Patients’ blood test results are different from one another hence Physicians need a reference measure that they can compare to in order to make the correct clinical decisions. These reference measures, obtained from a sample group in the healthy population, are the “normal ranges” for a specific measure. However normal ranges need to be established only for healthy individuals but also for specific physiologic conditions like pregnancy or ovulation or menopause and some pathologic conditions. The term “normal range” does not cover these conditions. Hence the term “normal range” was given up for the more appropriate term “reference interval”.
What is the exact terminology for “reference interval”?
According to the CLSI/IFCC1 C28-P3 document; Reference Individual is a selected person who meets the defined criteria for reference individuals for establishing a reference interval for a specific analyte. Reference population refers to all reference individuals in the general population. Reference sample groups are individuals within the reference population pool. Reference values are obtained from the test results of reference sample group. Reference distribution is evaluated from reference values. Reference limits are calculated from reference values. Finally reference intervals are those values that lie between the lower and upper of the reference limits (Figure 1).
Figure 1:
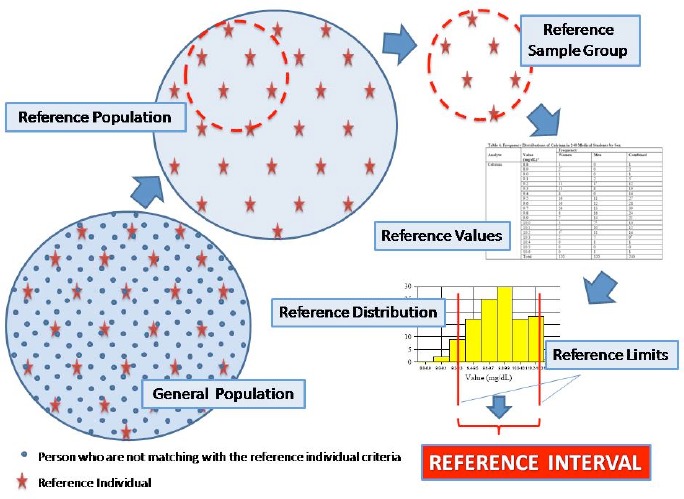
Reference individuals’ selection for reference interval studies
Why are there still the needs for describing or defining reference interval?
Emergence of new biomarkers for which reference interval has not yet been determined.
Reference intervals in-use, were established mainly for the Caucasian population, and are often inappropriate for the diverse population that many laboratories serve.
Existing reference intervals needs to be verified or validated because they were established decades ago with methods and instrumentations that are now obsolete.
Why do people find evaluating reference intervals a daunting task?
Because it requires at least 120 reference individuals. It is hard in some situations to obtain sufficient numbers of reference individuals, for example, newborns and geriatric populations. The cost of establishing comprehensive reference intervals for all of the analytes is just too prohibitive for the average laboratory. Due to these restrains laboratories have been relying on reference intervals provided by the instrument or reagent manufacturers. But these are often times inadequate due to less than optimum sample numbers and lack of details of the selection criteria used to select the reference individual. In 2008, CLSI/IFCC proposed a set of new guidelines for the establishment of reference intervals5 in which the non-parametric treatment of data is the preferred method if sample number is more than 120. However if the number of reference individual is less than 120, the robust method can be applied effectively1. This statistical method has been in use since the 1880s and the term “robust” was coined by Box in 195310. It was Horne and Pesce that applied the robust method to the field of reference intervals9 establishement. They demonstrated that accurate reference intervals can be established with less than 120 reference individuals. The Robust method is not easily applied to raw data but there are some calculation tools which have been developed to make it easier to use10. When comparing the two methods, the robust approach is easier to apply. Even though fewer samples are used in this method, confidence intervals are much higher than the classical approach.
How are reference intervals established? - A brief practical summary
The proposed 2008 CLSI/IFCC document C28-P31 lists the steps for reference interval establishment. They are as follows:
Analytical interferences and the source of biological variability should be considered. All preanalytical and analytical processes must be thoughtfully considered, documented and controlled.
-
The selection of reference individuals must be thoughtful and with advanced consideration given to exclusion and partitioning criteria. The health status of the reference individuals must be documented. The better the reference individual is described, the greater the value of the reference interval study. Two commonly used procedures for obtaining reference values in the past should be avoided;
Collecting samples from only healthy young adults and
Using laboratory test results from the patient population.
A reference individual questionnaire should be prepared appropriately.
The potential reference individuals should be categorized by using the data obtained from questionnaires and health investigations of reference individual candidates.
