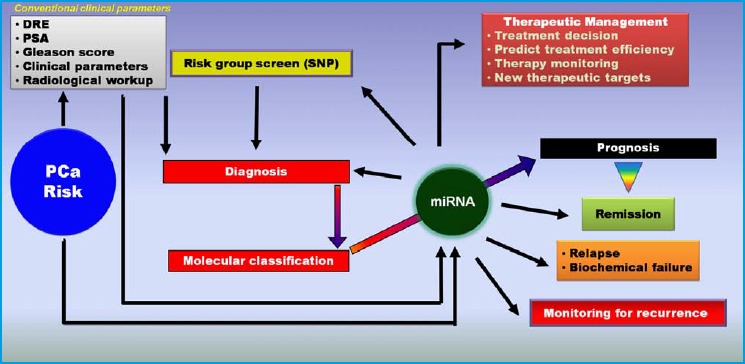
A schematic approach of the potential role of miRNAs in prostate cancer patient management.
Conventional clinical parameters have limited value for assessment of clinical outcome after diagnosis, and are not efficient for personalizing the treatment plan for individual patients. miRNAs, alone or in combination with clinical parameters, can be used to enhance patient management plans and this can lead to a significant improvement of outcome.
3.2 The prognostic utility of miRNAs in prostate cancer
Assessment of PCa prognosis is a challenge, and now relies on histopathological parameters (like Gleason score) together with PSA levels (Figure 3), which do not always correctly reflect disease status. Many studies have been reported the potential utility of specific miRNA expression profiles to assist in linking PCa with its aggressive behavior (21;24;31;48;51), either alone or combined with current prognostic tools (52). Schaefer et al. reported high miR-96 expression as a prognostic tissue biomarker associated with decreased recurrence free interval. High miR-96 was found to be associated with high Gleason score, tumour stage and biochemical failure (defined as elevation of PSA after surgery to = 0.2 ng/ml in two successive measurements) remains the only available marker, and Gleason score (22). Differential miRNA tissue expression has also been observed between high grade (Gleason score = 8) and low grade tumors (24).
Statistical survival analysis identified down-regulation of two miRNAs with prognostic importance expressed in prostate tissue, miR-221 and miR-96, which were found to be associated with clinical outcome and biochemical relapse (22;31). Table 2 summarizes the potential utility of miRNA expression to predict aggressive behavior, including extra-prostatic disease, biochemical failure, and CRPC. Recent studies have shown the ability of miRNAs to predict relapse and biochemical failure in PCa (13;48). Differential miRNA expression also correlated with metastases and stem cell formation (40).
A recent study identified 25 differentially expressed miRNAs between patients with high versus low risk of biochemical failure, including miR-331-3p, miR-193a, and miR-125a-b. miR-152 function through targeting ERBB signaling pathways, transforming growth factor-ß (TCF-P) signaling, focal adhesion, and extracellular matrix (ECM)-receptor interaction. ERBB signaling is a major steroid-independent activator of AR, which makes miR-152 a biomarker with therapeutic importance (48). miR-l0b and miR-222/miR-10b ratio were good predicators of PCa biochemical failure (13).
3.3 miRNAs as predictive markers for prostate cancer
Markers that can predict response to therapy allow physician to restrict treatment only to the subgroup of patients who are likely to respond, thus avoiding unnecessary cost and side effects of administering treatment to patients who will not experience a benefit. PCa is a hormone-dependent malignancy. A recent study showed that serum miR-21 levels are elevated in CRPC patients, especially in those resistant to docetaxel-based chemotherapy. This study suggested that miR-21 can be a marker to indicate the transformation to hormone refractory disease, and a potential predictor for the efficacy of docetaxel-based chemotherapy (53). Another study reported that SNPs inside miRNAs and miRNA target sites have potential value to improve outcome prediction in PCa patients receiving androgen deprivation therapy (54). A third study investigated miR-141 as a potential biomarker of therapeutic response in PCa. Serum miR-141 could be a new predictive biomarker to PCa progression, when compared to validated biomarkers such as PSA and CTC. However, it was less specific than PSA (55). Therefore, miRNAs should be combined with other validated biomarkers to increase their effectiveness. Directional changes in PSA, CTC, and miR-141 had sensitivity in predicting clinical outcome in 79 % of cases. Logistic regression modeling of the probability of clinical progression demonstrates that miR-141 levels predicted clinical outcomes with an odds ratio of at least 8.3 (55). More research studies are needed to assess the utility of miRNAs as predictive markers for radiotherapy, chemotherapy and androgen suppression therapy (56).
