Abstract
While the frequency of laboratory errors varies greatly, depending on the study design and steps of the total testing process (TTP) investigated, a series of papers published in the last two decades drew the attention of laboratory professionals to the pre- and post-analytical phases, which currently appear to be more vulnerable to errors than the analytical phase. In particular, a high frequency of errors and risk of errors that could harm patients has been described in both the pre-pre- and post-post-analytical steps of the cycle that usually are not under the laboratory control. In 2008, the release of a Technical Specification (ISO/TS 22367) by the International Organization for Standardization played a key role in collecting the evidence and changing the perspective on laboratory errors, emphasizing the need for a patient-centred approach to errors in laboratory testing.
Key words: quality, patient safety, laboratory medicine, diagnostic errors, brain-to-brain loop, laboratory-associated errors
A further step in the journey towards improved understanding of the issue is the recent demonstration that errors in laboratory medicine are part of a much wider issue, commonly known as “diagnostic error”, thus definitively linking laboratory-associated errors to patient safety problems. The current awareness of the nature of laboratory testing-associated errors, in particular the link between appropriate test ordering and result interpretation/utilization, and their potential in reducing diagnostic errors, should herald a change in the old paradigm which was focused only on errors detected within the laboratory walls. Evidence-based quality indicators represent a formidable tool for improving quality and decreasing the risk of errors in the total testing process.
INTRODUCTION
During the past decade, after the publication of the Institute of Medicine (IOM) report, To Err Is Human (1), patient safety has finally become the object of medical and public attention. Compared with other types of medical error, however, errors in laboratory medicine have received little attention. The reasons for this neglect are complex, but the difficulties largely arise from the number of steps and the time lapse which separate laboratory testing, physicians’ actions and patient outcomes (2). Moreover, usually only the analytical phase falls under laboratory control, while the pre- and post-analytic phases are the responsibility of stakeholders other than the laboratory such as the clinician, the nurse, the patient and others involved in patient identification, data entry, specimen collection and transport. In addition, most of the many different terms used in the literature to define errors in laboratory medicine (e.g. mistakes, blunders, defects, outliers, unacceptable results, quality failure) have negative connotations involving blame, individual failure and culpability and, even worse, pertain to studies focusing on a limited number of total testing process (TTP) steps. Taken together these are the “reasons for neglect” for errors in laboratory medicine, and should explain why the patient-centred viewpoint has been taken into account only in recent years (3).
A brief history of errors in laboratory medicine
Initial studies, starting from the seminal paper by Belk and Sunderman in 1947 (4), as well as other articles published before the 1990s, focused only on the analytical phase and demonstrated high rates and severity of analytical errors. However, despite the limited study design, they provided a wide range of opportunities to improve analytical performance, including the development of external quality assurance programs (EQA) and improved rules for internal quality control (IQC).
In the late nineties, a body of evidence was accumulated which documented: a) a dramatic decrease in the analytical error rates from 162,116 errors per million laboratory tests (parts per million, ppm) to 447 ppm (5, 6); b) high rates of errors in the pre- and post-analytical steps (7-9); and c) the risk of adverse events and inappropriate care due to laboratory errors, mainly for errors in pre-pre-analytical steps (10, 11).
In fact, over the past decades, a ten-fold reduction in the analytical error rate has been achieved thanks to improvements in the reliability and standardization of analytical techniques, reagents, and instrumentation. In addition, advances in information technology, quality control and quality assurance methods have made a valuable contribution to error reduction. However, although the state-of-the-art highlights that pre- and post-analytical phases are more vulnerable to errors, there is still evidence indicating that analytical quality remains a major issue. In particular, a relatively high frequency of analytical errors has been documented for immunoassays with associated adverse clinical outcomes, sometimes resulting in grossly erroneous results (2). The issue of analytical interference does not only affect immunoassays. As an example, monoclonal proteins may affect many laboratory measurements, including glucose, bilirubin, C-reactive protein, creatinine and albumin. The frequency of this type of error is variable and probably underreported (12). The lack of inter-changeability between different methods and clinical laboratories, although not considered an “analytical error” in the strict sense, may also confound both clinical reasoning and patient management. This, in turn, is the main driver for the increasing awareness and concern regarding the need of standardization and harmonization projects in laboratory medicine (13).
