Abstract
Precise, accurate and clear writing is essential for communicating in health sciences, as publication is an important component in the university criteria for academic promotion and in obtaining funding to support research. In spite of this, the development of writing skills is a subject infrequently included in the curricula of faculties of medicine and allied health sciences. Therefore clinical investigators require tools to fill this gap. The present paper presents a brief historical background to medical publication and practical guidelines for writing scientific papers for acceptance in good journals.
Key words: guidelines, scientific writing
INTRODUCTION
A scientific paper is the formal lasting record of a research process. It is meant to document research protocols, methods, results and conclusions derived from an initial working hypothesis. The first medical accounts date back to antiquity. Imhotep, Pharaoh of the 3rdDynasty, could be considered the founder of ancient Egyptian medicine as he has been credited with being the original author of what is now known as the Edwin Smith Papyrus (Figure 1). The Papyrus, by giving some details on cures and anatomical observations, sets the basis of the examination, diagnosis, treatment, and prognosis of numerous diseases. Closer to the Common Era, in 460 BCE, Hippocrates wrote 70 books on medicine. In 1020, the Golden age of the Muslim Culture, Ibn Sina, known as Avicenna (Figure 2a), recorded the Canon of medicine that was to become the most used medical text in Europe and Middle East for almost half a millennium. This was followed in the beginning of the 12th Century bytheextensivetreatiseofMaimonides(Figure 2b) (Moses ben Maimon) on Greek and Middle Eastern medicine. Of interest, by the end of the 11th Century Trotula di Ruggiero, a woman physician, wrote several influential books on women’s ailment. A number of other hallmark treatises also became more accessible, thanks to the introduction of the printing press that allowed standardization of the texts. One example is the De Humani Corporis Fabrica by Vesalius which contains hundreds of illustrations of human dissection. Thomas A Lang provides an excellent concise history of scientific publications [1]. These were the days when writing and publishing scientific or philosophical works were the privilege of the few and hence there was no or little competition and no recorded peer reviewing system. Times have however changed, and contemporary scientists have to compose with an increasingly harsh competition in attracting editors and publishers attention. As an example, the number of reports and reviews on obesity and diabetes has increased from 400 to close to 4000/year and 50 to 600/year respectively over a period of 20 years (Figure 3). The present article, essentially based on TA Lang’s guide for writing a scientific paper [1], will summarize the steps involved in the process of writing a scientific report and in increasing the likelihood of its acceptance.
Figure 1.

The Edwin Smith Papyrus (≈3000 BCE)
Figure 2.

Avicenna and Maimonides
Figure 3.
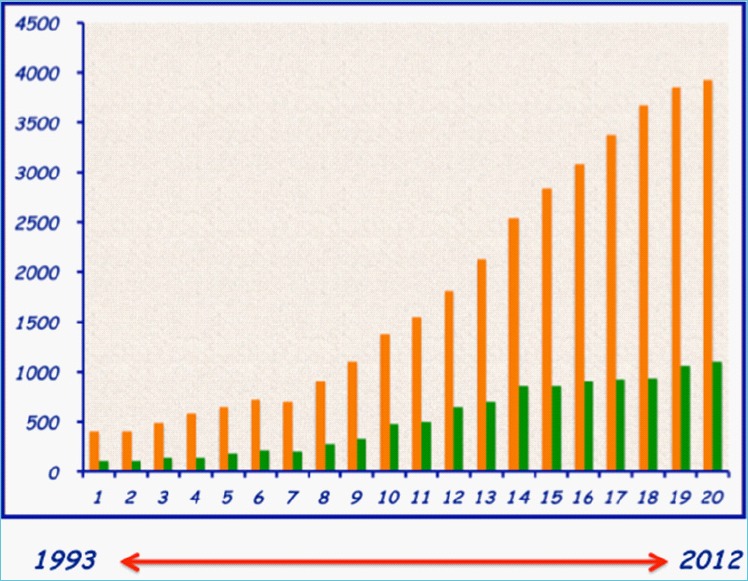
Annual publication load in the field of obesity and diabetes over 20 years.