4. miRNAs as non-invasive biomarkers
Cellular miRNAs may be released to body fluids such as serum, plasma, urine or saliva. These miRNAs are carried and protected from degradation in complexes with Argonaute proteins (catalytic components of RISC), high-density lipoprotein and microvesicles (57). Current studies are looking to use these cell-free, circulating miRNAs as noninvasive biomarkers for PCa (58). Extracellular miRNAs could be the product of dead cancer cells, circulating tumor cells (CTCs), as well as nonmalignant cells, such as platelets, or the product of nonmalignant cells tissue damage (59).
4.1 Circulating miRNAs in blood
miRNAs originating from prostate cancer can be released into the circulation and can be readily measured in plasma and serum from PCa patients (60). An earlier study showed that miRNAs are present in a remarkably stable form in blood and that they are protected from endogenous RNase activity (58). This study showed that miRNAs originating from human PCa xenografts are readily measured in plasma, and can robustly distinguish xenografted mice from controls. In addition, it was determined that serum levels of miR-141 can distinguish patients with PCa from healthy controls (58).
Table 3 summarizes circulatory miRNAs with diagnostic, prognostic and predictive importance. Recent studies have observed a correlation between circulating miRNA expression and risk assessment models. miR-20a was significantly overexpressed in plasma from patients with stage 3 tumors compared to stage 2 or below, and significant increases in miR-21 and miR-145 expression were also observed with intermediate or high risk D’Amico scores (Table 3) compared to low risk scores (51). Combining miRNAs with the current prognostic tools for risk assessment can improve the accuracy of these models (52).
Table 3.
Circulating miRNA biomarker applications
| miRNA applications (Samples) | Up-regulated miRNAs | Down-regulated iRNAs | Methods | Patients samples | Refs |
|---|---|---|---|---|---|
| Prognostic for D’Amico scores1 High vs. low risk (Plasma) | 20a, 21, 145, 221 | --------- |
|
82 PCa | (51) |
| Prognostic Biochemical failure (Serum) | 141, 146b-3p, 194 | --------- |
|
8PCa8 patients with recurrence | (52) |
| Prognostic for metastatic PCa (Serum) | 100, 125b, 141, 143, 296 | --------- |
|
25 patients with metastasis 25 healthy volunteers | (58) |
| Prognostic for PCa risk factor index1,2 (Serum) | 20b, 874, 1274a, 1207-5p, 93, 106a | 223, 26b, 30c, 24 |
|
36 patients with metastasis 12 healthy volunteers | (60) |
| Prognostic for metastatic PCa3 (Serum) | 375, 9*, 141, 516a3p, 629, 203, 429, 618, 212, 21, 545, 218, 422, 656, 655, 29c, 200b, 200c, 502-5p | --------- |
|
10 PCa7 patients with metastasis | (61) |
| Prognostic for metastatic castration-resistant PCa and diagnostic3 (Serum) | 141, 298, 375, 3461 | --------- |
|
25 patients with metastasis 25 healthy volunteers | (62) |
| Prognostic for metastatic PCa (Plasma) | 125b, 136, 1513p, 200a, 744a*, 9, 8*, 99a, 7d, 126, 142-5p, 15b, 27a, 27b, 30a* | 205, 106b, 16, 363 |
|
25 PCa25 patients with metastasis | (63) |
1. D›Amico scores: risk assessment: PSA level, Gleason and T stage. Low-risk: PSA less than or equal to 10, Gleason score less than or equal to 6, and clinical stage T1-2a Intermediate risk: PSA between 10 and 20, Gleason score 7, or clinical stage T2b High-risk: PSA more than 20, Gleason score equal or larger than 8, or clinical stage T2c-3a.
