Abstract
Harmonisation of reference intervals (RIs) refers to use of the same or common RI across different platforms and /or assays for a specified analyte. It occurs optimally for those analytes where there is sound calibration and traceability in place and evidence from a between-method comparison shows that bias would not prevent the use of a common RI. The selection of the RI will depend on various sources of information including local formal RI studies, published studies from the literature, laboratory surveys, manufacturer’s product information, relevant guidelines, and mining of databases. Pre-analytical and partitioning issues, significant figures and flagging rates, are assessed for each analyte.
Several countries and regions including the Nordic countries, United Kingdom, Japan, Turkey, and Australasia are using common RIs that have been determined either by direct studies or by a consensus process. In Canada, the Canadian Society of Clinical Chemists Taskforce is assessing the feasibility of establishing common reference values using the CALIPER (Canadian Laboratory Initiative on Pediatric Reference Intervals) and CHMS (The Canadian Health Measures Survey) databases as the basis. Development of platform-specific common reference values for each of the major analytical systems may be a more practical approach especially for the majority of analytes that are not standardised against a primary reference method and are not traceable to a primary or secondary reference material.
We encourage laboratories to consider adopting reference intervals consistent with those used by other laboratories in your region where it is possible and appropriate for your local population. Local validation of the adopted reference interval is also recommended as per CLSI guidelines.
Key words: harmonisation, standardisation, reference intervals, transference, adult, paediatric
INTRODUCTION
Despite studies having shown that the variation in reference intervals (RIs) for chemistry analytes may be greater than the analytical inaccuracy of the measurement, differences in RIs persist between laboratories that use the same platforms and the same reagents (1-3). This has implications for result interpretation and patient outcomes where the same values may be interpreted differently due to differences in RIs or decision limits hence leading to inappropriate over- or under-investigation or treatment of the patient.
One way to overcome this situation is to use the same interval. Harmonisation of RIs refers to use of the same or common RI across different platforms and /or assays for a specified analyte. Importantly, harmonisation of RIs occurs optimally for those analytes where there is sound calibration and traceability in place and evidence from a method comparison study shows that bias would not prevent the use of a common RI. The advantages of using a harmonised RI are less confusion and misinterpretation of results for both doctors and patients. Irrespective of the pathology provider or the method, provided the same RI, unit and terminology are used, an individual patient’s results can then be amalgamated.
An organisational plan is required before setting out on the sequence of practical processes that are required to achieve a major national change in pathology RIs. This is not a trivial matter and the importance of a structured approach cannot be overemphasised. Table 1 outlines the sequence of steps required to derive and validate common RIs that was used for the Australasian RIs study (3). The four key areas are: 1) seeking the evidence; 2) consultation; 3) verification; and 4) implementation (Fig.1 A and 1B). The Australasian Association of Clinical Biochemists (AACB) and the Royal College of Pathologists of Australasia (RCPA) invited pathologists and medical scientists to harmonise RIs at the same time as other RCPA initiatives for standardisation of pathology units, terminology, and report formatting and flagging were being undertaken (4). The input by main stakeholders, i.e. pathologists, scientists, clinical societies and government bodies, is central to the success of any harmonisation project and can provide helpful advice and guidance as was the case for the UK Pathology Harmony RIs project (5,6).
Table 1.
Sequence of events to derive and validate common reference intervals (RIs) through an evidence-based approach and extensive data analysis
| Identify problem |
| Agree to address common RIs |
| Identify relevant groups |
| Seek formal co-operation (if external bodies involved) |
| Form working group |
| Describe problem in detail |
| Allocate a budget and determine sources of funding |
| Gather information (surveys, RI studies, data mining, bias study, calibration traceability, RI verification laboratory information, flagging rates) |
| Consider solutions |
| Produce discussion paper, etc. |
| Seek feedback from stakeholders |
| Revise recommendations |
| Obtain formal endorsement |
| Publish |
| Promote |
| Monitor introduction |
Figure 1A.
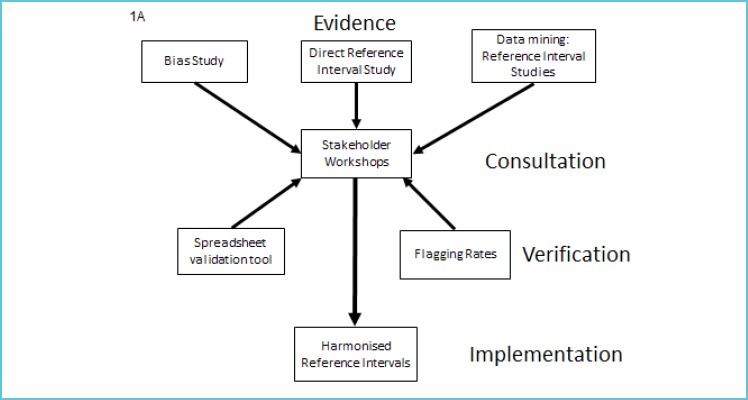
Implementation plan for the introduction of adult common reference intervals
Figure 1B.
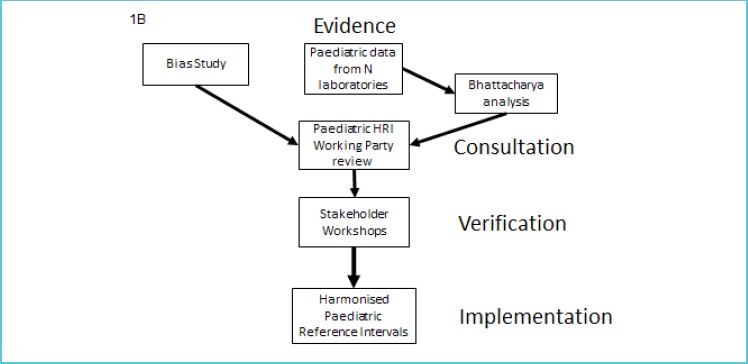
Implementation plan for the introduction of paediatric common reference intervals
REQUIREMENTS FOR USE OF HARMONISED REFERENCE INTERVALS
Seeking the evidence is paramount to the implementation of common RIs. One such approach used in Australasia to assess the feasibility of using common RIs was an evidence-based checklist approach. (7). It was based on the following criteria (8):
Define analyte (measurand)
Define assays used, accuracy base, analytical specificity, any method-based bias
Consider important pre-analytical differences, and actions in response to interference
Define the principle behind the RI (e.g. central 95%)
-
Describe evidence for selection of common RIs
data sources (literature, lab surveys, local RI studies, manufacturers’ product information)
data mining
bias goal as quality criterion for acceptance
Consider partitioning based on age, sex, etc.
