Abstract
Prostate cancer is a leading contributor to male cancer-related deaths worldwide. Kallikrein-related peptidases (KLKs) are serine proteases that exhibit deregulated expression in prostate cancer, with KLK3, or prostate specific antigen (PSA), being the widely-employed clinical biomarker for prostate cancer. Other KLKs, such as KLK2, show promise as prostate cancer biomarkers and, additionally, their altered expression has been utilised for the design of KLK-targeted therapies. There is also a large body of in vitro and in vivo evidence supporting their role in cancer-related processes. Here, we review the literature on studies to date investigating the potential of other KLKs, in addition to PSA, as biomarkers and in therapeutic options, as well as their current known functional roles in cancer progression. Increased knowledge of these KLK-mediated functions, including degradation of the extracellular matrix, local invasion, cancer cell proliferation, interactions with fibroblasts, angiogenesis, migration, bone metastasis and tumour growth in vivo, may help define new roles as prognostic biomarkers and novel therapeutic targets for this cancer.
Key words: prostate cancer, protease, kallikrein-related peptidase, biomarker
PROSTATE CANCER-ASSOCIATED DEREGULATION OF KALLIKREIN-RELATED PEPTIDASES (KLKS) AND THEIR USE AS CLINICAL BIOMARKERS
Prostate cancer is a leading cause of male cancer-related deaths in most developed nations. Although prostate cancer progresses through similar molecular and phenotypic ‘hallmarks’ as other endocrine-related cancers [1], the biological mechanisms driving its development are poorly understood. Locally-confined prostate tumours can be successfully treated by radical prostatectomy, androgen ablation and/or radiotherapy, although often with debilitating side effects. Despite the five-year survival rate for patients with localised tumours nearing 100%, there is a high degree of post-operative recurrence and many cancers progress to more advanced disease and ultimately incurable bone metastases [2]. Improved diagnostic and prognostic markers, as well as therapies, are required for effective prostate cancer detection and elimination.
Prostate cancer is accompanied by the aberrant expression of members of the KLK family of serine proteases, most notably KLK3 or prostate-specific antigen (PSA), which is the ‘gold-standard’ clinical biomarker for prostate cancer detection. PSA expression is largely prostate-specific, and this protease functions to liquefy the seminal clot in the healthy prostate [3]. In prostate cancer, although local PSA levels decrease with disease progression [4], serum PSA levels are elevated following its leakage into the bloodstream, resulting from a disrupted prostate glandular architecture. Total circulating PSA (tPSA) is measured in the ‘PSA test’ and, although there is no threshold for serum tPSA that definitively indicates prostate cancer, values ≥3-4ng/mL are generally accepted in the clinic, with a positive predictive value of ~25% at 3ng/mL [5]. Patients with
serum tPSA ≥3-4ng/mL are often referred for biopsy to diagnose the cancer, after accounting for patient ethnicity, family history of disease and results of a digital rectal examination. PSA testing has reduced mortality of men [6]; however, it countributes up to 42% of prostate cancer over-diagnosis, which often translates to over-treatment [7]. Even with a high incidence of over-diagnosis and earlier diagnosis, many prostate cancers progress to form metastases, primarily in bone [2]. Clearly, there is a requirement for improved diagnostic and prognostic indicators of prostate cancer establishment and progression.
Attempts to refine PSA testing primarily centre around discrimination of various PSA iso-forms in circulation, including complexed PSA (cPSA), that is PSA complexed to other circulating proteins [8], free PSA (fPSA), that is PSA not bound to other circulating proteins [9, 10], full-length or intact PSA (iPSA) [9, 11], internally cleaved or ‘nicked’ PSA (N-PSA) [11], iso-forms, such as [2-]ProPSA [10], and various differentially glycosylated PSA proteins [12]. For example, measuring the Prostate Health Index (PHI; [2-]ProPSA/fPSA × √tPSA) improved predictive accuracy in patients with familial prostate cancer history and in men aged 60 and below [10, 13]. Discriminating tPSA, fPSA and iPSA, as well as measuring circulating KLK2, advanced the predictive accuracy of PSA testing, in addition to improving discrimination of pathologically insignificant from aggressive disease [9, 14]. This ‘four kallikrein panel’ is under further examination in a clinical trial for its ability to predict biochemical recurrence [15]. Thus, despite down-regulation of KLK3 transcription in prostate cancer, certain post-transcriptional and -translational alterations to this peptidase appear to be enriched in diseased tissue, and detecting various PSA iso-forms may improve the specificity of clinical PSA testing. Additionally, as certain PSA regulatory pathways may be activated only in select disease stages, discriminating between PSA iso-forms may hold important prognostic value.
Anumber of other KLKs hold promise for prostate cancer diagnosis or prognosis, including KLK4-5, KLK10-11 and KLK14-15. These KLKs, along with KLK1-3, KLK9 and KLK13, are expressed and translated in prostate tissue [16] and have been detected in biological fluids, including serum (KLK1-8, KLK10-15) [17, 18], seminal plasma (KLK1-5, KLK7 and KLK9-15) [16] and extra-prostatic fluid (KLK1-3, KLK11 and KLK13) [19, 20]. KLK2, KLK4, KLK11 and KLK14-15 expression is increased in malignant prostate tissue, versus benign or normal tissue, and has been correlated with clinical disease parameters. KLK4 expression is associated with increased risk of prostate cancer and tumour stage [21], and KLK14-15 expression positively correlates with pathological stage [22, 23]. KLK11 expression is increased in prostate cancer and inversely correlates with tumour stage and grade [24], while KLK5 expression is inversely correlated with prostatic malignancy and Gleason score [25]. Additionally, DNA methylation of KLK10 positively correlates with pathological stage [26]. Larger studies are required to confirm these findings and to determine whether changes in mRNA concentrations in prostate tissue correlate with reproducibly detectable differences in secreted protein abundance in biological fluids.