Reasons for publishing are varied. One may write to achieve a post-graduate degree, to obtain funding for pursuing research or for academic promotion. While all 3 reasons are perfectly legitimate, one must ask whether they are sufficient to be considered by editors, publishers and reviewers. Why then should the scientist write? The main reason is to provide to the scientific community data based on hypotheses that are innovative and thus to advance the understanding in a specific domain. One word of caution however, is that if a set of experiments has not been done or reported, it does not mean that it should be. It may simply reflect a lack of interest in it.
DECIDING ON PUBLISHING AND TARGETING THE JOURNAL
In order to assist with the decision process, pres-ent your work orally first to colleagues in your field who may be more experienced in publishing. This step will help you in gauging whether your work is publishable and in shaping the paper.
Targeting the journal, in which you want to present your data, is also a critical step and should be done before starting to write. One hint is to look for journals that have published similar work to yours, and that aims readers most likely to be interested in your research. This will allow your article to be well read and cited. These journals are also those that you are most likely to read on a regular basis and to cite abundantly. The next step is to decide whether you submit your manuscript to a top-ranking impact factor journal or to a journal of lower prestige. Although it is tempting to test the waters, or to obtain reviewers comments, be realistic about the contribution your work provides and submit to a journal with an appropriate rank.
Do not forget that each rejection delays publication and that the basin of reviewers within your specialty is shallow. Thus repeated submission to different journals could likely result in having your work submitted for review to the same re-viewer.
DECIDING ON THE TYPE OF MANUSCRIPT
There are several types of scientific reports: observational, experimental, methodological, theoretical and review. Observational studies include 1) single-case report, 2) collective case reports on a series of patients having for example common signs and symptoms or being followed-up with similar protocols, 3) cross-sectional, 4) cohort studies, and 5) case-control studies. The latter 3 could be perceived as epidemiological studies as they may help establishing the prevalence of a condition, and identify a defined population with and without a particular condition (disease, injury, surgical complication). Experimental reports deal with research that tests a research hypothesis through an established protocol, and, in the case of health sciences, formulate plausible explanations for changes in biological systems. Methodological reports address for example advances in analytical technology, statistical methods and diagnostic approach. Theoretical reports suggest new working hypotheses and principles that have to be supported or disproved through experimental protocols. The review category can be sub-classified as narrative, systematic and meta-analytic. Narrative reviews are often broad overviews that could be biased as they are based on the personal experience of an expert relying on articles of his or her own choice. Systematic reviews and meta-analyses are based on reproducible procedures and on high quality data. Researchers systematically identify and analyze all data collected in articles that test the same working hypothesis, avoiding selection bias, and report the data in a systematic fashion. They are particularly helpful in asking important questions in the field of healthcare and are often the initial step for innovative research. Rules or guidelines in writing such report must be followed if a quality systematic review is to be published.
For clinical research trials and systematic reviews or meta-analyses, use the Consort Statement (Consolidated Standards Of Reporting Trials) and the PRISMA Statement (Preferred Reporting Items for Systematic reviews and Meta-Analyses) respectively [2,3]. This assures the editors and the reviewers that essential elements of the trials and of the reviews were tackled. It also speeds the peer review process. There are several other Statements that apply to epidemiological studies [4], non-randomized clinical trials [5], diagnostic test development (6) and genetic association studies (7). The Consortium of Laboratory Medicine Journal Editors has also published guidelines for reporting industry-sponsored laboratory research (8).
INITIAL STEPS IN THE PROCESS OF WRITING A SCIENTIFIC DOCUMENT
Literature review is the initial and essential step before starting your study and writing the scientific report based on it. In this process use multiple databases, multiple keyword combinations. It will allow you to track the latest development in your field and thus avoid you to find out that someone else has performed the study before you, and hence decrease the originality of your study. Do not forget that high-ranking research journals publish results of enough importance and interest to merit their publication.
Determining the authorship and the order of authorship, an ethical issue, is the second essential step, and is unfortunately often neglected. This step may avoid later conflicts as, despite existing guidelines, it remains a sensitive issue owing to personal biases and the internal politics of institutions. The International Committee of Medical Editors has adopted the following guidelines for the biomedical sciences (9).