Define degree of rounding
Consider the clinical implications of the RI
Consider use of common RI
Document and implement
An example of the checklist approach is shown for creatinine (Table 2).
Table 2.
Checklist reference interval (RI) approach for creatinine
| Analyte | Creatinine (plasma and serum) |
|---|---|
| Population RI | Based on healthy subjects not hospital patients. |
| eGFR used for decision making. | |
| Units | µmol/L |
| JCTLM-listed traceability or preferred method and reference material | ID-GC/MS and |
| ID-LC/MS (some methods require instrument factors). | |
| SRM 914 (pure creatinine). | |
| SRM 909, 967 (human serum). | |
| Pre-analytics 1. Serum/plasma 2. Sample collection 3. Interferences |
1. Interchangeable. 2. Increases with meat consumption. |
| Analytical differences | Analytically there are no differences. |
| Partitioning by 1. Gender 2. Age |
1. Gender differences. 2. Age-related increases above 60 years not agreed by Renal Physicians. |
| Reporting Interval | 1 µmol/L |
Assessment of method differences
Bias study
Any significant method bias will result in mis-classification of too many patients. The expected information derived from the combination of assay and RI must meet the appropriate clinical sensitivity and specificity required for each test. Hence a key requirement for the use of common RIs is the effect of methodological differences on bias and if this would affect the sharing of a common RI. Method differences are best assessed for bias using commutable patient-based samples. In the case of the Australasian Harmonised RI study specified performance limits based on biological variation were applied to determine whether bias would prevent the use of a common RI by assessing if all results fell within the allowable limits of agreement and if regression lines were all within allowable limits for the tested measurement procedures (10). The allowable limits of performance or allowable error specify that the imprecision and bias of a method must be within stated limits. Of 27 tested analytes among eight platforms/assays, 19 gave acceptable bias for a common RI (11). Note that where a RI is shared the analytical variation for more analysers in more laboratories using more methods will be larger than a singly-derived interval, resulting in a wider RI (12).
Calibration traceability
An initial assessment of methodology and calibration traceability of laboratory assays to be used to establish the common RI is required. Laboratories need to assess the traceability claims made by manufacturers including the reference material and reference measurement procedures used to assign values to master calibrators from which product calibrators are traceable in routine assays. Preliminary information can be gathered from the manufacturer, external quality assurance (EQA) programs and other published data. If a laboratory uses a method known to be biased compared with the method used to set the RI, a common RI cannot be used. Rather, for analytes with established traceability, traceable assays should be used to both set and to use the interval (13). Ideally, analytes should have a complete reference measurement system or a reference material and/or a reference measurement procedure listed on the Joint Committee for Traceability in Laboratory Medicine (JCTLM) website (14).
Selection of reference intervals
Various sources of information on RIs should be searched including local formal RI studies, published studies from the literature, laboratory surveys, manufacturer’s product information, relevant guidelines, and mining of databases. Pre-analytical and partitioning issues, significant figures and flagging rates, which provide an indication of the clinical considerations of the RI, should also be assessed for each analyte.
Common laboratory usage
A survey of local laboratories ideally through the national EQA provider provides the opportunity for laboratories to compare their RIs with those from other laboratories using the same and different methods. By linking RIs to results from measurements on commutable samples, it is also possible to see the effect of the intervals on between-laboratory differences. For the majority of common chemistry analytes the between-laboratory variation in RIs is usually greater than the variation in results (15). These types of data can be used to support the use of common RIs for many analytes.
Published studies
The Nordic Reference Interval Project (NORIP) established common RIs in apparently healthy adult populations from five Nordic countries for 25 of the most common clinical chemistry analytes (16). Results were traceable to higher-order reference measurement systems. More recently Nordic paediatric RIs have been determined for 21 common biochemistry analytes and intervals were suggested for combined age groups (17). In the United Kingdom, reference limits have been established by a survey of RIs in use followed by an assessment of analytical variability, any age and sex related variation, or other variances in populations where these were seen as relevant to the analyte (5,6). The aim was to remove unnecessary variation that was demonstrated to lack scientific validity prior to taking on new work to formally validate the consensus RIs (6).
Global formal reference interval studies
The CALIPER Initiative
The Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) (18) was established by a Canadian team of investigators to develop a new database of biomarker reference values (stratified by age, sex and ethnicity) determined from a large, healthy population of community children and adolescents. The CALIPER project was initiated as a result of several detailed gap analyses evaluating the availability of pediatric RIs in four clinical sub-specialties: bone markers (19), risk markers for cardiovascular disease and metabolic syndrome (19,20), hormones of the thyroid and growth hormone axes (21), and markers of inborn errors of metabolism (22). These analyses revealed major gaps in data available to clinical laboratories and paediatricians and highlighted the critical need for new initiatives. Since its inception in 2009, the CALIPER program has made considerable strides in establishing and publishing a new RI database for biochemical markers (23-33), however, the reference values were initially established on a single analytical system, the Abbott Architect assay system. To address this limitation, a series of transference studies (34-37) have recently been completed by the CALIPER program, allowing transference of paediatric reference values from the Abbott database to four other major analytical systems including Beckman, Ortho, Roche, and Siemens. Additional transference studies are in progress to complete transference of the entire CALIPER RI database to all major chemistry assay systems allowing widespread application of CALIPER reference standards in clinical laboratories worldwide using any one of the five major biochemical assay systems.