Overall, prostatic KLKs demonstrate useful bio-markers for prostate cancer. There is a clear clinical potential for measurement of the abundance of KLK4-5, KLK10-11 and/or KLK14-15 in biological fluids to improve prostate cancer diagnosis and prognosis. While prostate-specific expression of KLK2 and KLK3 is greater than other prostatic KLKs [27], prostate cancer-specific biomarkers, such as the aforementioned prostate cancer-associated KLKs, may be measured adjunct to prostate organ-specific biomarkers, for sensitive and specific detection of prostate cancer. However, larger clinical cohorts need to be evaluated before clinical translation of the apparent utility of these promising biomarker candidates. Identification of novel variants of these KLKs may serve to further enhance their biomarker potential, as has been demonstrated for PSA.
THE CLINICAL UTILITY OF KLK-TARGETED PROSTATE CANCER THERAPIES
Beyond their application as prostate cancer biomarkers, the tissue-specific, and/or deregulated, expression of KLKs has been utilised for the design of targeted cancer therapies. A range of anti-prostate cancer pro-drugs have been developed, whereby cytotoxic compounds have been coupled to KLK2- or PSA-activatable sequences, as the prostate-restricted expression of these KLKs allows for cytotoxicity selective to the prostate. Among these is L-377202, a PSA-activatable doxyrubicin-conjugate, which reduced tumour growth in a mouse model of prostate cancer and has completed Phase I clinical trials [28, 29]. KLKs also hold efficacy as antigens for immunotherapy. PROSTVAC® is one among available PSA-based vaccines, which is currently in Phase III clinical trials. It consists of vaccinia- and fowlpox-based vectors encoding transgenes for PSA and immune co-stimulatory molecules, which is administered to patients to elicit a T-cell response targeting PSA-expressing cells. Phase II clinical trials demonstrated that PROSTVAC® improved the overall survival at 3 years in men with low symptomatic multiple castration-resistant prostate cancer; progression-free survival was not affected [30]. Thus, novel therapies targeting the prostate cancer-enriched expression and/or activity of certain KLKs hold promise as cancer therapies.
Of note, an engineered variant of alpha-1-anti-chymotrypsin (MDPK67b), modified to inhibit a number of proteases, including KLK2, KLK4-5 and KLK14, is undergoing human trials [31]. This represents the first KLK inhibitor as a putative prostate cancer therapy evaluated in a human study. Pre-clinical evidence showed that this inhibitor reduced tumour growth, conferred by KLK2 over-expression, in a xenograft model of prostate cancer. This compound exhibited low toxicity in the animal host [31]. The anti-tumour efficacy of inhibitors targeting other KLKs, demonstrated experimentally, must be confirmed in future clinical studies. To our knowledge, there are no other clinical studies targeting the biological function of KLKs in prostate cancer progression, despite various in vitro and in vivo animal studies showing functional roles for KLKs in this disease.
Converse to KLK inhibition, KLK agonists targeting those KLKs, which possess anti-tumourigenic activity, have been proposed as a therapeutic strategy. PSA-binding peptides have been developed, which serve as functional agonists of the anti-angiogenic and hence anti-tumourigenic activity of PSA in prostate cancer [32]. Similar agonists may be developed for other anti-tumourigenic KLKs, as such functions are discovered.
While KLKs show deregulated expression in prostate cancer, there has been minimal translation from laboratory-based evidence of the cancer-associated functions of prostatic KLKs, to clinical therapeutics targeting these functions. To bridge this gap and inform the design of therapies targeting KLK-mediated proteolysis, a greater understanding of the mechanism of KLK action in prostate cancer progression is required. In addressing this issue, the remainder of this review combines biochemical evidence of KLK-mediated substrate proteolysis with data from in vitro and in vivo animal studies, where KLK expression has been found to affect cellular ‘hallmarks’ of cancer (see Figure 1). In doing so, we provide a theoretical mechanism for KLK action in prostate cancer, which may form the basis for studies validating KLK activity in prostate carcinogenesis, from deregulated KLK expression, through proteolysis of their substrate intermediates and the affected down-stream signalling pathways, to the resulting functional outcomes. Only with such an understanding will the utility of KLKs as therapeutic targets for this disease be realised.
Figure 1.
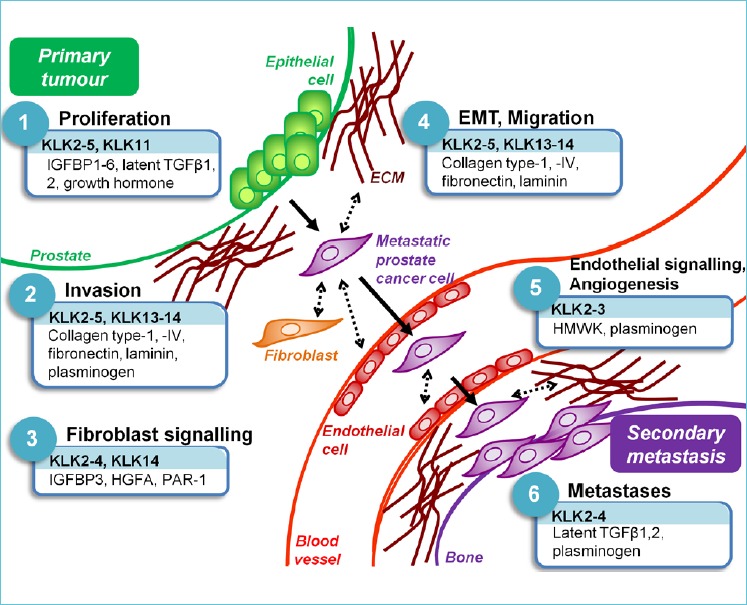
The role of KLK-related peptidase (KLK)-mediated proteolysis in prostate cancer progression.