Pre- and post-analytical phases
While the frequency of laboratory errors varies greatly, depending on the study design and the specific steps of the total testing process (TTP) investigated, a series of papers published between 1989 and 2007 drew the attention of laboratory professionals to the pre-, and post-analytical phases, which currently appear to be more vulnerable to errors than the analytical phase. In particular, two papers published in 1997 and 2007 (7, 8) used a study design that allowed us to investigate most TTP steps in the same clinical context (stat laboratory). In both studies, the pre-analytic phase had the highest error rate, the most frequent problems arising from mistakes in tube filling, inappropriate specimen containers, and requesting procedures. Identification errors were noted too, although the appropriateness of test request was not considered in the study design. Further studies confirmed these data and, currently, pre-analytical errors or more accurately pre-pre-analytical errors are estimated to account for up to 70% of all mistakes made in laboratory diagnostics, most of which arise from problems in patient preparation, and sample collection, transportation, preparation for analysis and storage (9-11), as shown in Figure 1.
Figure 1.
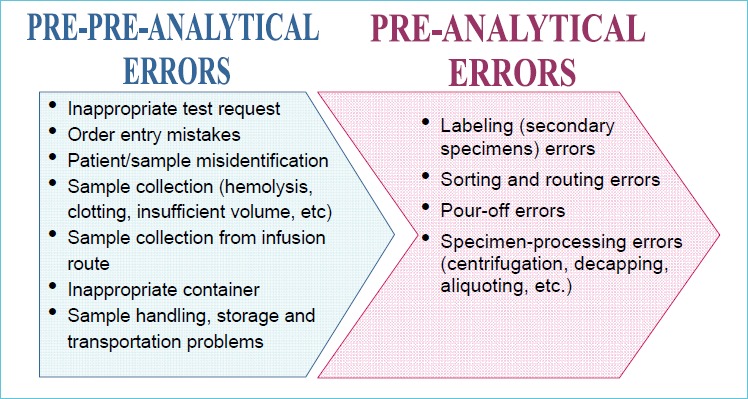
Most frequent sources of errors in the pre-pre- and pre-analytical steps (accounting for 48%-62% of total errors in laboratory medicine)
Laboratory errors and risk management
From a risk management viewpoint, the great majority of laboratory errors have little direct impact on patient care but provide important learning opportunities. In fact, any error, regardless of its apparently trivial nature, might indicate weaknesses in policies and procedures that may not lead to adverse events in their particular context, but might cause the patient harm in slightly different circumstances (14). The lesson we learnt is that the entire system (TTP) should be designed to consider not only the real patient harm sustained, but also the potential worst clinical outcome if such an error were to recur.
In 2008, the release of a Technical Specification (ISO/TS 22367) by the International Organization for Standardization played a key role in collecting the evidence and changing the perspective on laboratory errors, defining laboratory error as “failure of planned action to be completed as intended, or use of a wrong plan to achieve an aim, occurring at any part of the laboratory cycle, from ordering examinations to reporting results and appropriately interpreting and reacting to them” (15). In addition, according to this Technical Specification (15), any clinical laboratory should employ processes for: a) identifying high risk processes where the potential error could lead to a safety risk for patients; b) detecting actual incidents associated with deviations from standard requirements; c) estimating and evaluating the associated risks to patient safety; d) controlling the risks; and e) monitoring the effectiveness of the measure taken.
This inspired a patient-centred evaluation of errors in laboratory testing and an increased concern to identify weaknesses and vulnerability in procedures and processes, so that corrective and preventive actions can be activated before any adverse event or patient harm may occur.
A further step in the journey towards a better understanding of the issue is the recent proof that errors in laboratory medicine are part of a much wider issue, commonly known as “diagnostic error”, thus definitively linking laboratory-associated errors to patient safety problems, as shown in Table 1.