Canadian Health Measures Survey (CHMS)
The Canadian Health Measures Survey (CHMS) is the most comprehensive, direct health measures survey ever conducted in Canada. The study was launched in 2007 by Statistics Canada, in partnership with Health Canada and the Public Health Agency of Canada, to collect population-representative health information from Canadians aged 3-79 years. An initial household interview collected information about general health including nutrition, smoking habits, alcohol use, medical history, physical activity, and socioeconomic variables. Respondents then visited a mobile examination centre, where direct physical measures of health were taken, such as height, weight and blood pressure, and blood specimens were collected and analysed for biomarkers of health and disease (25). Individuals were selected in a systematic manner to be representative of 96.3% of the Canadian population. Data from CHMS samples were then weighted to ensure that the study population was truly representative of age, geographical distribution and ethnic origin of the Canadian population. In a recent collaboration between CALIPER and CHMS, laboratory data from approximately 12000 Canadian children and adults were used to establish a comprehensive database of paediatric and adult reference intervals for 24 chemistry (38), 13 endocrine/special chemistry (39), and 16 haematology markers (40). These reference intervals provide a valuable description of the changes in key biochemical parameters within the Canadian population. The use of common patient selection, pre-analytical, analytical and post-analytical methods allowed for assessment of fluctuations in ‘normal’ levels over time and prevalence of disease risk factors. Together, these studies provide a comprehensive description of the changes in important biomarkers within the Canadian population throughout the course of a lifetime, from childhood to adulthood to geriatrics.
The CALIPER and CHMS initiatives also provide a unique opportunity to strive towards establishment of common RIs across Canada. A taskforce has recently been developed by the Canadian Society of Clinical Chemists and discussions have begun among a number of opinion leaders across the country to assess the feasibility of establishing common reference values using the CALIPER and CHMS databases as the basis. The Canadian common reference interval initiative is also examining the potential development of platform-specific common reference values for each of the major analytical systems. This may be a more practical approach especially for the majority of analytes that are not standardised based on primary reference method and not traceable to a primary or secondary reference material.
Asian Studies
Although not attempting to define population reference intervals, Ichihara et al. (41) found unexpectedly large variations between the results obtained from samples sourced from 6 Asian cities (Hong Kong, Shanghai, Seoul, Kuala Lumpur, Taipei and Tokyo) for the 13 analytes tested suggesting that harmonised RIs would be difficult between these countries that these cities represent.
In contrast to the study by Ichihara, a Japanese multicentre study by Yamamoto et al. (42) involving 105 laboratories across Japan and using 4 different chemistry platforms demonstrated no regional differences and concluded that the RIs established in this study were also suitable for adoption nationwide (Table 3).
Table 3.
Adult reference intervals (RIs) for chemistry analytes determined by direct RI studies or by consensus
| Analyte | Unit | Australia44 | Turkey43 | Nordic countries16 | United Kingdom5 | Japan42 | Canada38 | Australasia3 |
|---|---|---|---|---|---|---|---|---|
| Cat 2a Direct | Cat 2a Direct | Cat 2a Direct | Cat 4 Consensus | Cat 2a Direct | Cat 2a Direct | Cat 4 Consensus | ||
| Architect | Architect | Multiple platforms | Multiple platforms | 4 main platforms | Architect | 8 main platforms | ||
| Sodium (M) | mmol/L | 136-145 | 137-144 | 137-145 | 133-146 | 137-144 | 16-49y: 137-142 | 135-145 |
| 50-79y: 136-143 | ||||||||
| Sodium (F) | mmol/L | 136-145 | 137-144 | 137-145 | 133-146 | 137-144 | 16-49y: 137-143 | 135-145 |
| 50-79y: 136-143 | ||||||||
| Potassium | mmol/L | 3.7-4.9 | 3.7-4.9 | 3.6-4.6 | 3.5-5.3 | 3.6-4.8 | 3.8-4.9 | 3.5-5.2 |
| Chloride | mmol/L | 101-110 | 99-107 | - | 95-108 | 101-108 | 30-79y: 102-108 | 95-110 |
| Bicarbonate | mmol/L | 20-29* | - | - | 22-29 | - | 19-26 | 22-32 |
| Creatinine (M) | µmol/L | <75y: 65-103 | 59-92 | 60-100 | 60-100 | 57-94 | 16-79y: 63-102 | 60-110*** |
| 75+y: 47-120 | ||||||||
| Creatinine (F) | µmol/L | <75y: 54-83 | 50-71 | 50-90 | 60-100 | 41-69 | 17-79y: 49-85 | 45-90*** |
| 75+y: 40-91 | ||||||||
| Calcium (M) | mmol/L | 2.19-2.56 | 2.15-2.47 | 2.15-2.51 | 2.2-2.6 (adjusted)** | 2.2-2.5 | 20-39y: 2.28-2.60 | 2-10-2.60 |