2. miRs-141, 298,375 are diagnostic and miRs-141 and 375 are prognostic (relapse)
3. Clinicopathology index: age, PSA level and Gleason score
Serum from patients with metastatic PCa showed up-regulation of five miRNAs; miR-375, miR-9*, miR-141, miR-200b, and miR-516a-3p. Also, miR-141 and miR-375 are associated with metastatic disease in other studies(61-63).Up-regulation of serum miR-93, miR-106a and down regulation of miR-24 are also linked to metastatic PCa (60). Combining circulating miRNAs associated with biochemical failure, such as miR-141, miR-146b-3p and miR-194, with the current prognostic tools can predict disease progression (52). Measuring tumor-derived miRNAs in blood was essential diagnostic step, but endogenous miRNAs baseline, tumour heterogeneity and other possible miRNAs sources should be considered.
4.2 miRNAs in urine
There is growing body of evidence supporting the clinical utility of urinary miRNAs as PCa biomarkers (4). miRNAs are reported to be stable in body fluids which contains RNases. They resist nuclease activity, as well as methylation, adenylation, or uridylation (64). Urine of PCa patients was found to have a higher concentration of miR-150 and -328, whereas miR-107, miR-574-3, miR-196b, miR-200b, miR-100, and miR-106a showed decreased concentrations (4;64). The diagnostic value of these miRNAs has been shown to outperform that of prostate cancer antigen3 (PCA3), a biomarker for PCa that is measured in urine samples. It is vital to realize that miRNAs released into body fluids do not necessarily reflect miRNAs abundance in the cell of origin. A recent study suggested the existence of cellular selection mechanisms for miRNA release, which should be an important consideration in the identification of circulating miRNA biomarkers (57). Extracellular miRNAs are feasible diagnostic and prognostic biomarkers. However, caution should be taken with study methodology and miRNA normalization reference to achieve concordant data and outcomes.
4.3 miRNAs in circulating tumor cells
Circulating tumor cells (CTCs) have been proven to be of significance as cancer biomarkers, especially as prognostic indicators and therapy-monitoring biomarkers (65). In PCa, CTC enumeration has been extensively studied and validated as a prognostic tool with FDA clearance for use in monitoring advanced disease (66). In addition to quantification of CTC in blood, recent evidence suggests the usefulness of CTCs as sources for DNA analysis. Molecular characterization of captured cells can serve as a “liquid biopsy” of the tumor, reflecting molecular changes in an individual’s malignancy over time.
Current evidence shows that EMT could occur in CTCs in PCa. Consequently, research groups are currently focusing on the development of new markers to detect CTCs with an EMT phenotype. Cells undergoing EMT produce mesenchymal proteins such as N-cadherin, vimentin, tenascin C, laminin_1, type VI_collagen and numerous proteinases, and lose epithelial E-cadherin, which protect cancer cells from anoikis. Additionally, expression of EMT transcription factors, Twist, Slug, Snail and SIP was found to protect CTCs from anoikis (65). Tumor-derived circulating miRNAs were studied in the plasma of PCa patients using centrifugation and filtration to exclude CTCs (58). Cell-free miRNAs were detected in the supernatant or filtrate. However, the authors could not exclude the possibility of cellular miRANs release during blood processing steps (58).
Recent studies in breast cancer documented the capacity of circulating miRNAs to indicate the CTC status and their potential as prognostic markers. CTC is a rapidly developing important biomarker in cancer (59). CTC-associated miRNAs could have higher specificity than the free circulating miRNAs. However, most of the CTC-associated miRNA study findings are preliminary that awaits further validation.
5. miRNAs as potential therapeutic targets
Recently, miRNAs are gaining attention as potential therapies for a wide array of diseases including hepatitis, hypercholesterolemia and cancer (67;68). Some miRNA-based therapies have already successfully passed phase II clinical trials. miRNA therapy has many advantages, as recently outlined (49). A major advantage of miRNAs is that their gene-silencing effects occur in the cytoplasm without disturbing nuclear molecules. Because of their small size, they are much easier to transfect without many side effects. Moreover, miRNAs regulate multiple gene networks, thus offering the advantage of simultaneous down-regulation of multiple cancer-promoting signaling pathways (69).