Table 1.
The journey towards a patient-centered view of errors in laboratory medicine
| Years | |
|---|---|
| 1950-1990 | ANALYTICAL ERRORS ↓ |
| 1990s | ERRORS IN CLINICAL LABORATORIES (including pre- and post-analytical phases) ↓ |
| 2000s | ERRORS IN THE TOTAL TESTING PROCESS (including pre-pre- and post-post-analytical phases) |
| AND | |
| Today | TESTING-RELATED DIAGNOSTIC ERRORS |
Diagnostic errors and laboratory testing
Diagnostic errors have been defined as “errors in which diagnosis was unintentionally delayed (while sufficient information was available earlier), wrong (another diagnosis made before the correct one), or missed (no diagnosis made) as judged from the eventual appreciation of more definitive information (e.g., autopsy studies)” (16). The evidence on the importance of and direct link between diagnostic errors and errors in laboratory medicine derives from a series of studies with a clinical starting point. In particular, studies performed on the pre-pre-analytical phase (initial procedures performed outside clinical laboratory or, at least in part, beyond the control of laboratory personnel) confirm that failure to order appropriate diagnostic tests (laboratory tests included) makes up 55% of observed breakdowns in missed and delayed diagnosis in the ambulatory setting (17-19) and 58% of errors in emergency departments (20).
Incorrect interpretation of diagnostic or laboratory tests in the end stages of the TTP loop was found to underlie a large percentage of errors in the ambulatory setting and in emergency departments. Failure to inform patients of clinically significant abnormal test results or to record the delivery of relevant information is relatively common, occurring in 1 out of every 14 tests; for example, patients not being informed of a total cholesterol value of 8.2 mmol/L (318 mg/dL), hematocrit of 28.6% or a potassium level of 2.6 mmol/L. The overall rate of failure to inform the patient or to record communication of information was 7.1%, in different practices, ranging from 0 to 26% (21). As revealed in a systematic review of the literature, failure to follow-up test results markedly compromises patient safety, yet the rate of abnormal laboratory results (for INR and PSA) without follow-up ranges from 6.8% to 62%. (22). Further evidence of inappropriate response to laboratory information is provided in a study evaluating the prescription of potassium in cases of hyperkalemia (23). Moreover, findings in another study (24) showed that over 2% (2.6% in 2000, 2.1% in 2007) of patients with thyrotropin (TSH) levels exceeding 20 mU/L were not followed up. Yet another study revealed that of 1,095 discharged patients, almost half had pending laboratory and radiology test results, 9% of which potentially required action (25). In another study, approximately one-third of sub-acute care patients had laboratory tests (microbiology tests in particular), which were pending at discharge, but few of these cases were recorded in hospital discharge forms (26). Overall, data reported demonstrate that the initial and final steps of the TTP process, above all test requesting and reaction to laboratory results, are not only more error-prone than all the other steps, but are also the most important causes of potential adverse outcomes for patients. Moreover, the data confirm that a significant number of failures occur in the interface between clinical practice and laboratories, thus emphasizing the need for laboratory professionals and physicians to “understand their mutual ownership and work together to ensure that patients are more safe” (27).