LABORATORY-BASED EVIDENCE FOR THE ROLE OF KLKS IN PROSTATE CANCER PROGRESSION
KLKs in extracellular matrix (ECM) degradation and local invasion
Primarily, the role of KLKs in degrading ECM proteins facilitates tumour expansion and invasion. The ECM plays a key role in tissue homeostasis, acting not only as a structural scaffold, but as a barrier to suppress malignant outgrowth, under healthy conditions. As with any tissue, ECM turnover is integral for healthy tissue maintenance, and a range of proteases, including KLKs, actively participate in this process. In prostatic malignancy, cancer proteases breakdown the basal lamina and facilitate physical clearance through the ECM to foster tumour outgrowth and entry into the vasculature [1].
Whether luminal or basal epithelial cells are the source of prostate cancer initiation is heavily debated as cancer cells often express mixed basal and luminal markers, the latter including KLK2 and PSA. KLK4, however, is expressed by both basal and luminal secretory epithelial cells and cleaves the basal lamina component, collagen type-IV, and the ECM components collagen type-I and fibronectin in vitro [33]. Thus, KLK4 is likely involved in the early breakdown of the basal lamina in prostate cancer. Luminal KLK2 and PSA may also function in this process, as cancerous outgrowth eventually brings cancer cells in contact with the basal lamina and the surrounding fibromuscular ECM. PSA cleaves laminin [34], while KLK2-4 also degrade fibronectin in vitro. Other prostatic KLKs also cleave fibronectin and laminin (KLK5 and KLK13-14), as well as collagens type-1 (KLK4-5 and KLK13-14) and type-IV (KLK4-5 and KLK14) [33, 35-37]. KLKs proteolytically process other KLKs and other protease classes in vitro, which may amplify KLK-induced ECM degradation, should this occur in vivo [38, 39].
Despite these in vitro observations, strikingly, bone metastatic prostate cancer, PC-3, cells made to over-express KLK2, PSA or KLK4 did not exhibit an altered invasive behaviour [40]. Additionally, and perhaps counter-intuitively, KLK4 over-expressing PC-3 cells showed increased attachment to collagens type-1 and type-IV [41], although this could be a transient feature of migration and invasion. The proteolytic activity of KLKs secreted by these cells was not confirmed. KLK4 over-expressing prostate cancer cell lines generally display an enhanced migratory phenotype [40, 41] and PSA and KLK4 over-expressing PC-3 cells transition from an epithelial to a mesenchymal phenotype, characterised by loss of E-cadherin, gain of vimentin and acquisition of an elongated morphology, promoting increased migration in vitro [40].
KLKs and cancer cell proliferation
KLK-mediated ECM degradation can release matrix-tethered growth factors to facilitate cancer growth. Indeed, the direct or indirect activation of mitogenic proteins is key to sustained tumour growth [1]. Particularly, KLK2-5 and KLK11 degrade insulin-like growth factor binding protein 3 (IGFBP3) [35, 37, 42-44], KLK4-5 hydrolyse IGFBP4-6 [43, 45], and KLK5 and KLK14 process IGFBP2 [35, 37], with KLK5 also processing IGFBP1 [37]. Hydrolysis of IGFBPs can reduce the binding of these proteins to insulin-like growth factors (IGFs), thus increasing cell proliferation. Up-regulated levels of free, versus bound, IGF-1 positively correlates with prostate cancer occurrence [46]. Additionally, KLK2, KLK5 and KLK14 may activate latent transforming growth factor (TGF) β1, while PSA activates TGFβ2, which in turn act as tumour suppressors or promoters, depending on the tumour stage [47, 48].
KLKs degrade hormones and hormonal regulators, at least in a biochemical setting. For example, KLK4-5 and KLK13-14 cleave human growth hormone (GH). GH proteolysis from a 22kDa single-chain form to a disulphide-linked 2-chain form may impede cell proliferation and angiogenesis [49].
KLKs at the tumour-stroma interface: fibroblasts
The prostate epithelial cell microenvironment is a complex, dynamic milieu that plays an integral role in prostate cancer establishment and maintenance. The prostate stromal niche consists of a number of resident or recruited cell populations, including endothelial cells, pericytes, adipocytes, preadipocytes, fibroblasts, nerve cells, myofibroblasts, smooth muscle and immune cell populations. Stromal cells, particularly myofibroblasts, interact with healthy or transformed prostate epithelium, which passively or actively influences prostate cancer establishment and progression, through one or more of its ‘hallmarks’ [1]. KLKs expressed by the invasive tumour may interact with proteins from each of these cell classes to regulate the tumour microenvironment.
Myofibroblasts secrete a significant proportion of the ECM in the cancerous stromal niche, including many of the KLK-targeted ECM substrates outlined above. In addition to degrading fibroblast-derived ECM, KLK2 activates latent TGFß1, one of the primary growth factors implicated in activation of the prostate cancer-adjacent fibroblasts, a process that renders fibroblasts permissive to, and accommodating of, prostate cancer growth [48, 50]. Prostatic stromal-derived fibroblasts respond to IGF signalling, and fibroblasts treated with PSA alone, or combined with IGFBP3 and IGF-1, induced stromal fibroblast expansion, at levels additive of that induced by PSA and IGF-1 individually, thus abrogating the inhibitory effect of IGFBP3 on IGF-1-induced stromal cell growth. This activity was clearly proteolytic, because it could be abrogated by zinc inhibition [51]. KLK4-5 are able to activate the pro-form of hepatocyte growth factor activator, which subsequently activates stromal-derived hepatocyte growth factor to induce an invasive phenotype [52]. KLK2, KLK4 and KLK14 can activate protease-activated receptor-1, which is highly expressed on prostate fibroblasts, inducing mitogenic cellular response [53-55].
