Abstract
Results from clinical laboratory measurement procedures must be equivalent to enable effective use of clinical guidelines for disease diagnosis and patient management. Analytical results that are harmonized and independent of the measurement system, time, and location of testing is essential for providing adequate patient care. The key to generating harmonized results is establishing traceability to an accepted reference standard where available. Awareness of the benefits of having traceable measurement results that are harmonized has increased along with efforts to develop approaches to enable and facilitate the implementation of harmonization. Although several organizations are addressing harmonization of test procedures, centralized and cooperative global oversight is needed to ensure that the most important tests are being addressed and resources are optimally used. Working with its domestic and international partners, the American Association for Clinical Chemistry (AACC) has created an International Consortium for Harmonization of Clinical Laboratory Results. Advances in this area will improve the quality of patient care.
Key words: harmonization, traceability, harmonization consortium, measurand, comparable test results
THE PROBLEM: INTRODUCTION
Many clinical decisions are based upon clinical guidelines that use a fixed laboratory test result for treatment decisions. A basic problem in laboratory medicine is that different laboratory measurement procedures that intend to measure the same measurand may give different results for the same specimen. If different laboratories get different results, clinical guidelines become compromised and a patient may get the wrong treatment. Many clinical studies may use a central laboratory with a single method; however, guidelines resulting from such a study cannot be effectively implemented until all other methods are harmonized to the central laboratory procedure. Other types of clinical studies may use multiple laboratories that use different methods in which case data cannot be aggregated to develop guidelines until the results from the different methods are harmonized.
Over the past two decades, there have been a number of harmonization successes that have contributed to significant improvements in identifying and managing individuals with chronic diseases, such as diabetes and heart disease (1, 2). But despite these successes, the total number of laboratory tests for which a reference system is available remains very small (approximately 80).
WHAT IS HARMONIZATION
Harmonization is achieving uniform results among different measurement procedures for the same laboratory test. Harmonization usually implies there is no reference measurement procedure available. Harmonization includes consideration of nomenclature, patient preparation, specimen collection and handling, result value, reporting units and interpretative information. The topic for this report focuses on achieving harmonized test results.
ACHIEVING HARMONIZED LABORATORY TEST RESULTS
There are basically two important aspects or requirements to achieve harmonized or equivalent results. The first requirement is that all measurement procedures must measure the same quantity. Secondly all measurement procedures should be traceable to a common reference system. There is an ISO Standard 17511 that describes traceability and puts forth a pathway for establishing a traceable link to a reference system, where one exists (3). Figure 1 shows the traceability scheme based on the ISO Standard. Since it is not practical in the clinical laboratory to use a reference measurement procedure for routine testing, it is important that the patient’s result is traceable to the reference measurement procedure. Establishing this “traceability chain” is accomplished through the materials and methods depicted in Figure 1. In many instances it may be necessary to substitute a panel of patient samples when reference materials are not available or deficiencies of the reference materials limit their use. For example, many existing reference materials are not commutable with patient samples and therefore not suitable to be used to calibrate routine clinical laboratory test procedures (4).
Figure 1.