| 40-79y: 2.24-2.56 | ||||||||
| Calcium (F) | mmol/L | 2.19-2.56 | 2.15-2.47 | 2.15-2.51 | 2.2-2.6 (adjusted)** | 2.2-2.5 | 20-39y: 2.24-2.53 | 2-10-2.60 |
| 40-79y: 2.24-2.56 | ||||||||
| Magnesium | mmol/L | 0.77-1.04 | 0.77-1.06 | 0.71-0.94 | 0.7-1.0 | 0.7-1.0 | - | 0.7-1.1 |
| Phosphate (M) | mmol/L | 0.83-1.36 | 0.80-1.40 | <50y: 0.75-1.65 | 0.8-1.5 | - | 16-47y: 0.95-1.52 | 0.75-1.50 |
| 50+y: 0.75-1.35 | 48-79y: 0.89-1.52 | |||||||
| Phosphate (F) | mmol/L | 0.88-1.44 | 0.80-1.40 | 0.85-1.50 | 0.8-1.5 | - | 16-47y: 0.95-1.52 | 0.75-1.50 |
| 48-79y: 0.99-1.54 | ||||||||
| LDH (M) | U/L | 130-230 | 126-220 | <70y: 105-205 | - | 124-226 [JSCC] | - | 120-250 (L-P [IFCC]) |
| 70+y: 115-255 | ||||||||
| LDH (F) | U/L | 122-232 | 126-220 | <70y: 105-205 | - | 124-226 [JSCC] | - | 120-250 (L-P [IFCC]) |
| 70+y: 115-255 | ||||||||
| U/L | <45y: 52-340 | 48-227 | <50y: 50-400 | 40-320 | 61-257 [JSCC] | - | <60y: 45-250 | |
| CK (M) | 45-65y: 55-357 | 50+y: 40-280 | 60+y: 40-200 | |||||
| 65+y: 49-207 | ||||||||
| U/L | <45y: 37-247 | 34-131 | 35-210 | 25-200 | 43-157 [JSCC] | - | 30-150 | |
| CK (F) | 45-65y: 39-230 | |||||||
| 65+y: 36-190 | ||||||||
| ALP (M) | U/L | <75y: 43-112 | 43-116 | 35-105 | 30-130 | 122-330 [JSCC] | 16-21y: 56-167 | 30-110 |
| 75+y: 42-126 | 22-79y: 50-116 | |||||||
| ALP (F) | U/L | <45y: 32-96 | <50y: 34-97 | 35-105 | 30-130 | 104-299 [JSCC] | 16-29y: 44-107 | 30-110 |
| 45-75y: 40-132 | 50+y: 47-133 | 30-79y: 46-122 | ||||||
| 75+y: 44-146 | ||||||||
| ALT (M) | U/L | <75y: 11-41 | 9-57 | 10-70 | 10-42 [JSCC] | 18-49y: 18-78 | 5-40 (no P5P) | |
| 75+y: 9-48 | 50-79y: 20-62 | |||||||
| ALT (F) | U/L | <75y: 9-35 | 7-28 | 10-45 | 7-27 [JSCC] | 12-49y: 14-41 | 5-35 (no P5P) | |
| 75+y: 8-33 | 50-79y: 16-44 | |||||||
| AST (M) | U/L | <75y: 14-36 | 13-30 | 15-45 | 14-32 [JSCC] | 18-54y: 18-54 | 5-35 (no P5P) | |
| 75+y: 14-34 | 55-79y: 18-39 | |||||||
| AST (F) | U/L | <75y: 13-31 | 11-25 | 15-35 | 12-27 [JSCC] | 20-54y: 18-34 | 5-30 (no P5P) | |
| 75+y: 14-35 | 55-79y: 18-39 | |||||||
| GGT (M) | U/L | <45y: 9-63 | 11-69 | <40y: 10-80 | 12-65 [JSCC] | 20-35y: 12-62 | 5-50 | |
| 45-75y: 13-72 | 40+y: 15-115 | 36-79y: 13-109 | ||||||
| 75+y: 15-78 | ||||||||
| GGT (F) | U/L | <45y: 9-49 | 7-33 | <40y: 10-45 | 9-38 [JSCC] | 18-35y: 12-38 | 5-35 | |
| 45-75y: 9-55 | 40+y: 10-75 | 36-79y: 10-54 | ||||||
| 75+y: 9-57 | ||||||||
| Total Protein | g/L | 62-79 | 66-82 | 62-78 | 60-80 | 66-80 | 20-29y: 65-83 | 60-80 |
| 30-79y: 65-78 | ||||||||
| Total Bilirubin (M) | µmol/L | 5-20 | 3.8-24.1 | 5-25 | <21 | 6.4-24.8 | 16-48y: 3-18 | 1-20 |
| 49-79y: 2-20 | ||||||||
| Total Bilirubin (F) | µmol/L | 5-21 | 2.7-15.9 | 5-25 | <22 | 6.4-24.8 | 16-48y: 1-16 | 1-20 |
| 49-79y: 1-17 |
* Bicarbonate measured prior to Abbott recalibration
** Calcium is adjusted for albumin
*** Creatinine has harmonised RIs for adults up to the age of 60 y.
ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; Cat: category according to Stockholm Hierarchy; CK: creatine kinase; GGT: γ-glutamyltransferase; IFCC: International Federation of Clinical Chemistry and Laboratory Medicine; JSCC: Japan Society of Clinical Chemistry; LDH: lactate dehydrogenase; P5P: pyridoxal 5’-phosphate.
Turkish Study: Similar to the Japanese study, a multi-centre study by Ozarda et al. (43) determining RIs for 25 commonly tested analytes showed similar results between the seven Turkish geographical regions in 28 laboratories where the samples were sourced. They concluded that the intervals determined by this study using the same Architect 8000 analysers were suitable for use in all Turkish clinical chemistry laboratories that used the same platforms (Table 3).
Australian Study
The Aussie Normals study was a formal reference interval study of 1876 male and female healthy adult Australians in the age group 18 to 95 years (44). Up to 91 biochemistry analytes were measured by Abbott Architect analysers. Partitioning was done according to the effects of gender, age and body mass index (BMI) on these RIs. For the most part these differences were statistically small such as for lactate dehydrogenase and phosphate where they were less than day to day biological variation. As shown in Table 3, reference intervals for the Aussie Normals formal RI study were in general similar to those for the Australasian common RIs study although somewhat tighter as they were determined using one platform only. However, γ-glutamyltransferase (GGT) upper reference limits were notably higher in the Aussie Normals study which demonstrated BMI differences with increasing age in men and women (44). For the 18-<45y age group and BMI <25 kg/m2, GGT was 12-37 U/L for men and 9-38 U/L for women. However, it is difficult to adopt RIs in association with BMI at this stage as this parameter is not routinely provided to the laboratory.