KLKs at the tumour-stroma interface: angiogenesis
Tumour-stroma interactions that are widely recognised as integral to cancer progression are those between tumour cells and endothelial cells, as well as neighbouring smooth muscle cells. KLK2 cleaves high molecular weight kininogen (HMWK) to release bradykinin, a factor that can induce smooth muscle cell contraction, facilitating vasodilation and cancer cell intravasation [56]. PSA releases a kinin-like molecule from seminal fluid, although this likely involves activation of a HMWK-activating intermediate, as recombinant PSA could not directly activate HMWK [57]. Conversely, PSA can cleave Lys-plasminogen to release bioactive angiostatin-like fragments, and these purified peptides inhibit human umbilical vein endothelial, HUVEC, cell tube formation [58]. PSA affects the expression of a number of HUVEC-derived genes, inversely regulating genes that are integral in tube formation [59]. Furthermore, PSA reduces the expression and production of the pro-angiogenic vascular endothelial growth factor, along with regulating other pro- and anti-angiogenic factors, in a metastatic derivative of bone metastasis-derived prostate cancer, PC-3M, cells in vitro and following subcutaneous implantation into mice [60].
Whether the role of PSA in angiogenesis depends on its proteolytic activity is heavily debated [61-63]. Inhibition of PSA activity with small molecule inhibitors abrogated its anti-angiogenic effects compared to active PSA alone[62]. It was postulated that PSA is the functional reason that prostate cancers grow slowly, relative to other cancers, given the dependence of expanding tumours on neo-angiogenesis [63]. This has led to the rationale of using PSA agonists as prostate cancer therapies [32].
KLKs in bone metastasis
The primary site of prostate cancer metastasis is the bone [2]. KLK2-4 are expressed in bone metastatic lesions; however, their expression is not necessary for cancer metastasis, but may be responsible for the predominating osteoblastic (bone-forming) versus osteolytic (bone-degrading) phenotype almost exclusive to prostate cancer [64]. KLK4 is perhaps the most interesting KLK with regard to bone metastasis, as ameloblast-expressed KLK4 degrades the dental enamel constituent, amelogenin, in maturing mouse molars, in vivo [65]. In humans, a mutation in the KLK4 gene, predicted to encode a truncated KLK4 variant lacking a functional active site, is linked to amelogenesis imperfecta, a disease characterized by hypomaturation of the dental enamel [66]. Therefore, KLK4 is functionally active in mineralised dentine tissue, and by extension, is likely proteolytically active in the mineralised bone matrix of prostate cancer metastasis.
In accordance with the predilection of prostate cancer cells to metastasise to bone, KLK4 over-expressing PC-3 cells migrate preferentially to osteoblast-like, SaOS2, cell conditioned media compared to vector controls, which is abrogated by the serine protease inhibitor, aprotinin [41]. Our group has identified a range of novel KLK4 sub-strates in a mineralised bone matrix model [67] and validation of the functional consequences of these interactions are underway (unpublished data).
Other KLKs may also function to promote bone metastases, through the aforementioned ability of KLK2 and KLK14 to cleave and activate latent TGFβ1, respectively, as TGFβ1 regulates osteoblast differentiation and bone formation. PSA may indirectly activate latent TGFβ1 by processing plasminogen into the TGFβ-activating protease, plasmin [58]. KLK2 and KLK4 activate pro-urokinase plasminogen activator (uPA), which activates plasminogen [39, 68]. KLK2 also activates PSA [39], and KLK4 activates both KLK2 and PSA [38, 68], thus providing a proteolytic cascade which amplifies TGFβ1 activation.
PSA is mitogenic for human and rodent osteoblast cell lines, which can be abrogated by the addition of a TGFβ1 and TGFβ2 neutralizing antibody [69]. PSA activates latent TGFβ2 produced by PC-3 cells [47], and incubation of PC-3 cell conditioned media with PSA induced proliferation of rat osteoblast-derived osteosarcoma, UMR106, cells, which was reversed by TGFβ neutralisation [70]. TGFβ1 has a well-recognised effect in bone formation, and PSA over-expressing rat prostate cancer, MatLyLu, cells, injected into mouse femur, displayed increased osteoblastic properties and decreased evidence of osteolysis, although tumour burden was the same as vehicle controls. At least one of the three subcutaneously inoculated PSA over-expressing clones showed evidence of PSA activity in murine sera compared to controls [71]. Active PSA directly injected into human bone, which had been subcutaneously implanted into mice, induced osteoblastic properties, such as increased bone volume, osteoid surface and osteoblast number, concomitant with a decreased osteoclast population [69]. Strikingly, the effect of PSA in osteoblast cell mitogenesis in vitro and osteogenesis in vivo was inhibited by serine protease inhibitors [69], suggesting that these outcomes were a result of PSA-mediated proteolysis.
KLKs and tumour growth in animal models
The roles of KLKs in tumour progression are confounded by their proteolytic activity not being reported in many cell culture or animal models, and, where reported, the discrepancy between levels of active KLK in these models as compared to patient tissue. PSA is believed to possess a high level of enzymatic activity around the prostate [72]. The efficacy of KLK2 and KLK4 activity surrounding the prostate is supported by known complex formations between active forms of these KLKs and serpins in seminal fluid, as serpins only inhibit active proteases [56, 73]. Androgen-sensitive prostate cancer, LNCaP, cells express KLK2-4 at higher levels than other commonly used prostate cancer cell lines; however, KLK2 and PSA in LNCaP secretions have low levels of activity [72]. Activity of endogenous KLK4 has not been confirmed in LNCaP or other cell lines. PSA knockdown reduced LNCaP cell proliferation in vitro and reduced tumour size by 10-fold relative to controls in vivo [74]. Despite these effects, levels of active PSA in xenografts are lower than those derived from patient tissue [72]. Knockdown of KLK4 in LNCaP cells similarly reduced xenograft tumour volume, although its proteolytic activity was not assessed [75].