Towards a patient-centred approach to laboratory-associated errors
The awareness of the current nature of laboratory testing-associated errors, in particular the link between appropriateness in test ordering and result interpretation/utilization, and their potential in addressing diagnostic errors, should herald a change in the old paradigm which was focused only on errors detected within the laboratory walls. In order to translate the concept of “patient-centred care” from theory to practice it is of the utmost importance to investigate, and improve upon, not only those procedures and processes performed under the direct control of the clinical laboratory, but also the initial and final steps of the testing cycle that are usually managed by other healthcare personnel. Projects aiming to improve quality and patient safety must therefore be based upon a total quality perspective, in particular the accreditation of clinical laboratory services according to the International Standard ISO 15189:2012(28) and the search for valuable quality indicators (Qls) for all phases of the testing process. In particular, the identification and implementation of valuable Qls are requested as mandatory for clinical laboratory accreditation according to the International Standard (ISO 15189:2012). In this document quality indicators are defined as “ a measure of the degree to which a set of inherent characteristics fulfils requirements” and “can measure how well an organization meets the needs and requirements of users and the quality of all operational processes” (28). The second definition is emphasised in the context of the present paper, and specifically the fact that “all operational processes” requires the inclusion of pre- and post-analytical steps. However, a major problem is the lack of consensually defined Qls, particularly for extra-analytical phases. The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) launched in 2004 a new project, implementing a Working Group on Laboratory Errors and Patient Safety (WG LEPS) that promoted and developed a model of quality indicators (MQI) (29, 30). This model is divided into process and outcome measures, mainly based on measures of the pre-, intra-and post-analytical procedures and processes, and has been revised in a Consensus Conference organized to establish a list of Qls that should be evidence-based, feasible for most laboratories around the world and actionable (31). The list of Qls is available on line at www.ifcc-mqi.com
CONCLUSIONS
According to recent data from malpractice claims, diagnostic errors appear to be the most common, most costly and most dangerous of medical mistakes both in inpatients and outpatients (32, 33). Failure in the ordering of appropriate laboratory test and the application of laboratory test results are major contributors to diagnostic errors, along with residual problems in test performances (analytical errors) (34). Therefore, the main message is the need to improve the quality of laboratory services, avoiding errors and improving patient safety, employing a global approach across the TTP, according to the seminal concept of the brain-to-brain loop (35). The use of a consensually-defined list of evidence-based Qls to be applied in the accreditation programs of clinical laboratories according to the current International Standard (ISO 15189:2012) is an effective tool for improving quality, decreasing the risk of errors and increasing patient safety.
REFERENCES
- 1.Institute of Medicine (US) Committee on Quality of Health Care in America Kohn LT, Corrigan JM, Donaldson MS, editors. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US); 2000. [PubMed] [Google Scholar]
- 2.Plebani M. The CCLM contribution to improvements in quality and patient safety. Clin Chem Lab Med 2013;51:39-46. [DOI] [PubMed] [Google Scholar]
- 3.Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 2010;47:101-110. [DOI] [PubMed] [Google Scholar]
- 4.Belk WP, Sunderman FW. A survey of the accuracy of chemical analyses in clinical laboratories. Am J Clin Pathol 1947;17:853-861. [DOI] [PubMed] [Google Scholar]
- 5.Steindel SJ, Howanitz PJ, Renner SW. Reasons for proficiency testing failures in clinical chemistry and blood gas analysis: a College of American Pathologists Q-Probes study in 665 laboratories. Arch Pathol Lab Med 1996;120:1094-1101. [PubMed] [Google Scholar]
- 6.Witte VanNess SA, Angstadt DS, Pennell BJ. Errors, mistakes, blunders, outliers, or unacceptable results: how many? Clin Chem 1997;43:1352-1356. [PubMed] [Google Scholar]
- 7.Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997;43: 1348-1351. [PubMed] [Google Scholar]