A number of studies high lighted the potential therapeutic applications of miRNAs in PCa(70). lnhibition of cancer cell growth and migration with genistein, a small biologically active flavanoid, has been found to act by inhibiting oncogenic miRNAs such as miR-21, 151, 221 and 222, which inactivate Notch signaling, RAC1/VEGF mediating angiogenesis and increase expression of tumour suppression gene ARHI, respectively (71). On contrary, genistein inhibited cell growth by tumor suppressor miR-574-3p up-regulation (71). A natural agent, isoflavone, was found to alter methylation sites of miR-29a and miR-1256, increasing their levels and decreasing expression of TRIM68 and PGK-1, which inhibits PCa cell growth and invasion (72). Moreover, vitamin D was found to up-regulate tumour suppressor miR-98, which suppressed tumour growth by inducing G2/M arrest (73). The aim of these therapeutic maneuvers is manipulating oncogenic and tumour suppressor miRNAs to control tumorigenesis.
6. Future prospects
The current efforts to define PCa diagnostic and prognostic miRNAs are still evolving (Figure 3). The consensus on high value miRNAs as specific biomarkers has not yet been established due to multiple factors. Observed discrepancies among studies could be due to differences in case series examined, specimen type (formalin-fixed vs. frozen tissue and blood), tumor heterogeneity and sampling issues, RNA isolation protocols and method of detection (microarray vs. qRT-PCR, etc). Large scale high quality studies with patients’ clinical and pathological information is an important step in this regard. Further validation of the potential miRNA biomarkers should be conducted as well (3).
The accumulating evidence shows that miRNAs are actively involved in PCa pathogenesis, tumor progression and metastasis (17), and can be used as potential biomarkers for patient management (5). Among promising miRNAs which could be used in future (Figure 2) as biomarkers for tumour proliferation miRs-15, 16, 20a, 21, 23, 32, invasion miRs-143, 145, 205, 221/222 and androgen-independent growth miRs-125b, 146, 205, 221/222 (13;14;21;22;28;30;51).
In the era of personalized medicine, a great hope relies upon the integration of multiple clinical and molecular parameters to establish a patient-specific risk profile useful for clinical decision making (Figure 3) (74). Multiparametric approaches utilizing different types of molecules hold the promise of enhancing the sensitivity and specificity of molecular markers as diagnostic tests. In addition to their value as disease and therapeutic biomarkers, miRNAs have great potential as therapeutic targets.
Glossary
Non-standard abbreviations
- PCa
prostate cancer
- miRNA
microRNA
- CSCs
cancer stem cells
- AR
androgen receptor
- CTC
circulating tumor cells
- CRPC
castration-resistant prostate cancer
Reference List
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120-134. [DOI] [PubMed] [Google Scholar]
- 3.Pasic MD, Samaan S, Yousef GM. Genomic medicine: new frontiers and new challenges. Clin Chem 2013;59:158-167. [DOI] [PubMed] [Google Scholar]
- 4.Sapre N, Selth LA. Circulating MicroRNAs as Biomarkers of Prostate Cancer: The State of Play. Prostate Cancer 2013;2013:539680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer A, Stephan C, Busch J, Yousef GM, Jung K. Diagnostic, prognostic and therapeutic implications of microRNAs in urologic tumors. Nat Rev Urol 2010;7:286-297. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev Clin Oncol 2011;8:467-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winter J, Jung S, Keller S, Gregory Rl, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009;11:228-234. [DOI] [PubMed] [Google Scholar]
- 9.Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AG02. Nature 2013;497:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasic MD, Olkhov E, Bapat B, Yousef GM. Epigenetic regulation of kallikrein-related peptidases: there is a whole new world out there. Biol Chem 2012;393:319-330. [DOI] [PubMed] [Google Scholar]