“Authorship credit should be based only on: 1) Substantial contributions to the conception and design, or acquisition of data, or analysis and interpretation of data; 2) Drafting the article or revising it critically for important intellectual content; and 3) Final approval of the version to be published. Conditions 1, 2 and 3 must be all met. Acquisition of funding, the collections of data, or general supervision of the research group, by themselves, do not justify authorship.” (9,10)
The order of authorship should reflect the individual contribution to the research and to the publication, from most to least (11). The first author usually carries out the lead for the project reported. However the last author is often mistakenly perceived as the senior author. This is perpetuated from the European tradition and is discouraged. As there are divergent conventions among journals, the order of authorship order may or may not reflect the individual contributions; with the exception that the first author should be the one most responsible for the work.
WRITING EFFECTIVELY
Effective writing requires that the text helps the readers 1) understand the content and the context, 2) remember what the salient points are, 3) find the information rapidly and, 4) use or apply the information given. These cardinal qualities should be adorned with the precise usage of the language, clarity of the text, inclu-siveness of the information, and conciseness. Effective writing also means that you have to focus on the potential readers’ needs. Readers in science are informed individuals who are not passive, and who will formulate their own opinion of your writing whether or not the meaning is clear. Therefore you need to know who your audience is. The following 4 questions should help you writing a reader-based text, meaning written to meet the information needs of readers [12].
What do you assume your readers already know? In other words, which terms and concepts can you use without explanation, and which do you have to define?
What do they want to know? Readers in science will read only if they think they will learn something of value.
What do they need to know? Your text must contain all the information necessary for the reader to understand it, even if you think this information id obvious to them.
What do they think they know that is not so? Correcting misconceptions can be an important function of communication, and persuading readers to change their minds can be a challenging task.
WRITING THE SCIENTIFIC PAPER
Babbs and Tacker’s advice to write as much of the paper before performing the research project or experimental protocol may, at first sight, seem unexpected and counterintuitive [13], but in fact it is exactly what is being done when writing a research grant application. It will allow you to define the authorship alluded to before. The following section will briefly review the structure of the different sections of a manuscript and describe their purpose.
Reading the instructions to authors of the Journal you have decided to submit your manuscript is the first important step. They provide you with the specific requirements such as the way of listing the authors, type of abstract, word, figure or table limits and citation style. The Mulford Library of University of Toledo website contains instructions to authors for over 3000 journals (http://mulford.meduoiho.edu/instr/).
The general organization of an article follows the IMRAD format (Introduction, Methods, Results, and Discussion). These may however vary. For instance, in clinical research or epidemiology studies, the methods section will include details on the subjects included, and there will be a statement of the limitation of the study. Although conclusions may not always be part of the structure, we believe that it should, even in methodological reports.
Title page
The tile page provides essential information so that the editor, reviewers, and readers will identify the manuscript and the authors at a glance as well as enabling them to classify the field to which the article pertains.
The title page must contain the following:
The tile of the article – it is an important part of the manuscript as it is the most often read and will induce the interested readers to pursue further. Therefore the title should be precise, accurate, specific and truthful;
Each author’s given name (it may be the full name or initials) and family name;
Each author’s affiliation;
Some journals ask for highest academic degree;
A running title that is usually limited to a number of characters. It must relate to the full title;
Key words that will serve for indexing;
For clinical studies, the trial’s registration number;
The name of the corresponding author with full contact information.
Abstract
The abstract is also an important section of your manuscript. Importantly, the abstract is the part of the article that your peers will see when consulting publication databases such as PubMed. It is the advertisement to your work and will strongly influence the editor deciding whether it will be submitted to reviewers or not. It will also help the readers decide to read the full article. Hence it has to be comprehensible on its own. Writing an abstract is challenging. You have to carefully select the content and, while being concise, assure to deliver the essence of your manuscript.
Without going into details, there are 3 types of abstracts: descriptive, informative and structured. The descriptive abstract is particularly used for theoretical, methodological or review articles. It usually consists of a single paragraph of 150 words or less. The informative abstract, the most common one, contains specific information given in the article and, are organized with an introduction (background, objectives), methods, results and discussion with or without conclusion. They usually are 150 to 250 words in length. The structured abstract is in essence an informative abstract with sections labeled with headings. They may also be longer and are limited to 250 to 300 words. Recent technology also allows for graphical or even video abstracts. The latter are interesting in the context of cell biology as they enable the investigator to illustrate ex vivo experiment results (phagocytosis process for example).