Data mining
Expert groups can provide RI information through their data mining of millions of data points from primary care patients. This method has advantages over the direct RI validation process by providing large amounts of data on the local population being tested and reflects the actual analytical and pre-analytical conditions for the tested population. This approach is valid only if there is a majority of results from the primary care population such that the healthy distribution of values can be clearly identified in the midst of a smaller number of non-healthy values. Bhattacharya analysis to determine underlying distributions in the presence of outlier results can be used to assess proposed RIs. For example, in Australasia data mining of over 200,000 paediatric data points provided by 15 laboratories for the main general chemistry analytes from birth to 18 years of age was used for establishing partitioned paediatric RIs (3).
Final selection of the common reference interval
One approach to the setting of a common RI that was used in Australasia is described as follows. The starting point to develop a common RI was to do a national survey of laboratory RIs and determine the predominant RI in use. Then a method comparison study across the major chemistry platforms using commutable samples from healthy subjects was used to assess if bias would prevent use of a common RI with acceptability based on the specified allowable limits of performance such as those based on biological variation for example (11). For analytes where bias may prevent use of a common RI for one or two main platforms, it may be possible for other platforms to share a common RI, e.g. lactate dehydrogenase methods that use pyruvate to lactate [P to L] rather than the IFCC-recommended [L to P] method cannot be combined.
The next step involves gathering supportive date for the proposed common RI using data from formal local RI studies, if available, and from data mining. In Australia values from the Aussie Normals adult RI study were used to confirm the common RIs recommended for use in Australia and New Zealand (44). Note that reference intervals are wider for the common RIs that have been established for eight platforms compared with those obtained using the one platform; inclusion of between-method variation results in wider intervals than for a singly-derived RI (Table 3). Further mining of hundreds of thousands of data points from primary care patients who are relatively healthy was then employed to show the biochemical physiology from childhood to adulthood through to geriatric age, according to age and gender (45). In order to compare partitioning according to the continuous variables of age and pregnancy, and whether merged or separate partitions will affect clinical outcomes, there must be an understanding of the physiological processes affecting an analyte. Without the knowledge of clinical outcomes and their association with partitioned RIs, the lesser approaches of clinical opinion, statistics or laboratory consensus are used to determine the suitability of partitioning (45).
Once RIs are agreed upon, the proposed reference limits should be supported by flagging rates which provide an indication of the clinical considerations of the RI. Excess flagging of results can lead to inappropriate testing due to decreased specificity of the RI. Horowitz suggests that laboratories should be mindful of excess partitioning which is due to minor changes in physiology not to pathology (46). Hence local laboratories should assess flagging rates to determine if a change to historical RIs will create higher flag rates. For example, the preanalytical effect of delayed sample transport would impact on potassium levels and hence for pragmatic reasons laboratories may choose to have a higher upper reference limit (URL) of 5.5 mmol/L rather than 5.2 mmol/L (Fig. 2A) (3).
Figure 2A.
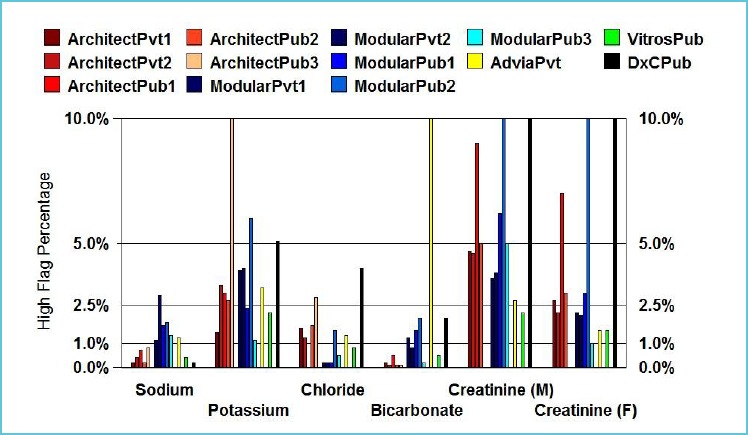
Typical high flagging rates for the first measurement in outpatient adults (18y – 60y) for sodium, potassium, chloride, bicarbonate, creatinine (M), creatinine (F)
Reproduced from Tate et al. (3) with permission from the AACB.
Final agreement by a majority of stakeholders is required to support the selected common RI and a laboratory’s intention to implement it, as described in the next section. The consensus process for deriving common RIs is not perfect and there are limitations. As noted above, intervals are usually wider than for singly-derived RIs obtained on the same platform, pre-analytical issues can cause elevated flagging rates, and elevated BMI in the population is not factored into clinical interpretation by the routine laboratory of GGT for example. Traceable analytes with JCTLM-listed reference materials and reference measurement procedures are more likely to share common RIs. However, countries may not be using IFCC recommended methods for enzymes as is the case in Australia where non-pyridoxal-5’-phosphate (P5P) AST and ALT methods are predominantly in use (Table 3). A harmonised RI with non-P5P methods is better than no harmonised RI and a future goal is for Australian laboratories to use P5P methods for AST and ALT.
FINAL ACCEPTANCE, ADOPTION AND IMPLEMENTATION OF HARMONISED REFERENCE INTERVALS
Communication and discussion by all stakeholders
Laboratory acceptance should be sought at a national level prior to introduction of common RIs. Various approaches can be used to assess the likely adoption rates for the panel of RIs including a survey as to whether the laboratory is using the common RI already, would accept the RI, or ask for comments and their reason if they do not accept the common RI. Representation is required from the whole nation and from public and private pathology, small and large laboratories and networks if harmonised RIs are to have any chance of being implemented. National acceptance of a change to pathology RIs requires that there is an on-going discussion by all involved stakeholders especially those at the highest management level who are responsible for patient pathology results and their interpretation. Harmonisation workshops provide a forum for presenting and discussing the evidence and reaching a consensus decision.