- 11.Lin PC, Chiu YL, Banerjee S, Park K, Mosquera JM, Giannopoulou E, et al. Epigenetic repression of miR-31 disrupts androgen receptor homeostasis and contributes to prostate cancer progression. Cancer Res 2013;73:1232-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khella HW, Bakhet M, Lichner Z, Romaschin AD, Jewett MA, Yousef GM. MicroRNAs in kidney disease: an emerging understanding. Am J Kidney Dis 2013;61:798-808. [DOI] [PubMed] [Google Scholar]
- 13.Fendler A, Jung M, Stephan C, Honey RJ, Stewart RJ, Pace KT, et al. miRNAs can predict prostate cancer biochemical relapse and are involved in tumor progression. Int J Oncol 2011;39:1183-1192. [DOI] [PubMed] [Google Scholar]
- 14.Leite KR, Tomiyama A, Reis ST, Sousa-Canavez JM, Sanudo A, Camara-Lopes LH, Srougi M. MicroRNA expression profiles in the progression of prostate cancer--from high--grade prostate intraepithelial neoplasia to metastasis. Urol Oncol 2013;31:796-801. [DOI] [PubMed] [Google Scholar]
- 15.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res 2007;67:6130-6135. [DOI] [PubMed] [Google Scholar]
- 16.White NM, Fatoohi E, Metias M, Jung K, Stephan C, Yousef GM. Metastamirs: a stepping stone towards improved cancer management. Nat Rev Clin Oncol 2011;8:75-84. [DOI] [PubMed] [Google Scholar]
- 17.Fendler A, Stephan C, Yousef GM, Jung K. MicroRNAs as regulators of signal transduction in urological tumors. Clin Chem 2011;57:954-968. [DOI] [PubMed] [Google Scholar]
- 18.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 2006;103:2257-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res 2008;68:6162-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008;27:1788-1793. [DOI] [PubMed] [Google Scholar]
- 21.Tong AW, Fulgham P, Jay C, Chen P, Khalil I, Liu S, et al. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther 2009;16:206-216. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer 2010;126:1166-1176. [DOI] [PubMed] [Google Scholar]
- 23.Wach S, Nolte E, Szczyrba J, Stohr R, Hartmann A, Orntoft T, et al. MicroRNA profiles of prostate carcinoma detected by multiplatform microRNA screening. Int J Cancer 2012;130:611-621. [DOI] [PubMed] [Google Scholar]
- 24.Walter BA, Valera VA, Pinto PA, Merino MJ. Comprehensive microRNA Profiling of Prostate Cancer. J Cancer 2013;4:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White NM, Yousef GM. Translating molecular signatures of renal cell carcinoma into clinical practice. J Urol 2011;186:9-11. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Kelnar K, Vlassov AV, Brown D, Wang J, Tang DG. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7. Cancer Res 2012;72:3393-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang YX, Gao WQ. Roles of microRNAs during prostatic tumorigenesis and tumor progression. Oncogene 2013. [DOI] [PubMed] [Google Scholar]
- 28.Musumeci M, Coppola V, Addario A, Patrizii M, Maugeri-Sacca M, Memeo L, et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene 2011;30:4231-4242. [DOI] [PubMed] [Google Scholar]
- 29.Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther 2012;11:1166-1173. [DOI] [PubMed] [Google Scholar]
- 30.Jalava SE, Urbanucci A, Latonen L, Waltering KK, Sahu B, Janne OA, et al. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene 2012;31:4460-4471. [DOI] [PubMed] [Google Scholar]
- 31.Spahn M, Kneitz S, Scholz CJ, Stenger N, Rudiger T, Strobel P, et al. Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. Int J Cancer 2010;127:394-403. [DOI] [PubMed] [Google Scholar]
- 32.Ostling P, Leivonen SK, Aakula A, Kohonen P, Makela R, Hagman Z, et al. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res 2011;71:1956-1967. [DOI] [PubMed] [Google Scholar]
- 33.Shi XB, Xue L, Ma AH, Tepper CG, Gandour-Edwards R, Kung HJ, deVere White RW. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene 2013;32:4130-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med 2004;10:33-39. [DOI] [PubMed] [Google Scholar]