To increase PSA activity in LNCaP cells to a level similar to that observed in human prostatic fluid, the wild-type PSA pro-region was substituted for a pro-region susceptible to activation by furin proteases, which are constitutively active in LNCaP cells. Subcutaneous transplantation of LNCaP cells over-expressing this furin-activating PSA construct into mice rendered circulating PSA, whereby 90% was active (bound to serpins), compared to 12% being active in xenografts expressing wild-type PSA [74]. These tumours were increased in size compared to controls, validating in vitro findings that demonstrated increased cell proliferation [74]. To the contrary, when active PSA, at concentrations similar to that of patient tissues, was injected into tumours formed in mice upon subcutaneous injection of PC-3M cells, smaller xenografts were formed compared to saline-injected controls, concomitant with the down-regulation of platelet-derived growth factor β and uPA receptor [60]. PSA activity in these tissues post injection was not reported; hence, it is not known for how long, if at all, PSA retained activity.
CHALLENGES AND OPPORTUNITIES IN DEFINING KLK FUNCTION, TOWARDS TARGETING KLKS FOR CANCER THERAPY, AND THEIR UTILITY AS BIOMARKERS
KLKs show therapeutic promise as many prostatic KLKs drive cancer-related ‘hallmarks’, including epithelial-to-mesenchymal transition, migration, invasion, and local and distal stroma interaction, to facilitate tumour expansion and spread. PSA has served as the long-standing ‘gold-standard’ biomarker for prostate cancer, and there is evidence that other KLKs may add benefit as adjunct or stand-alone biomarkers for this disease. Particularly, KLK2 has shown the greatest clinical efficacy as a PSA-adjunct biomarker, in addition to discriminating between free, intact and total circulating PSA. This biomarker efficacy is emphasised by the effective application of KLK2- and PSA-activatable pro-drugs, the latter presently in clinical trials. Additionally, PSA appears to be an effective antigen for immune therapy. There exists only a single clinical trial targeting KLK function as a means of inhibiting prostate cancer progression, particularly using an engineered protein inhibitor of proteases, including KLK2, KLK4 and KLK14. Mapping the mechanism of KLK action in prostate cancer is the next step forward in the rationale design of targeted, novel therapies for this fatal disease.
To this end, laboratory-based studies of KLK function must address some key limiting issues. Firstly, it is imperative that the proteolytic activity of KLKs in cell culture and animal models be assessed in order to discriminate proteolytic from non-proteolytic functions. This is also important to determine if a lack of observed functional outcomes are due only to the inactivity of KLKs tested in these models. Furthermore, a current shortcoming, which presents also as a future opportunity for KLK research, is that functional studies to date nearly exclusively assess the action of a single KLK protease. However, multiple KLKs are simultaneously secreted by the cancerous prostate, and knowledge of the temporal expression and activity of each KLK, as well as redundancies in their proteolytic substrates and effector pathways, will be important for therapeutic design. Similarly, most functional studies have focused only on the epithelial cell component or the bone metastatic site. However, cross-talk of epithelial cell-derived KLKs with the local stroma, particularly activated fibroblasts, constitutes a myriad of interlinkages yet to be mapped that will likely greatly enhance knowledge of KLK function in prostate cancer. The next generation of cancer therapies will need to target both the tumour and activated stroma [76]; hence, it is crucial to outline those pathways in the latter compartment, as it is affected by deregulated KLK expression. Microenvironmental regulation of KLK activity is likely a key contributor to whether multifunctional KLKs act to promote or suppress tumour progression, at given disease stages. Thus, as much as possible, KLK function should be studied in humanised cell culture and animal models of prostate cancer to accelerate translation of key findings into the clinic.
Overall, understanding the functional consequences of deregulated KLK expression in prostate cancer will underpin the effective application and targeting of this protease family for prostate cancer treatment. This will also refine the utility of prostatic KLKs as prognostic bio-markers in these cancers if they are shown to be promising translational targets.