Validation of reference intervals by local laboratories
Responsibility for adoption of common RIs lies with each laboratory. Advice on how to do this is found in guidelines from the Clinical and Laboratory Standards Institute (CLSI) (47). Key questions are: ‘Is this RI suitable for my method and for my population?’ Validations of RIs may be by subjective assessment assuming the same method and the same population are used or by a simple validation using 20 normal subjects representing the local population (47,48). Alternatively, you can mine your laboratory’s existing data. The most useful parameter is the midpoint of the extracted data, which can be used to assess analytical or population bias by comparison with the corresponding midpoint of the data used to set the reference interval. Bhattacharya analysis can also be used to assess the proposed intervals (12).
Validation of flagging rates for local population
Based on the principle of minimum, desirable and optimal categories used to define allowable bias limits, flag rates may range from 1.0% to 1.8% for low flagging rates, and 5.7% to 3.3% for high flagging rates, respectively. Flag rates however, may be quite complex to interpret depending on the population used to derive them. For example in the Australasian common RIs project, a URL of 110 U/L for alkaline phosphatase may result in a flag rate of 7-8% (3). However, the clinical benefit of using the URL of 110 U/L is to detect pathology in postmenopausal women. Increasing the URL to 115 U/L did not have any significant impact due to the logarithmic distribution of reference values. In contrast, the flag rate at the URL for sodium was 1% indicating that hypernatraemia is uncommon (Fig. 2A and 2B). Data mining of local population values also allows for an assessment of the expected number of results outside the RI (12). The laboratory can then compare the expected flagging rates with their current rates.
Figure 2B.
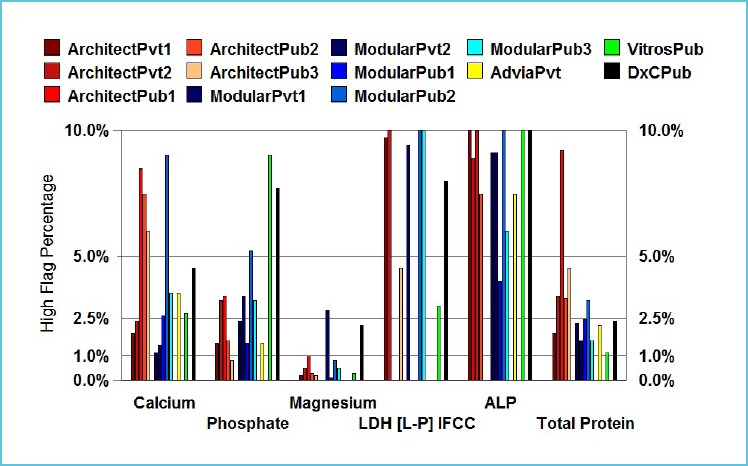
Typical high flagging rates for the first measurement in outpatient adults (18y – 60y) for calcium, phosphate, magnesium, lactate dehydrogenase, alkaline phosphatase, total protein
Reproduced from Tate et al. (3) with permission from the AACB.
CLOSING THE HARMONISED REFERENCE INTERVAL LOOP
Following the endorsement of common RIs by pathologists and scientists, formal endorsement by the profession is sought from the National Pathology College and National Clinical Chemistry, Biochemistry or Laboratory Medicine Society. Support by the National Testing Authorities for Laboratory Medicine, who should be included in meetings on harmonisation, is via formal recommendations to laboratories that they use these intervals, or if not, to provide supporting evidence for other references.
Continuing work is required to produce and validate common RIs, to manage ongoing issues, e.g. problems with implementation of RIs by the local laboratory Information Technology unit into the Laboratory Information System. These issues may be changes to reporting units, significant figures, rounding, report formatting, etc. Consultation with clinical societies and education of local clinicians are imperative if the new RIs are to be used. Other flow-on effects can be those regarding the reimbursement of pharmaceutical benefits according to national government benefit schemes that use specific RIs or decision limit values when assessing the provision of a treatment drug.
The level of uptake of common RIs can be readily surveyed through EQA programs. One such scheme that also surveys the bias of methods within the reference interval measuring range by using commutable samples from healthy subjects is the RCPA Quality Assurance Program Liquid Serum Chemistry program (15). The scheme allows assessment of the between-laboratory variation in results, RIs and the information transmitted by the combination of these factors. For most common chemistry analytes, use of common RIs has improved the variation seen in the information produced by different laboratories.
CONCLUSION
Consideration should be given by laboratories to adopting RIs consistent with those used by other laboratories in the region where it is possible and appropriate for the local population. These may be common RIs for use across several major platforms in the region, e.g. United Kingdom, Nordic countries, Japan, Australasia, or for use with one specific platform, e.g. Canada, Asia, Turkey. Scientific evidence supports the use of common RIs for many general chemistry analytes especially those with sound calibration and traceability in place. For other non-harmonised immunoassay analytes where either there is currently no secondary reference material or reference measurement procedure for value assignment, it seems logical to use platform-specific RIs and decision limits across regions, provided that laboratories have acceptable assay precision, until such time when methods become harmonised internationally.
REFERENCES
- 1.Zardo L, Secchiero S, Sciacovelli L, Bonvinci P, Plebani M. Reference intervals: are interlaboratory differences appropriate? Clin Chem Lab Med 1999;37:1131-1133. [DOI] [PubMed] [Google Scholar]
- 2.Jones GR, Barker A, Tate J, Lim C-F, Robertson K. The case for common reference intervals. Clin Biochem Rev 2004;25:99-104. [PMC free article] [PubMed] [Google Scholar]
- 3.Tate JR Sikaris KA Jones GRD Yen T Koerbin G Ryan J et al.on behalf of the AACB Committee for Common Reference Intervals . Harmonising adult and paediatric reference intervals in Australia and New Zealand: an evidence-based approach for establishing a first panel of chemistry analytes. Clin Biochem Rev 2014;35:213-235. [PMC free article] [PubMed] [Google Scholar]
- 4.Australian Pathology Units AND Terminology (APUTS) Standards and Guidelines (v2.2). http://www.rcpa.edu.au/Library/Practising-Pathology/PTIS/APUTS-Downloads (Accessed 5 November, 2015).