- 35.Huang S, Guo W, Tang Y, Ren D, Zou X, Peng X. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep 2012;28:1831-1837. [DOI] [PubMed] [Google Scholar]
- 36.Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One 2010;5:e12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu Y, Li WC, Hellem MR, Rostad K, Popa M, McCormack E, et al. MiR-182 and miR-203 induce mesenchymal to epithelial transition and self-sufficiency of growth signals via repressing SNAI2 in prostate cells. Int J Cancer 2013;133:544-555. [DOI] [PubMed] [Google Scholar]
- 38.Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A 2012;109:15312-15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan X, Liu S, Su F, Pan Q, Lin T. Effective enrichment of prostate cancer stem cells from spheres in a suspension culture system. Urol Oncol 2012;30:314-318. [DOI] [PubMed] [Google Scholar]
- 40.Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med 2013;17:30-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salzman DW, Weidhaas JB. SNPing cancer in the bud: microRNA and microRNA-target site polymorphisms as diagnostic and prognostic biomarkers in cancer. Pharmacol Ther 2013;137:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agalliu I, Wang Z, Wang T, Dunn A, Parikh H, Myers T, et al. Characterization of SNPs associated with prostate cancer in men of Ashkenazic descent from the set of GWAS identified SNPs: impact of cancer family history and cumulative SNP risk prediction. PLoS One 2013;8:e60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin HY, Amankwah EK, Tseng TS, Qu X, Chen DT, Park JY. SNP-SNP interaction network in angiogenesis genes associated with prostate cancer aggressiveness. PLoS One 2013;8:e59688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Bi J, Liu X, Li K, Di J, Wang B. Has-miR-146a polymorphism (rs2910164) and cancer risk: a meta-analysis of 19 case-control studies. Mol Biol Rep 2012;39:4571-4579. [DOI] [PubMed] [Google Scholar]
- 45.Feng N, Xu B, Tao J, Li P, Cheng G, Min Z, et al. A miR-125b binding site polymorphism in bone morphogenetic protein membrane receptor type IB gene and prostate cancer risk in China. Mol Biol Rep 2012;39:369-373. [DOI] [PubMed] [Google Scholar]
- 46.George GP, Gangwar R, Mandal RK, Sankhwar SN, Mittal RD. Genetic variation in microRNA genes and prostate cancer risk in North Indian population. Mol Biol Rep 2011;38:1609-1615. [DOI] [PubMed] [Google Scholar]
- 47.Kim HK, Prokunina-Olsson L, Chanock SJ. Common genetic variants in miR-1206 (8q24.2) and miR-612 (11q13.3) affect biogenesis of mature miRNA forms. PLoS One 2012;7:e47454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lichner Z, Fendler A, Saleh C, Nasser AN, Boles D, AI-Haddad S, et al. MicroRNA Signature Helps Distinguish Early from Late Biochemical Failure in Prostate Cancer. Clin Chem 2013;59:1595-1603. [DOI] [PubMed] [Google Scholar]
- 49.Metias SM, Lianidou E, Yousef GM. MicroRNAs in clinical oncology: at the crossroads between promises and problems. J Clin Pathol 2009;62:771-776. [DOI] [PubMed] [Google Scholar]
- 50.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-838. [DOI] [PubMed] [Google Scholar]
- 51.Shen J, Hruby GW, McKiernan JM, Gurvich I, Lipsky MJ, Benson MC, Santella RM. Dysregulation of circulating microRNAs and prediction of aggressive prostate cancer. Prostate 2012;72:1469-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selth LA, Townley SL, Bert AG, Strieker PD, Sutherland PD, Horvath LG, et al. Circulating microRNAs predict biochemical recurrence in prostate cancer patients. Br J Cancer 2013;109:641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang HL, Yang LF, Zhu Y, Yao XD, Zhang SL, Dai B, et al. Serum miRNA-21: elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate 2011;71:326-331. [DOI] [PubMed] [Google Scholar]