REFERENCES
- 1.Hanahan D., Weinberg R. A., Hallmarks of cancer: the next generation. Cell 2011,144, 646-674. [DOI] [PubMed] [Google Scholar]
- 2.Schroder F. H., Hugosson J., Carlsson S., Tammela T., et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012, 62, 745-752. [DOI] [PubMed] [Google Scholar]
- 3.Lilja H., Oldbring J., Rannevik G., Laurell C. B., Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest 1987, 80, 281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pretlow T. G., Pretlow T. P., Yang B., Kaetzel C. S., et al. , Tissue concentrations of prostate-specific antigen in prostatic carcinoma and benign prostatic hyperplasia. Int J Cancer 1991, 49, 645-649. [DOI] [PubMed] [Google Scholar]
- 5.Bokhorst L P., Zhu X., Bul M., Bangma C. H., et al. , Positive predictive value of prostate biopsy indicated by prostate-specific-antigen-based prostate cancer screening: trends over time in a European randomized trial. BJU Int 2012, 110, 1654-1660. [DOI] [PubMed] [Google Scholar]
- 6.Schroder F. H., Hugosson J., Roobol M. J., Tammela T L, et al. , Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012, 366, 981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draisma G., Etzioni R., Tsodikov A., Mariotto A., et al. , Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 2009,101, 374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partin A. W., Brawer M. K., Bartsch G., Horninger W., et al. , Complexed prostate specific antigen improves specificity for prostate cancer detection: results of a prospective multicenter clinical trial. J Urol 2003, 170, 1787-1791. [DOI] [PubMed] [Google Scholar]
- 9.Vickers A., Cronin A., Roobol M., Savage C, et al. , Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol 2010, 28, 2493-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazzeri M., Haese A., Abrate A., de la Taille A., et al. , Clinical performance of serum prostate-specific antigen isoform [-2] proPSA (p2PSA) and its derivatives, %p2PSA and the prostate health index (PHI), in men with a family history of prostate cancer: results from a multicentre European study, the PROMEtheuS project. BJU Int 2013, 112, 313-321. [DOI] [PubMed] [Google Scholar]
- 11.Peltola M. T, Niemela P., Vaisanen V., Viitanen T., et al. , Intact and internally cleaved free prostate-specific antigen in patients with prostate cancer with different pathologic stages and grades. Urology 2011, 77, 1009 e1001-1008. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima K., Satoh T, Baba S., Yamashita K., alpha1,2-Fucosylated and beta-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostatic cancer. Glycobiology 2010, 20, 452-460. [DOI] [PubMed] [Google Scholar]
- 13.Fossati N., Lazzeri M., Haese A., McNicholas T, et al. , Clinical performance of serum isoform [-2] proPSA (p2PSA) and its derivatives, namely %p2PSA and PHI (Prostate Health Index) in men younger than 60 years of age: results from a multicentric European study. BJU Int 2014. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson S., Maschino A., Schroder F., Bangma C, et al. , Predictive Value of Four Kallikrein Markers for Pathologically Insignificant Compared With Aggressive Prostate Cancer in Radical Prostatectomy Specimens: Results From the European Randomized Study of Screening for Prostate Cancer Section Rotterdam. Eur Urol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center M. S.-K. C, : ClinicalTrials.gov [Internet], Bethesda (MD): National Library of Medicine (US) 2014. [Google Scholar]
- 16.Shaw J. L, Diamandis E. P., Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem 2007, 53, 1423-1432. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Valentine S. J., Plasencia M. D., Trimpin S., et al. , Mapping the human plasma proteome by SCX-LC-IMS-MS. J Am Soc Mass Spectrom 2007, 18, 1249-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Planque C, Li L, Zheng Y, Soosaipillai A., et al. , A multiparametric serum kallikrein panel for diagnosis of non-small cell lung carcinoma. Clin Cancer Res 2008, 14, 1355-1362. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Ignatchenko V, Yao C. Q., Kalatskaya I., et al. , Identification of differentially expressed proteins in direct expressed prostatic secretions of men with organ-confined versus extracapsular prostate cancer. Mol Cell Proteomics 2012, 11, 1870-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Principe S., Kim Y, Fontana S., Ignatchenko V., et al. , Identification of prostate-enriched proteins by in-depth proteomic analyses of expressed prostatic secretions in urine. J Proteome Res 2012, 11, 2386-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avgeris M., Stravodimos K., Scorilas A., Kallikrein-related peptidase 4 gene (KLK4) in prostate tumors: quantitative expression analysis and evaluation of its clinical significance. Prostate 2011, 71, 1780-1789. [DOI] [PubMed] [Google Scholar]
- 22.Yousef G. M., Stephan C, Scorilas A., Ellatif M. A., et al. , Differential expression of the human kallikrein gene 14 (KLK14) in normal and cancerous prostatic tissues. Prostate 2003, 56, 287-292. [DOI] [PubMed] [Google Scholar]
- 23.Mavridis K., Stravodimos K., Scorilas A., Quantified KLK15 gene expression levels discriminate prostate cancer from benign tumors and constitute a novel independent predictor of disease progression. Prostate 2013, 73, 1191-1201. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T., Stephan C, Scorilas A., Yousef G. M., et al. , Quantitative analysis of hippostasin/KLK11 gene expression in cancerous and noncancerous prostatic tissues. Urology 2003, 61, 1042-1046. [DOI] [PubMed] [Google Scholar]
- 25.Yousef G. M., Scorilas A., Chang A., Rendl L, et al. , Down-regulation of the human kallikrein gene 5 (KLK5) in prostate cancer tissues. Prostate 2002, 51, 126-132. [DOI] [PubMed] [Google Scholar]