- 5.Berg J, Lane V. Pathology Harmony; a pragmatic and scientific approach to unfounded variation in the clinical laboratory. Ann Clin Biochem 2011;48:195-197. [DOI] [PubMed] [Google Scholar]
- 6.Berg J. The UK Pathology Harmony initiative; the foundation of a global model. Clin Chim Acta 2014;432:22-26. [DOI] [PubMed] [Google Scholar]
- 7.Koerbin G, Sikaris KA, Jones GRD, Ryan J, Reed M, Tate J, On behalf of the AACB Committee for Common Reference Intervals Evidence-based approach to harmonised reference intervals. Clin Chim Acta. 2014, 432:99-107. [DOI] [PubMed] [Google Scholar]
- 8.Jones GD, Barker T. Reference intervals. Clin Biochem Rev. 2008;29 Suppl:S93-S97. [PMC free article] [PubMed] [Google Scholar]
- 9.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Chapter 1. Kidney Int Suppl 2013;3:19-62. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf (Accessed on 5 November, 2015). [DOI] [PubMed] [Google Scholar]
- 10.Jones GR, Sikaris K, Gill J. Allowable Limits of Performance for External Quality Assurance Programs - an approach to application of the Stockholm criteria by the RCPA Quality Assurance Programs. Clin Biochem Rev 2012;33:133-139. [PMC free article] [PubMed] [Google Scholar]
- 11.Koerbin G, Tate JR, Ryan J, Jones GRD, Sikaris KA, Kanowski D, et al. Bias assessment of general chemistry analytes using commutable samples. Clin Biochem Rev 2014;35:203-211. [PMC free article] [PubMed] [Google Scholar]
- 12.Jones GRD. Validating common reference intervals in routine laboratories. Clin Chim Acta 2014;432:119-121. [DOI] [PubMed] [Google Scholar]
- 13.Panteghini M, Ceriotti F. Obtaining reference intervals traceable to reference measurement systems: is it possible, who is responsible, what is the strategy? Clin Chem Lab Med 2012;50:813-817. [DOI] [PubMed] [Google Scholar]
- 14.JCTLM: Joint Committee for Traceability in Laboratory Medicine. Available at: http://www.bipm.org/jctlm (Accessed on 5 November, 2015).
- 15.Jones GRD, Koetsier SDA. RCPAQAP first combined measurement and reference interval survey. Clin Biochem Rev 2014;35:243-250. [PMC free article] [PubMed] [Google Scholar]
- 16.Rustad P, Felding P, Franzson L, Kairisto V, Lahti A, Mårtensson A, et al. The Nordic Reference Interval Project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest 2004;64:271-284. [DOI] [PubMed] [Google Scholar]
- 17.Hilsted L, Rustad P, Aksglaede L, Sorensen K, Juul A. Recommended Nordic paediatric reference intervals for 21 common biochemical properties. Scand J Clin Lab Invest 2013;73:1-9. [DOI] [PubMed] [Google Scholar]
- 18.Adeli K. Closing the gaps in pediatric reference intervals: The CALIPER initiative. Clin Biochem 2011;44:480-482. [DOI] [PubMed] [Google Scholar]
- 19.Mansoub S, Chan MK, Adeli K. Gap analysis of pediatric reference intervals for risk biomarkers of cardiovascular disease and the metabolic syndrome. Clin Biochem 2006;39:569-587. [DOI] [PubMed] [Google Scholar]
- 20.Davis GK, Bamforth F, Sarpal A, Dicke F, Rabi Y, Lyon ME. B-type natriuretic peptide in pediatrics. Clin Biochem 2006;39:600-605. [DOI] [PubMed] [Google Scholar]
- 21.Delvin EE, Laxmi G, V, Vergee Z. Gap analysis of pediatric reference intervals related to thyroid hormones and the growth hormone-insulin growth factor axis. Clin Biochem 2006;39:588-594. [DOI] [PubMed] [Google Scholar]
- 22.Lepage N, Li D, Kavsak PA, Bamforth F, Callahan J, Dooley K, Potter M. Incomplete pediatric reference intervals for the management of patients with inborn errors of metabolism. Clin Biochem 2006;39:595-599. [DOI] [PubMed] [Google Scholar]
- 23.Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012;58:854-868. [DOI] [PubMed] [Google Scholar]
- 24.Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D, Adeli K. Marked biological variance in endocrine and biochemical markers in childhood: Establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem 2013;59:1393-1405. [DOI] [PubMed] [Google Scholar]
- 25.Konforte D, Shea JL, Kyriakopoulou L, Colantonio D, Cohen AH, Shaw J, et al. Complex biological pattern of fertility hormones in children and adolescents: A study of healthy children from the CALIPER cohort and establishment of pediatric reference intervals. Clin Chem 2013;59:1215-1227. [DOI] [PubMed] [Google Scholar]
- 26.Bevilacqua V, Chan MK, Chen Y, Armbruster D, Schodin B, Adeli K. Pediatric population reference value distributions for cancer biomarkers and covariate-stratified reference intervals in the CALIPER cohort Clin Chem 2014;60:1532-1542. [DOI] [PubMed] [Google Scholar]
- 27.Raizman JE, Cohen AH, Teodoro-Morrison T, Wan B, Khun-Chen M, Wilkenson C, et al. Pediatric reference value distributions for vitamins A and E in the CALIPER cohort and establishment of age-stratified reference intervals. Clin Biochem 2014;47:812-815. [DOI] [PubMed] [Google Scholar]
- 28.Bailey D, Bevilacqua V, Colantonio DA, Pasic MD, Perumal N, Chan MK, Adeli K. Pediatric within-day biological variation and quality specifications for 38 biochemical markers in the CALIPER cohort. Clin Chem 2013;60:518-529. [DOI] [PubMed] [Google Scholar]