Inappropriate individuals should be excluded according to pre-defined exclusion criteria.
The specimens should be collected and handled in a manner consistent with the routine practice for patient specimen.
The specimens should be analyzed by an appropriate analytical methodology under well defined conditions.
After all data are collected, a frequency histogram should be prepared and examined visually in order to decide on outliers. Additionally Dixon-Reed or Tukey test should be applied to detect outliers.
Although the nonparametric method is strongly recommended, considering the difficulties of obtaining 120 samples for individual laboratories the robust method is also recommended.
There are many ongoing studies for defining reference interval throughout the world. There are several projects throughout Europe. TURA, is one of such project being conducted by The Association of Clinical Biochemist (KBUD) in Turkey. This organization also established the national external quality control programme and successfully been directing it since 2005 in Turkey. TURA is not the first reference interval study in Turkey but it is the latest and the most ambitious one. A number of university hospital laboratories, public hospital laboratories and private laboratories are collaborating on the project. The initial goal of the project is to establish reference interval for several analytes with specific focus on the Turkish population but eventually to update all outdated reference intervals for all analytes.
Appropriate reference intervals are required for accurate diagnosis and monitoring of patients. There are some good examples of well designed and successful reference interval studies throughout Europe which were completed within the last ten years. NORIP (NOrdic Reference Interval Project)11 is one of the most successful ones1. Five Nordic countries participated in this project. 25 of the most common chemistry analytes were measured, 102 laboratories participated, resulting in about 200,000 measurements (125,000 reference values and 75,000 control values) from 3,036 reference individuals12,8. The Italian REALAB Project7 is another comprehensive project which uses patients’ results. In this study 15,000,000 records related to 197,350 individuals were collected. After exclusion criteria’s were applied only 61,246 samples constituting the reference sample group study were used. Reference intervals for the 23 most common chemistry analytes plus an additional 13 analytes were established from this set of 61,246 results. Even though this method is not recommended by the recent CLSI / IFCC guideline, many laboratories still use this method to establish reference intervals.
CALIPER is another project focusing on development of paediatric reference interval involving paediatric hospitals across Canada4. The initial aim of this study is to establish instruments and ethnic specific reference intervals for analytes that are needed most urgently. CALIPER is also aiming to collaborate with paediatric laboratory centres around the world for sample collection.
Laboratories throughout the world are realising that their reference intervals are either not accurate or in-appropriate for the population they serve and are updating them by either the transferance method or conducting a full scale reference intervals study. The recent CLSI/IFCC document is an excellent aid for design and implementation of such studies1. The IFCC being an international organisation is the perfect body to co-ordinate this most important task of establishing accurate and appropriate reference intervals. It could help to establish a tool in which the laboratory communities throughout the world could tap into; like an interactive web based forum where scientists can share their experiences. This platform may also help to harmonise the observed data where possible. The IFCC also has a key role to play in the standardization of assays so reference data are transferable.
References
- 1.IFCC&CLSI C28-P3 Guideline for Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Proposed Guideline—Third Edition [Google Scholar]
- 2.Method Validation Reference Interval Transference. http://www.westgard.com.
- 3. http://www.aacc.org
- 4. http://www.caliperproject.ca
- 5. http://www.clsi.org
- 6. http://www.ifcc.org
- 7.The REALAB Project: A New Method for the Formulation of Reference Intervals Based on Current Data E. Grossi, R. Colombo, S. Cavuto, C. Franzini Clinical Chemistry 51:7 1232-1240(2005) [DOI] [PubMed] [Google Scholar]
- 8.NORDIC Reference Interval Project. P, Rustad. 2002. http://www.furst.no/norip/, 2003.
- 9.A robust approach to reference interval estimation and evaluation Paul S. Horn, Amadeo J. Pesce, and Bradley E. Copeland Clinical Chemistry 44:3,622-631 (1998) [PubMed] [Google Scholar]
- 10.Simon Newcomb, Percy Daniell and the History of Robust Estimation 1885-1920 Stephen M. Stigler Journal of the American Statistical Association, Vol. 68, No. 344 (Dec., 1973), pp. 872-879 [Google Scholar]
- 11.Suggested Reference Intervals For 8 Serum Enzymes Based On Data From NORIP Database Stromme J. et al. : http://www.furst.no/norip/reports, 2002
- 12.The Nordic Reference Interval Project 2000: Recommended Reference Intervals for 25 Common Biochemical Properties. Scand J. Clin. Lab. Invest 2004; 64:285-292. [DOI] [PubMed] [Google Scholar]