Qualities of abstracts:
Understood without reading the full paper. Shoul dcontain no abbreviations.lf abbreviations are used, they must be defined. This however removes space for more important information;
Contains information consistent with the full report. Conclusions in the abstract must match those given in the full report;
Is attractive and contains information needed to decide whether to read the full report.
Introduction
The introduction has 3 main goals: to establish the need and importance of your research, to indicate how you have filled the knowledge gap in your field and to give your readers a hint of what they will learn when reading your paper. To fulfil these goals, a four-part introduction consisting of a background statement, a problem statement, an activity statement and a forecasting statement, is best suited. Poorly defined background information and problem setting are the 2 most common weaknesses encountered in introductions. They stem from the false perception that peer readers know what the issue is and why the study to solve it is necessary. Although not a strict rule, the introduction in clinical science journals should target only references needed to establish the rationale for the study and the research protocol. This differ from more basic science or cell biology journals, for which a longer and elaborate introduction may be justified because the research at hand consists of several approaches each requiring background and justification.
The 4-part introduction consists of:
A background statement that provides the context and the approach of the research;
A problem statement that describes the nature, scope and importance of the problem or the knowledge gap;
An activity statement, that details the research question, sets the hypothesis and actions undertaken for the investigation;
A forecasting statement telling the readers whattheywillfìndwhen readingyourarticle [14].
Methods section
This section may be named “Materials and Methods”, “Experimental section” or “Patients and Methods” depending upon the type of journal. Its purpose to allow your readers to provide enough information on the methods used for your research and to judge on their adequacy. Although clinical and “basic” research protocols differ, the principles involved in describing the methods share similar features. Hence, the breadth of what is being studied and how the study can be performed is common to both. What differ are the specific settings. For example, when a study is conducted on humans, you must provide, up front, assurance that it has received the approval of you Institution Ethics Review Board (IRB) and that participants have provided full and informed consent. Similarly when the study involves animals, you must affirm that you have the agreement from your Institutional Animal Care and Use Committee (IACUC). These are too often forgotten, and Journals (most of them) abiding to the rules of the Committee on Publication Ethics (COPE) and World Association of Medical Editors (WAME) will require such statement. Although journals publishing research reports in more fundamental science may not require such assurance, they do however also follow to strict ethics rules related to scientific misconduct or fraud such as data fabrication, data falsification. For clinical research papers, you have to provide information on how the participants were selected, identify the possible sources of bias and confounding factors and how they were diminished.
In terms of the measurements, you have to clearly identify the materials used as well as the suppliers with their location. You should also be unambiguous when describing the analytical method. If the method has already been published, give a brief account and refer to the original publication (not a review in which the method is mentioned without a description). If you have modified it, you have to provide a detailed account of the modifications and you have to validate its accuracy, precision and repeatability. Mention the units in which results are reported and, if necessary, include the conversion factors [mass units versus “système international” (S.I.)]. In clinical research, surrogate end-points are often used as biomarkers. Under those circumstances, you must show their validity or refer to a study that has already shown that are valid.
In cases of clinical trials, the Methods section should include the study design, the patient selection mode, interventions, type of outcomes.
Statistics are important in assuring the quality of the research project. Hence, you should consult a biostatistician at the time of devising the research protocol and not after having performed the experiments or the clinical trial.
The components of the section on statistics should include:
The way the data will be reported (mean, median, centiles for continuous data);
Details on participant assignments to the different groups (random allocation, consecutive entry);
Statistical comparison tools (parametric or non parametric statistics, paired or unpaired t-tests for normally distributed data and so on);
The statistical power calculation when determining the sample size to obtain valid and significant comparisons together with the a level;
The statistical software package used in the analysis.
Results section
The main purpose of the results section is to report the data that were collected and their relationship. It should also provide information on the modifications that have taken place because of unforeseen events leading to a modification of the initial protocol (loss of participants, reagent substitution, loss of data).