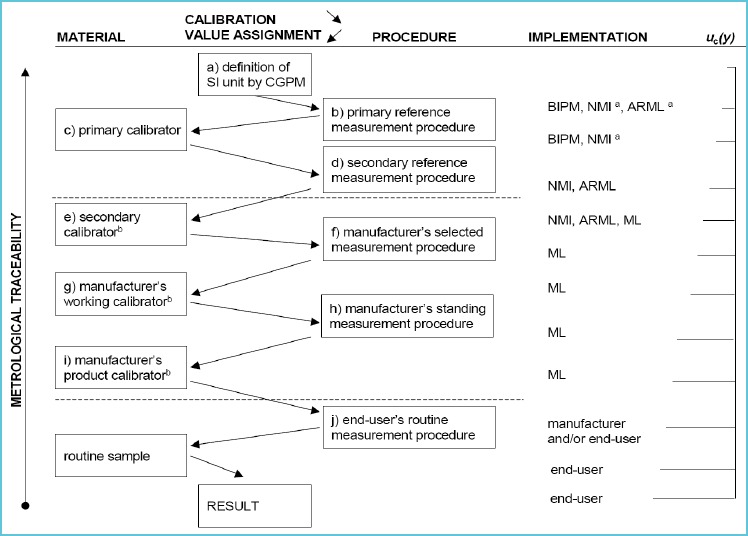
Traceability scheme
Source: ISO 17511
It is important to recognize that calibration traceability does not ensure accuracy for an individual patient’s sample. The imprecision of the measurement procedure may be too large, the measurement procedure may not be specific for the measurand, interfering substances may influence the result or the measurand itself may not be well defined and the molecular form of clinical interest may not be understood. Consequently different methods may be measuring something a little bit different making it impossible to achieve harmonization of results.
WHAT TO DO
The AACC convened an international leadership conference in 2010 to address some of the issues that hamper calibration and traceability in laboratory medicine. Professional organizations and in vitro diagnostics (IVD) manufacturers were invited to send representatives to participate in this leadership conference. Ninety individuals from 12 countries representing 62 organizations and IVD manufacturers participated in the conference to review the issues and come up with recommendations for improving calibration traceability in laboratory medicine. The output from this conference was a proposed roadmap that established a pathway to address unmet needs for harmonization of clinical laboratory measurement procedures (5). The key point in the roadmap was a recommendation to develop an infrastructure to coordinate harmonization activities worldwide. The infrastructure needed to address the following key points: 1) prioritizing measurands by medical importance, 2) coordinating the work of different organizations, 3) developing technical processes to achieve harmonization when there is no reference measurement procedure or no reference material and 4) promoting surveillance of the successes of harmonization. These four goals were intended to address the key issues identified by the conference participants.
One of the key attributes of this new infrastructure is a focus on cooperation with other organizations already actively working to improve harmonization of laboratory test results. Cooperation is accomplished in part by establishing a communication portal that provides information on what harmonization activities are being conducted by organizations in different countries. A communication portal is essential to minimize duplication of effort and resources.
FORMATION OF A HARMONIZATION CONSORTIUM
Following the international leadership conference, a steering committee was established to fully develop the consortium organization.
Figure 2 shows the organizational infrastructure for the International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR) created to fulfil the roadmap recommendations. The AACC, which supported the development work, agreed to serve as the Secretariat and host organization for this new consortium. The principal components of the ICHCLR include; a Council made up of a small number of professional organizations which is responsible for the governance and administration of the program, a Harmonization Oversight Group (HOG) which is the principle group responsible for the operation and management of harmonization activities, an Organizational Member category which provides an opportunity for organizations (e.g., IVD manufacturers, professional societies, standard-setting organizations, etc.) to become a member of the ICHCLR and appoint a representative to be a member of the HOG, and a Strategic Partners Group which is open to interested stakeholders to officially join and contribute to the consortium by submitting measurands in need of harmonization and nominating experts for consideration to serve on the HOG.
Figure 2.