- 29.Shaw JL, Cohen A, Konforte D, Binesh-Marvasti T, Colantonio DA, Adeli K. Validity of establishing pediatric reference intervals based on hospital patient data: A comparison of the modified Hoffmann approach to CALIPER reference intervals obtained in healthy children. Clin Biochem 2014;47:166-172. [DOI] [PubMed] [Google Scholar]
- 30.Kyriakopoulou L, Yazdanpanah M, Colantonio DA, Chan MK, Daly CH, Adeli K. A sensitive and rapid mass spectrometric method for the simultaneous measurement of eight steroid hormones and CALIPER pediatric reference intervals. Clin Biochem 2013;46:642-651. [DOI] [PubMed] [Google Scholar]
- 31.Teodoro-Morrison T, Kyriakopoulou L, Chen YK, Raizman JE, Bevilacqua V, Chan MK, Wan B, Yazdanpanah M, Schulze A, Adeli K. Dynamic biological changes in metabolic disease biomarkers in childhood and adolescence: A CALIPER study of healthy community children. Clin Biochem 2015;48:828-836. [DOI] [PubMed] [Google Scholar]
- 32.Raizman JE, Quinn F, Armbruster DA, Adeli K. Pediatric reference intervals for calculated free testosterone, bioavailable testosterone and free androgen index in the CALIPER cohort. Clin Chem Lab Med. 2015;53:e239-e243. [DOI] [PubMed] [Google Scholar]
- 33.Devgun MS, Chan MK, El-Nujumi AM, Abara R, Armbruster D, Adeli K. Clinical decision limits for interpretation of direct bilirubin - A CALIPER study of healthy multiethnic children and case report reviews. Clin Biochem;48:93-96. [DOI] [PubMed] [Google Scholar]
- 34.Estey MP, Cohen AH, Colantonio DA, Chan MK, Marvasti TB, Randell E, et al. CLSI-based transference of the CALIPER database of pediatric reference intervals from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem 2013;46:1197-1219. [DOI] [PubMed] [Google Scholar]
- 35.Araújo PA, Thomas D, Sadeghieh T, Bevilacqua V, Chan MK, Chen Y, et al. CLSI based transference of the CALIPER database of pediatric reference intervals to Beckman Coulter DxC biochemical assays. Clin Biochem 2015;48:870-880. [DOI] [PubMed] [Google Scholar]
- 36.Abou El Hassan M, Stoianov A, Araújo PA, Sadeghieh T, Chan MK, Chen Y, et al. CLSI-based transference of CALIPER pediatric reference intervals to Beckman Coulter AU biochemical assays. Clin Biochem 2015. May 13. pii: S0009-9120(15)00170-8. [DOI] [PubMed] [Google Scholar]
- 37.Higgins V, Chan MK, Nieuwesteeg M, Hoffman BR, Bromberg IL, Gornall D, et al. Transference of CALIPER pediatric reference intervals to biochemical assays on the Roche cobas 6000 and the Roche Modular P. Clin Biochem 2015. Aug 19. pii: S0009-9120(15)00398-7. [DOI] [PubMed] [Google Scholar]
- 38.Adeli K, Higgins V, Nieuwesteeg M, Raizman JE, Chen Y, Wong SL, Blais D. Biochemical marker reference values across pediatric, adult, and geriatric ages: Establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem 2015;61:1049-1062. [DOI] [PubMed] [Google Scholar]
- 39.Adeli K, Higgins V, Nieuwesteeg M, Raizman JE, Chen Y, Wong SL, Blais D. Complex reference values for endocrine and special chemistry biomarkers across pediatric, adult, and geriatric ages: Establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem 2015;61:1063-1074. [DOI] [PubMed] [Google Scholar]
- 40.Adeli K, Raizman JE, Chen Y, Higgins V, Nieuwesteeg M, Abdelhaleem M, et al. Complex Biological profile of hematologic markers across pediatric, adult, and geriatric Ages: Establishment of robust pediatric and adult reference intervals on the asis of the Canadian Health Measures Survey. Clin Chem 2015;61:1075-1086. [DOI] [PubMed] [Google Scholar]
- 41.Ichihara K, Itoh Y, Lam CWK, Poon PMK, Kim J-H, Kyono H, et al. : Science Committee for the Asian-Pacific Federation of Clinical Biochemistry. Sources of variation of commonly measured serum analytes in 6 Asian cities and consideration of common reference intervals. Clin Chem 2008;54:356-365. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto Y, Hosogaya S, Osawa S, Ichihara K, Onuma T, Saito A, et al. Nationwide multicenter study aimed at the establishment of common reference intervals for standardized clinical laboratory tests in Japan. Clin Chem LabMed 2015;51:1663-1672. [DOI] [PubMed] [Google Scholar]
- 43.Ozarda Y, Ichihara K, Aslan D, Aybek H, Ari Z, Taneli F, et al. A multicenter nationwide reference intervals study for common biochemical analytes in Turkey using Abbott analyzers. Clin Chem Lab Med 2014;52:1823-1833. [DOI] [PubMed] [Google Scholar]
- 44.Koerbin G, Cavanaugh JA, Potter JM, Abhayaratna WP, West NP, Glasgow N, et al. ‘Aussie normals’: an a priori study to develop clinical chemistry reference intervals in a healthy Australian population. Pathology 2015;47:138-144. [DOI] [PubMed] [Google Scholar]
- 45.Sikaris KA. Physiology and its importance for reference intervals. Clin Biochem Rev 2014;35:3-14. [PMC free article] [PubMed] [Google Scholar]
- 46.Horowitz GL. The power of asterisks [Editorial]. Clin Chem 2015;61:1009-1011. [DOI] [PubMed] [Google Scholar]
- 47.CLSI. Defining, establishing and verifying reference intervals in the clinical laboratory; approved guideline-third edition. Wayne (PA): CLSI; 2008. CLSI document C28-A3. [Google Scholar]
- 48.Tate Jr, Yen T, Jones GRD. Transference and validation of reference intervals [Editorial]. Clin Chem 2015;61:1012-1015. [DOI] [PubMed] [Google Scholar]