Report results as tables and figures whenever possible, avoid duplication in the text. The text should summarize the findings;
Report the data with the appropriate descriptive statistics;
Report any unanticipated events that could affect the results;
Report a complete account of observations and explanations for missing data (patient lost).
Discussion
The discussion should set your research in context, reinforce its importance and show how your results have contributed to the further understanding of the problem posed. This should appear in the concluding remarks. The following organization could be helpful.
Briefly summarize the main results of your study in one or two paragraphs, and how they support your working hypothesis;
Provide an interpretation of your results and show how they logically fit in an overall scheme (biological or clinical);
Describe how your results compare with those of other investigators, explain the differences observed;
Discuss how your results may lead to a new hypothesis and further experimentation, or how they could enhance the diagnostic procedures.
Provide the limitations of your study and steps taken to reduce them. This could be placed in the concluding remarks.
Acknowledgements
The acknowledgements are important as they identify and thank the contributors to the study, who do not meet the criteria as co-authors. They also include the recognition of the granting agency. In this case the grant award number and source is usually included.
Declaration of competing interests
Competing interests arise when the author has more than one role that may lead to a situation where there is a conflict of interest. This is observed when the investigator has a simultaneous industrial consulting and academic position. In that case the results may not be agreeable to the industrial sponsor, who may impose a veto on publication or strongly suggest modifications to the conclusions. The investigator must clear this issue before starting the contracted research. In addition, the investigator may own shares or stock in the company whose product forms the basis of the study. Such conflicts of interest must be declared so that they are apparent to the readers.
Acknowledgments
The authors thank Thomas A Lang, for his advice in the preparation of this manuscript.
REFERENCES
- 1.Lang TA. How to write, publish, and present in the health sciences: A guide for clinicians and laboratory researchers. Lang TA, , ACP Press, Philadelphia, PA, 2010, :1-25. [Google Scholar]
- 2.Moher D, Schulz KF, Altman DG, et al. The CONSIRT statement: Revised recommendations for improving the quality of reports of parallel group randomized trials. Lancet 2001;357:1191-1194. [PubMed] [Google Scholar]
- 3.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of meta-analyses of randomized controlled studies: the QUORUM statement. Lancet 1999;354:1896-1900. [DOI] [PubMed] [Google Scholar]
- 4.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 2007;147:573-577. [DOI] [PubMed] [Google Scholar]
- 5.Des Jarlais DC, Lyles C, Crepaz N. and the TREND Group. Improving the reporting quality of non-randomized evaluations of behavioural and public health interventions: The TREND statement. Am J Publ Health 2004;94:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossyut PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD initiative. BMJ 2003;326:41-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little J, Higgings JPT’ loannidis JPA et al. Strengthening the reporting of genetic association studies (STREGA) – An extension of the STROBE statement. PLos Med 2009;6:1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rifai N, Plebani M, Wu AH, et al. Full disclosure in industry-sponsored laboratory medicine research studies: Statement by the Consortium of Laboratory Medicine Journal Editors. Clin Biochem. 2011. February;44:149-150. [DOI] [PubMed] [Google Scholar]
- 9.International Committee of Medical Editors. Uniform requirements for manuscripts submitted to biomedical journals: Writing and editing for biomedical publications. http://www.icmie.Org/index.html#top. [Google Scholar]
- 10.Council of Science Editors. CSE’s White paper on promoting integrity in scientific journal publications, http://www.councilofscienceeditors.org. [Google Scholar]
- 11.Rennie D, Yank V, Emanuel L. When authorship fails: A proposal to make contributors accountable. JAMA 1997;278:579-585. [DOI] [PubMed] [Google Scholar]
- 12.Lang TA. How to write, publish, and present in the health sciences: A guide for clinicians and laboratory researchers. Lang TA, , ACP Press, Philadelphia, PA, 2010, :32-34. [Google Scholar]
- 13.Babbs CF, Tacker MM. Writing a scientific paper prior to the research. Am J Emerg Med 1985;3:360-363. [DOI] [PubMed] [Google Scholar]
- 14.Lang TA. How to write, publish, and present in the health sciences: A guide for clinicians and laboratory researchers. Lang TA, , ACP Press, Philadelphia, PA, 2010, pl48. [Google Scholar]