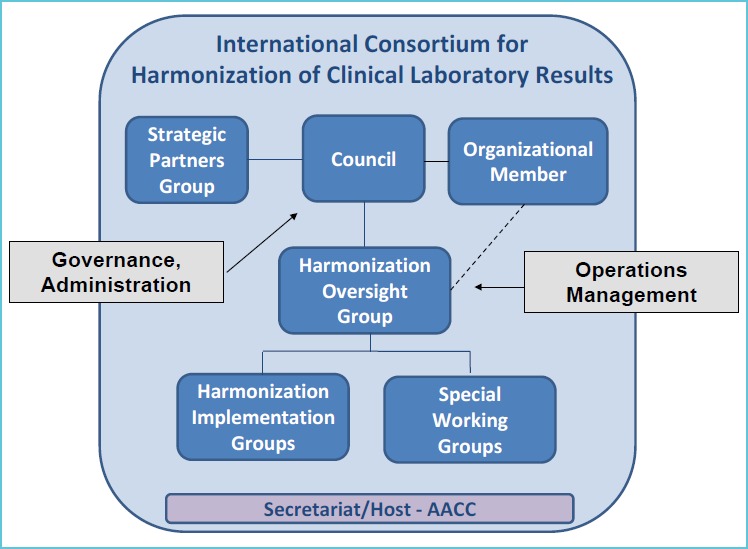
An infrastructure for harmonization
As the HOG is the central organizing body for managing harmonization activities in the Consortium, a key responsibility is to communicate with strategic partners, which include clinical practice groups, laboratory practice groups, IVD manufacturers, public health organizations, metrology institutes, standards organizations, regulatory organizations and proficiency testing and external quality assessment organizations. It is extremely important that all of these organizations are engaged in the process and know what is going on. Another major responsibility of the HOG is to evaluate measurand proposals submitted by interested stakeholders and determine their priority and technical feasibility for harmonization. To accomplish this, a Special Working Group of experts can be convened to evaluate a submitted proposal and make recommendations back to the HOG. Criteria for prioritization include: medical need, is the test associated with a particular clinical practice guideline, frequency of testing, and performance of routine tests methods in proficiency testing and external quality assurance schemes (EQAS) programs. The HOG will post prioritization information on the Consortium website so that stakeholders around the world will be aware of what measurands are in need of harmonization. The prioritized list will allow standards organizations and IVD companies to decide how to direct limited resources to improve harmonization of clinical laboratory test results.
The web site also includes information on what organization is pursuing harmonization of a given measurand. If an organization, such as the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) that is active in harmonization work, is interested to accept a project, then the HOG will refer a project to that organization and provide a link on the web site so progress can be tracked. Alternatively, the HOG may recommend that a project to harmonize the measurand be initiated. The HOG will then identify a champion and appoint a Harmonization Implementation Group (HIG) to develop a technical plan for harmonization with the ultimate goal to achieve listing in the database maintained by the Joint Committee for Traceability in Laboratory Medicine (JCTLM).
The JCTLM uses ISO standards to review reference measurement procedures (ISO 15193), reference materials (ISO 15194) and reference measurement laboratories (ISO 19195) for conformance to ISO criteria. The JCTLM database (www.bipm.org/jctlm/) lists approved reference measurement procedures, reference materials, and reference measurement services that the IVD industry can use as the basis for measurement procedure calibration traceability. What is missing from the ISO standards is a standard that addresses traceability to a harmonization protocol that does not use a reference measurement procedure or certified reference material. To fill this need the HOG developed and submitted to the ISO Technical Committee 212, a preliminary work item proposal on harmonized measurement procedures. This proposal is being addressed by Working Group 2 as a new standard that will allow JCTLM listing of processes to achieve harmonization.
WEBSITE PORTAL
As mentioned previously, a key attribute of the ICHCLR is the establishment of a communication portal to share information on harmonization activities from around the world. A website portal for this purpose has been established at www.harmonization.net. Figure 3 is a screen shot of the harmonization website resources page. The site provides information on the Council, the HOG, and the Strategic Partners Group. The site contains resources to support global harmonization of clinical laboratory measurement procedures including: a link to the “Roadmap” paper, an AACC position statement on harmonization, minutes from meetings of the Council and HOG, Strategic Partners Update Reports, operating procedures for the ICHCLR and a copy of the toolbox of technical procedures to be considered when developing a process to achieve harmonization for a measurand. There is a separate section dedicated to measurands which provides information on the status of harmonization and standardization of measurands from organizations around the world. Individuals or organizations can submit a measurand to the Consortium for inclusion on the priority list through the website. A fully electronic process provides an efficient mechanism for submitting measurands for consideration.
Figure 3.