- 26.Olkhov-Mitsel E., Van der Kwast T., Kron K. J., Ozce-lik H., et al. , Quantitative DNA methylation analysis of genes coding for kallikrein-related peptidases 6 and 10 as biomarkers for prostate cancer. Epigenetics 2012, 7, 1037-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence M. G., Lai J., Clements J. A., Kallikreins on Steroids: Structure, Function, and Hormonal Regulation of Prostate-Specific Antigen and the Extended Kallikrein Locus. Endocr Rev 2010. [DOI] [PubMed] [Google Scholar]
- 28.DeFeo-Jones D. Garsky V. M. Wong B. K. Feng D. M. et al. , A peptide-doxorubicin ‘prodrug’ activated by prostate-specific antigen selectively kills prostate tumor cells positive for prostate-specific antigen in vivo. Nat Med 2000, 6, 1248-1252. [DOI] [PubMed] [Google Scholar]
- 29.DiPaola R.S., Rinehart J., Nemunaitis J., Ebbinghaus S., et al. , Characterization of a novel prostate-specific antigen-activated peptide-doxorubicin conjugate in patients with prostate cancer. J Clin Oncol 2002, 20, 1874-1879. [DOI] [PubMed] [Google Scholar]
- 30.Kantoff P. W., Schuetz T. J., Blumenstein B. A., Glode L M., et al. , Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010, 28, 1099-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deperthes D., Kundig C, Kallikrein-related peptidases, De Gruyter; 2012, 161-186. [Google Scholar]
- 32.Mattsson J. M., Narvanen A, Stenman U. H., Koistinen H., Peptides binding to prostate-specific antigen enhance its antiangiogenic activity. Prostate 2012, 72, 1588-1594. [DOI] [PubMed] [Google Scholar]
- 33.Obiezu C. V., Michael I. P., Levesque M. A., Dia-mandis E. P., Human kallikrein 4: enzymatic activity, inhibition, and degradation of extracellular matrix proteins. Biol Chem 2006, 387, 749-759. [DOI] [PubMed] [Google Scholar]
- 34.Webber M. M., Waghray A., Bello D., Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin Cancer Res 1995, 1, 1089-1094. [PubMed] [Google Scholar]
- 35.Borgono C. A., Michael I. P., Shaw J. L, Luo L Y, et al. , Expression and functional characterization of the cancer-related serine protease, human tissue kallikrein 14. J Biol Chem 2007, 282, 2405-2422. [DOI] [PubMed] [Google Scholar]
- 36.Kapadia C, Ghosh M. C, Grass L, Diamandis E. P., Human kallikrein 13 involvement in extracellular matrix degradation. Biochem Biophys Res Commun 2004, 323, 1084-1090. [DOI] [PubMed] [Google Scholar]
- 37.Michael I. P., Sotiropoulou G., Pampalakis G., Magklara A., et al. , Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J Biol Chem 2005, 280, 14628-14635. [DOI] [PubMed] [Google Scholar]
- 38.Yoon H., Laxmikanthan G., Lee J., Blaber S. I., et al. , Activation profiles and regulatory cascades of the human kallikrein-related peptidases. J Biol Chem 2007, 282, 31852-31864. [DOI] [PubMed] [Google Scholar]
- 39.Takayama T. K., Fujikawa K., Davie E. W, Characterization of the precursor of prostate-specific antigen. Activation by trypsin and by human glandular kallikrein. J Biol Chem 1997, 272, 21582-21588. [DOI] [PubMed] [Google Scholar]
- 40.Veveris-Lowe T. L Lawrence M. G. Collard R. L Bui L et al. , Kallikrein 4 (hK4) and prostate-specific antigen (PSA) are associated with the loss of E-cadherin and an epithelial-mesenchymal transition (EMT)-like effect in prostate cancer cells. Endocr Relat Cancer 2005, 12, 631-643. [DOI] [PubMed] [Google Scholar]
- 41.Gao J., Collard R. L, Bui L, Herington A. C, et al. , Kallikrein 4 is a potential mediator of cellular interactions between cancer cells and osteoblasts in metastatic prostate cancer. Prostate 2007, 67, 348-360. [DOI] [PubMed] [Google Scholar]
- 42.Sano A., Sangai T., Maeda H., Nakamura M., et al. , Kallikrein 11 expressed in human breast cancer cells releases insulin-like growth factor through degradation of IGFBP-3. Int J Oncol 2007, 30, 1493-1498. [PubMed] [Google Scholar]
- 43.Matsumura M., Bhatt A. S., Andress D., Clegg N, et al. , Substrates of the prostate-specific serine protease prostase/KLK4 defined by positional-scanning peptide libraries. Prostate 2005, 62, 1-13. [DOI] [PubMed] [Google Scholar]
- 44.Koistinen H., Paju A., Koistinen R., Finne P., et al. , Prostate-specific antigen and other prostate-derived proteases cleave IGFBP-3, but prostate cancer is not associated with proteolytically cleaved circulating IGFBP-3. Prostate 2002, 50, 112-118. [DOI] [PubMed] [Google Scholar]
- 45.Michael I. P., Pampalakis G., Mikolajczyk S. D., Malm J., et al. , Human tissue kallikrein 5 is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression. J Biol Chem 2006, 281, 12743-12750. [DOI] [PubMed] [Google Scholar]
- 46.Monti S., Proietti-Pannunzi L., Sciarra A., Lolli F., et al. , The IGF axis in prostate cancer. Curr Pharm Des 2007, 13, 719-727. [DOI] [PubMed] [Google Scholar]
- 47.Dallas S. L, Zhao S., Cramer S. D., Chen Z., et al. , Preferential production of latent transforming growth factor beta-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J Cell Physiol 2005, 202, 361-370. [DOI] [PubMed] [Google Scholar]
- 48.Emami N., Diamandis E. P., Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGF beta 1 in semen. Biol Chem 2010, 391, 85-95. [DOI] [PubMed] [Google Scholar]
- 49.Struman I., Bentzien F., Lee H., Mainfroid V., et al. , Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci U S A 1999, 96, 1246-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coulson-Thomas V. J., Gesteira T. F., Coulson-Thom-as Y. M., Vicente C. M., et al. , Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-beta, and extracellular matrix down-regulation. Exp Cell Res 2010, 316, 3207-3226. [DOI] [PubMed] [Google Scholar]
- 51.Sutkowski D. M., Goode R. L, Baniel J., Teater C., et al. , Growth regulation of prostatic stromal cells by prostate-specific antigen. J Natl Cancer Inst 1999, 91, 1663-1669. [DOI] [PubMed] [Google Scholar]
- 52.Mukai S., Fukushima T., Naka D., Tanaka H., et al. , Activation of hepatocyte growth factor activator zymogen (pro-HGFA) by human kallikrein 1-related peptidases. FEBS J 2008, 275, 1003-1017. [DOI] [PubMed] [Google Scholar]