- 8.Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem 2007;53:1338-1342. [DOI] [PubMed] [Google Scholar]
- 9.Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chim Acta 2009;404: 16-23. [DOI] [PubMed] [Google Scholar]
- 10.Howanitz PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med 2005;129:1252-1261. [DOI] [PubMed] [Google Scholar]
- 11.Carraro P, Zago T, Plebani M. Exploring the initial steps of the testing process: frequency and nature of pre-ana-lytic errors. Clin Chem 2012;58:638-642. [DOI] [PubMed] [Google Scholar]
- 12.Dalai Bl, Bridgen ML. Factitious biochemical measurements resulting from hematologic conditions. Am J Clin Pathol 2009;131:195–204. [DOI] [PubMed] [Google Scholar]
- 13.Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med 2013;5:7411-7451. [DOI] [PubMed] [Google Scholar]
- 14.O’Kane M. The reporting, classification and grading of quality failures in the medical laboratory. Clin Chim Acta 2009; 404:28-31. [DOI] [PubMed] [Google Scholar]
- 15.ISO/TS 22367:2008. Medical laboratories - reduction of error through risk management and continual improvement. Geneva, Switzerland: International Organization for Standardization, 2008. [Google Scholar]
- 16.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005,165:1493-1499. [DOI] [PubMed] [Google Scholar]
- 17.Hickner J, Graham DG, Elder NC, Brandt E, Emsermann CB, Dovey S, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194-200. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi TK, Kachalia A, Thomas EJ, Puopolo AL, Yoon C, Brennan TA, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med 2006;145:488-496. [DOI] [PubMed] [Google Scholar]
- 19.Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kachalia A, Gandhi TK, Pupolo AL, Yoon C, Thomas EJ, Griffey R, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med 2007;49:196-205. [DOI] [PubMed] [Google Scholar]
- 21.Casalino LP, Dunham D, Chin MH, Bielang R, Kistner EO, Karrison TG, et al. Frequency of failure to inform patient of clinically significant outpatient test results. Arch Int Med 2009;169:1123-1129. [DOI] [PubMed] [Google Scholar]
- 22.Callen JL, Westbrook JI, Georgiou A, Li J. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiff GD, Aggarwal HC, Kumar S, McNutt RA. Prescribing potassium despite hyperkalemia: medication errors uncovered by linking laboratory and pharmacy information systems. Am J Med 2001;109:494-497. [DOI] [PubMed] [Google Scholar]
- 24.Schiff GD, Kim S, Krosnjar N, Wisniewski MF, Bult J, Fogelfeld L, et al. Missed hypothyroidism diagnosis uncovered by linking laboratory and pharmacy data. Arch Intern Med 2005;165(5):574-577. [DOI] [PubMed] [Google Scholar]
- 25.Roy CL, Poon EG, Karson AS, Ladak-Merchant Z, Johnson RE, Maviglia SM, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005;143:121-128. [DOI] [PubMed] [Google Scholar]
- 26.Walz SE, Smith M, Cox E, Sattin J, Kind AJ. Pending laboratory tests and the hospital discharge summary in patients discharged to sub-acute care. J Gen Intern Med 2011;26:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graber ML. The physician and the laboratory. Partners in reducing diagnostic error related to laboratory testing. Am J Clin Pathol 2005;124 (Suppl 1):S1-S4. [Google Scholar]
- 28.ISO 15189:2012. Medical laboratories- Requirements for quality and competence. Geneva, Switzerland: International Organization for Standardization, 2012. [Google Scholar]
- 29.Sciacovelli L, O’Kane M, Skaik YA, Caciagli P, Pellegrini C, Da Rin G, et al. IFCC WG-LEPS. Quality Indicators in Laboratory Medicine: from theory to practice. Preliminary data from the IFCC Working Group Project “Laboratory Errors and Patient Safety”. Clin Chem Lab Med 2011;49:835-844. [DOI] [PubMed] [Google Scholar]
- 30.Plebani M, Chiozza ML, Sciacovelli L. Towards harmonization of quality indicators in laboratory medicine. Clin Chem Lab Med 2013;51:187-195. [DOI] [PubMed] [Google Scholar]
- 31.Plebani M, Astion ML, Barth JH, Chen W, de Oliveira Galoro CA, Escuer Ml, et al. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med 2014;52: 951-958. [DOI] [PubMed] [Google Scholar]
- 32.Saber Tehrani AS, Lee H, Mathews SC, Shore A, Makary MA, Pronovost PJ, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf 2013;22:672-680. [DOI] [PubMed] [Google Scholar]
- 33.Singh H, Giardina TD, Meyer AN, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med 2013;173:418-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ Qual Saf 2013;22:ii6-ii10 doi: 10.1136/bmjqs-2012-001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plebani M, Laposata M, Lundberg GD. The brain-to-brain loop concept for laboratory testing 40 years after its introduction. Am J Clin Pathol 2011;136:829-833. [DOI] [PubMed] [Google Scholar]