Harmonization website resources page
TOOLBOX FOR HARMONIZATION
Special attention is drawn to the toolbox of technical procedures to be considered when developing a process to achieve harmonization for a measurand. The toolbox was created by a task force during the formation of the Consortium and contains useful information as a starting point for harmonization. There are two key protocols detailed in the toolbox, 1) the integrated harmonization protocol and 2) a step-up design for harmonization. The integrated protocol is meant to be an assessment study which is a very carefully designed experiment incorporating clinical samples, pooled clinical samples, admixed clinical samples to assess linearity and any candidate reference materials that may be available. The protocol integrates into one carefully designed experiment the ability to obtain information to enable decisions on feasibility to achieve harmonization given the tools available, the preferred approach to harmonization that is likely to succeed and based on this information a commitment to proceed by interested stakeholders.
The Step-up design is intended for use when there is no reference measurement procedure and no reference material. This particular protocol was developed under the leadership of Professor Linda Thienpont in the context of the IFCC Committee for Standardization of Thyroid Function Tests (6). The step-up design is a sequence of patient sample comparisons between clinical laboratory procedures where success at one phase allows the harmonization process to “step up” to the next phase. The phases are designed to determine whether the methods correlate with each other, which is an essential pre-requisite to achieve harmonization, if there is an adequate response over the measuring interval, if there is adequate specificity for the measurand and an adequate value assignment, such as an all methods mean or a trimmed all methods mean that may be agreeable on a consensus basis. After several qualification phases, a panel of patient sera is fit for purpose to harmonize a set of clinical laboratory measurement procedures. Sustainability is assured by a second panel to harmonize new methods entering the market and to be used to transfer values to subsequent panels to maintain consistency of the scheme.
PATH FORWARD
Harmonizing a greater number of clinical laboratory tests will contribute to improved healthcare in many important ways. Harmonized test results will ensure that clinical guidelines that call for the use of laboratory tests can be appropriately implemented. Reliable screening to detect diseases early, when they are easier to treat; appropriate diagnoses of diseases; correct and consistent treatment decisions; and effective monitoring of responses to treatment will be important outcomes of more extensive harmonization of clinical laboratory test results. Furthermore, by reducing incorrect interpretations of laboratory test results, harmonization can help prevent treatment errors and unnecessary – and expensive – follow-up diagnostic procedures and treatments based on inaccurate laboratory test results. The ICHCLR encourages all interested stakeholders to recognize the critical role of clinical laboratory testing in improving health outcomes and to join the ICHCLR in promoting the need for achieving harmonization of laboratory tests results.
REFERENCES
- 1.Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE for the NGSP Steering Committee The National Glycohemoglobin Standardization Program: A five-year progress report. Clin Chem 2001;47:1985–1992. [PubMed] [Google Scholar]
- 2.Warnick GR, Kimberly MM, Waymack PP, Leary ET, Myers GL. Standardization of measurements for cholesterol, triglycerides and major lipoproteins. LABMEDICINE 2008;39:481–490. [Google Scholar]
- 3.ISO. In vitro diagnostic medical devices – measurement of quantities in biological samples – metrological traceability of values assigned to calibrators and control materials. 1st ed. ISO17511:2003(E). Geneva: ISO; 2003.ISO 17511 [Google Scholar]
- 4.Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem 2006;52:553–554. [DOI] [PubMed] [Google Scholar]
- 5.Miller WG, Myers GL, Gantzer ML, Kahn SE, Schönbrunner ER, Thienpont LM, Bunk DM, Christenson RH, Eckfeldt JH, Lo SF, Nübling CM, Sturgeon CM. Roadmap for harmonization of clinical laboratory measurement procedures. Clin Chem 2011;57:1108–1117. [DOI] [PubMed] [Google Scholar]
- 6.Van Uytfanghe K, De Grande LA, Thienpont LM. A “Step-Up” approach for harmonization. Clin Chim Acta 2014;432:62–67. [DOI] [PubMed] [Google Scholar]