- 54.Bao BY, Pao JB, Huang CN, Pu YS, Chang TY, Lan YH, et al. Polymorphisms inside microRNAs and microRNA target sites predict clinical outcomes in prostate cancer patients receiving androgen-deprivation therapy. Clin Cancer Res 2011;17:928-936. [DOI] [PubMed] [Google Scholar]
- 55.Gonzales JC, Fink LM, Goodman OB, Jr., Symanowski JT, Vogelzang NJ, Ward DC. Comparison of circulating MicroRNA 141 to circulating tumor cells, lactate dehydrogenase, and prostate-specific antigen for determining treatment response in patients with metastatic prostate cancer. Clin Genitourin Cancer 2011;9:39-45. [DOI] [PubMed] [Google Scholar]
- 56.O’Kelly F, Marignol L, Meunier A, Lynch TH, Perry AS, Hollywood D. MicroRNAs as putative mediators of treatment response in prostate cancer. Nat Rev Urol 2012;9:397-407. [DOI] [PubMed] [Google Scholar]
- 57.Huang X, Liang M, Dittmar R, Wang L. Extracellular microRNAs in urologic malignancies: chances and challenges. Int J Mol Sci 2013;14:14785-14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008;105:10513-10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mostert B, Sieuwerts AM, Martens JW, Sleijfer S. Diagnostic applications of cell-free and circulating tumor cell-associated miRNAs in cancer patients. Expert Rev Mol Diagn 2011;11:259-275. [DOI] [PubMed] [Google Scholar]
- 60.Moltzahn F, Olshen AB, Baehner L, Peek A, Fong L, Stoppler H, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res 2011;71:550-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brase JC, Johannes M, Schlomm T, Faith M, Haese A, Steuber T, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer 2011;128:608-616. [DOI] [PubMed] [Google Scholar]
- 62.Selth LA, Townley S, Gillis JL, Ochnik AM, Murti K, Macfarlane RJ, et al. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int J Cancer 2012;131:652-661. [DOI] [PubMed] [Google Scholar]
- 63.Watahiki A, Macfarlane RJ, Gleave ME, Crea F, Wang Y, Helgason CD, Chi KN. Plasma miRNAs as Biomarkers to Identify Patients with Castration-Resistant Metastatic Prostate Cancer. Int J Mol Sci 2013;14:7757-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mlcochova H, Hezova R, Stanik M, Slaby O. Urine microRNAs as potential noninvasive biomarkers in urologic cancers?. Urol Oncol 2013. [DOI] [PubMed] [Google Scholar]
- 65.Liberko M, Kolostova K, Bobek V. Essentials of circulating tumor cells for clinical research and practice. Crit Rev Oncol Hematol 2013;88:338-356. [DOI] [PubMed] [Google Scholar]
- 66.Hu B, Rochefort H, Goldkorn A. Circulating tumor cells in prostate cancer. Cancers (Basel) 2013;5:1676-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbarotto E, Calin GA. Potential therapeutic applications of miRNA-based technology in hematological malignancies. Curr Pharm Des 2008;14:2040-2050. [DOI] [PubMed] [Google Scholar]
- 68.Tazawa H, Kagawa S, Fujiwara T. MicroRNAs as potential target gene in cancer gene therapy of gastrointestinal tumors. Expert Opin Biol Ther 2011;11:145-155. [DOI] [PubMed] [Google Scholar]
- 69.Frankel LB, Lund AH. MicroRNA regulation of autophagy. Carcinogenesis 2012;33:2018-2025. [DOI] [PubMed] [Google Scholar]
- 70.Deng JH, Deng Q, Kuo CH, Delaney SW, Ying SY. MiRNA targets of prostate cancer. Methods Mol Biol 2013;936:357-369. [DOI] [PubMed] [Google Scholar]
- 71.Chiyomaru T, Yamamura S, Fukuhara S, Hidaka H, Majid S, Saini S, et al. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS One 2013;8:e58929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Kong D, Ahmad A, Bao B, Dyson G, Sarkar FH. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics 2012;7:940-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ting HJ, Messing J, Yasmin-Karim S, Lee YF. Identification of microRNA-98 as a therapeutic target inhibiting prostate cancer growth and a biomarker induced by vitamin D. J Biol Chem 2013;288:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diamandis M, White NM, Yousef GM. Personalized medicine: marking a new epoch in cancer patient management. Mol Cancer Res 2010;8:1175-1187. [DOI] [PubMed] [Google Scholar]