- 53.Wang W., Mize G. J., Zhang X., Takayama T. K., Kallikrein-related peptidase-4 initiates tumor-stroma interactions in prostate cancer through protease-activated receptor-1. Int J Cancer 2010, 126, 599-610. [DOI] [PubMed] [Google Scholar]
- 54.Mize G. J., Wang W, Takayama T. K., Prostate-specific kallikreins-2 and -4 enhance the proliferation of DU-145 prostate cancer cells through protease-activated receptors-1 and -2. Mol Cancer Res 2008, 6, 1043-1051. [DOI] [PubMed] [Google Scholar]
- 55.Oikonomopoulou K., Hansen K. K., Saifeddine M., Vergnolle N., et al. , Kallikrein-mediated cell signalling: targeting proteinase-activated receptors (PARs). Biol Chem 2006, 387, 817-824. [DOI] [PubMed] [Google Scholar]
- 56.Charlesworth M. C, Young C. Y, Miller V. M., Tindall D. J., Kininogenase activity of prostate-derived human glandular kallikrein (hK2) purified from seminal fluid. J Androl 1999, 20, 220-229. [PubMed] [Google Scholar]
- 57.Fichtner J., Graves H. C, Thatcher K., Yemoto C., Shortliffe L M., Prostate specific antigen releases a kininlike substance on proteolysis of seminal vesicle fluid that stimulates smooth muscle contraction. J Urol 1996, 155, 738-742. [PubMed] [Google Scholar]
- 58.Heidtmann H. H., Nettelbeck D. M., Mingels A., Jager R., et al. , Generation of angiostatin-like fragments from plasminogen by prostate-specific antigen. Br J Cancer 1999, 81, 1269-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattsson J. M., Laakkonen P., Kilpinen S., Stenman U. H., Koistinen H., Gene expression changes associated with the anti-angiogenic activity of kallikrein-related peptidase 3 (KLK3) on human umbilical vein endothelial cells. Biol Chem 2008, 389, 765-771. [DOI] [PubMed] [Google Scholar]
- 60.Bindukumar B., Schwartz S. A., Nair M. P., Aalinkeel R., et al. , Prostate-specific antigen modulates the expression of genes involved in prostate tumor growth. Neoplasia 2005, 7, 241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chadha K. C, Nair B. B., Chakravarthi S., Zhou R., et al. , Enzymatic activity of free-prostate-specific antigen (f-PSA) is not required for some of its physiological activities. Prostate 2011, 71, 1680-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koistinen H., Wohlfahrt G., Mattsson J. M., Wu P., et al. , Novel small molecule inhibitors for prostate-specific antigen. Prostate 2008, 68, 1143-1151. [DOI] [PubMed] [Google Scholar]
- 63.Mattsson J. M., Valmu L., Laakkonen P., Stenman U. H., Koistinen H., Structural characterization and anti-angiogenic properties of prostate-specific antigen isoforms in seminal fluid. Prostate 2008, 68, 945-954. [DOI] [PubMed] [Google Scholar]
- 64.Koeneman K. S., Yeung F., Chung L W, Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 1999, 39, 246-261. [DOI] [PubMed] [Google Scholar]
- 65.Yamakoshi Y, Richardson A. S., Nunez S. M., Yama-koshi F., et al. , Enamel proteins and proteases in Mmp20 and Klk4 null and double-null mice. Eur J Oral Sci. 2011, 119 Suppl 1, 206-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hart P. S., Hart T.C., Michalec M. D., Ryu O. H., et al. , Mutation in kallikrein 4 causes autosomal recessive hypomaturation amelogenesis imperfecta. J Med Genet 2004, 41, 545-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reichert J. C, Quent V. M., Burke L. J., Stansfield S. H., et al. , Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials 2010, 31, 7928-7936. [DOI] [PubMed] [Google Scholar]
- 68.Takayama T. K., McMullen B. A., Nelson P. S., Mat-sumura M., Fujikawa K., Characterization of hK4 (prostase), a prostate-specific serine protease: activation of the precursor of prostate specific antigen (pro-PSA) and single-chain urokinase-type plasminogen activator and degradation of prostatic acid phosphatase. Biochemistry 2001, 40, 15341-15348. [DOI] [PubMed] [Google Scholar]
- 69.Yonou H., Aoyagi Y, Kanomata N., Kamijo T., et al. , Prostate-specific antigen induces osteoplastic changes by an autonomous mechanism. Biochem Biophys Res Commun 2001, 289, 1082-1087. [DOI] [PubMed] [Google Scholar]
- 70.Killian C. S., Corral D. A., Kawinski E., Constantine R. I., Mitogenic response of osteoblast cells to prostate-specific antigen suggests an activation of latent TGF-beta and a proteolytic modulation of cell adhesion receptors. Biochem Biophys Res Commun 1993, 192, 940-947. [DOI] [PubMed] [Google Scholar]
- 71.Cumming A. P., Hopmans S. N., Vukmirovic-Popovic S., Duivenvoorden W. C., PSA affects prostate cancer cell invasion in vitro and induces an osteoblastic phenotype in bone in vivo. Prostate Cancer Prostatic Dis 2011, 14, 286-294. [DOI] [PubMed] [Google Scholar]
- 72.Denmeade S. R., Sokoll L J., Chan D. W., Khan S. R., Isaacs J. T., Concentration of enzymatically active prostate-specific antigen (PSA) in the extracellular fluid of primary human prostate cancers and human prostate cancer xenograft models. Prostate 2001, 48, 1-6. [DOI] [PubMed] [Google Scholar]
- 73.Obiezu C. V., Shan S. J., Soosaipillai A., Luo L. Y., et al. , Human kallikrein 4: quantitative study in tissues and evidence for its secretion into biological fluids. Clin Chem 2005, 51, 1432-1442. [DOI] [PubMed] [Google Scholar]
- 74.Williams S. A., Jelinek C. A., Litvinov I., Cotter R. J., et al. , Enzymatically active prostate-specific antigen promotes growth of human prostate cancers. Prostate 2011, 71, 1595-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jin Y., Qu S., Tesikova M., Wang L, et al. , Molecular circuit involving KLK4 integrates androgen and mTOR signaling in prostate cancer. Proc Natl Acad Sci U S A 2013, 110, E2572-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J., Liu J., Tumor stroma as targets for cancer therapy. Pharmacol Ther 2013, 137, 200-215. [DOI] [PMC free article] [PubMed] [Google Scholar]


