Abstract
Laboratory medicine is the single highest volume medical activity in healthcare and demand for laboratory testing is increasing disproportionately to medical activity. It has been estimated that $6.8 billion of medical care in the US involves unnecessary testing and procedures that do not improve patient care and may even harm the patient. Physicians face many challenges in accurately, efficiently and safely ordering and interpreting diagnostic tests. In order to improve patient outcomes, laboratory tests must be appropriately ordered, properly conducted, reported in a timely manner, correctly interpreted and affect a decision for future diagnosis and treatment of the patient.
Key words: test utilization, test ordering, physician education, computerized physician order entry, guidelines, clinical outcomes
This paper discusses factors influencing test ordering by physicians, strategies for modifying physicians’ ordering patterns, and ways to implement policies to improve laboratory utilization and thereby improve patient outcome.
Successful management of laboratory test utilization requires the entire laboratory team to use their skills and knowledge to identify utilization issues, implement a programme that will achieve more effective testing and establish appropriate processes from the beginning to the end of the test cycle.
Laboratory medicine is the single highest volume medical activity in healthcare and demand for laboratory testing is increasing disproportionately to medical activity. Over the past 20 years, the number of laboratory tests available to clinicians has more than doubled, to at least 3,500 tests (1). The global IVD market, valued at $49 billion in 2012, is expected to grow by 7% over the period 2012-2017, and represents 3-5% of all healthcare costs (2).
A major component of US healthcare expenditure is an estimated $65 billion spent each year to perform more than 4.3 billion laboratory tests (3) but it has been estimated that $6.8 billion of medical care in the US involves unnecessary testing and procedures that do not improve patient care and may even harm the patient (4). Physicians face many challenges in accurately, efficiently and safely ordering and interpreting diagnostic tests. (The term ‘ordering’ will be used throughout this paper for consistency. However, tests are ‘requested’, not ‘ordered’ in many countries, and ‘requesting’ better reflects the collaboration between clinician and laboratory). To improve patient outcomes, laboratory tests must be appropriately ordered, properly conducted, reported in a timely manner, correctly interpreted and affect a decision for future diagnosis and treatment of the patient (5).
However, the use of laboratory diagnostics varies between countries and in the US it was 5 times greater (as a proportion of medical expenditure) than in the UK in 2006(2). Large differences between individual practitioners in laboratory utilization have been reported in several countries (6-9) The recent publication in England of the ‘National Health Service Atlas of Variation’ (10) demonstrated the variation in ordering rates for diagnostic tests across 151 primary care organizations. There may be valid reasons to explain some of the observed variation, such as different populations or case mix, incidence of deprivation, disease prevalence, local policy decisions on specific services and the availability of relatively new or high-technology tests. However, despite these factors, the variation in ordering rates is so large that it must reflect considerable differences in the individual ordering patterns of doctors within each primary care organization. An example is shown in Figure 1 for B-type natriuretic peptide (BNP). This test has been advocated for many years as a first line screening test for patients with symptoms of heart failure. UK national guidance commends its use (11) and recommends that the test is used to support the decision-making process as to whether a patient should be referred for echocardiography and/or to a specialist cardiologist.
Figure 1.
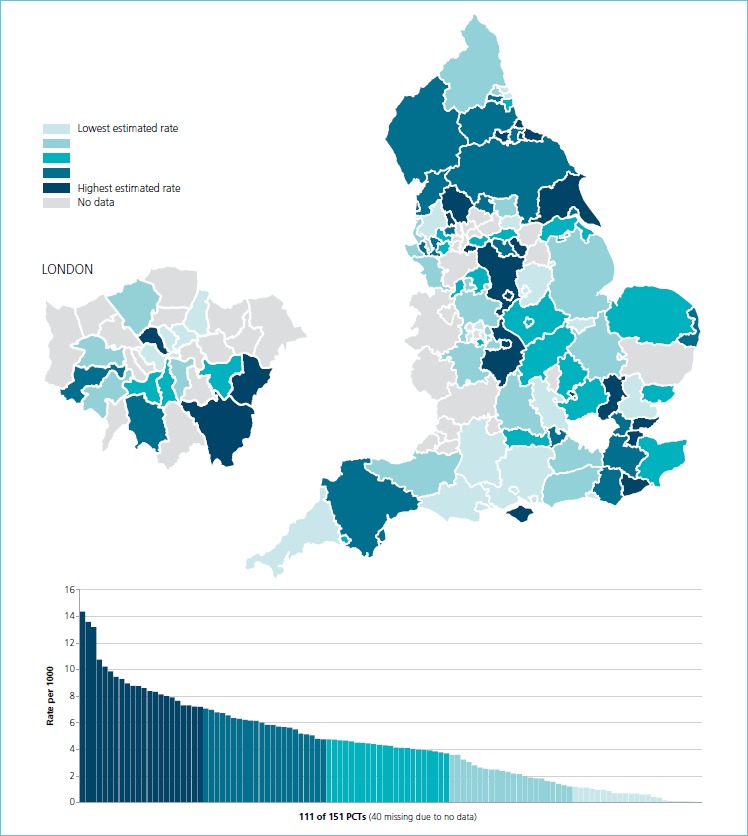
Brain natriuretic peptide (BNP or NTproBNP) ordering rates across primary care organizations (primary care trusts [PCTs]) in England in 2012
Figure 1 shows an 89-fold difference in ordering rates for BNP between different primary care organizations. This may represent failure of guideline uptake or the unavailability of the test in some areas due to cost pressures. Variation in utilization of this test can have a real impact on patient care and subsequent morbidity and mortality.
There are many factors which determine a physician’s test ordering practices. In literature surveys (12, 13), physicians mostly cite fear of legal (malpractice) complaints as the primary driver of over-testing. A recent article by Hoffman et al. (14) states that the main driver of over-diagnosis and over treatment is zero tolerance for error and uncertainty. Addressing the widespread intolerance of uncertainty requires a cultural change both within the medical profession and by the public.
This paper will examine the following:
Factors influencing test ordering by physicians;
Strategies for modifying physicians’ ordering pattern;
Ways to implementing policies to improve laboratory utilization and thereby improve patient outcome.
FACTORS INFLUENCING TEST ORDERING BY PHYSICIANS
Users of the clinical laboratory want information to allow them to make better decisions about patients. They want to be assured that the investigations they order will be quick, accurate and inexpensive and they want ‘new’ tests to be readily available. They want to be able to do the right investigation on the right patient at the right time, with results reaching the right clinician at the right time and in the right format and medium. In addition, availability of the right interpretation is essential to ensure the optimum patient outcome. Hopefully, the clinician is also concerned with patient safety, clinical accountability and clinical governance.
However, the clinician faces huge problems in getting test ordering right. There are too many tests, they have different names, they are reported in different units, there are different reference intervals between laboratories, there are different decision limits and guidelines are often inconsistent. Clinicians want tests with high diagnostic accuracy, good predictive value and proven clinical utility in decision making.
Two literature reviews (15, 16) are in broad agreement on the reasons for ordering diagnostic tests. These include diagnostic factors, such as rule-in or rule-out disease, therapeutic and prognostic factors, such as help in deciding on appropriate treatment, as well as patient-related factors such as patient reassurance, doctor-related factors such as clinical experience, confidence in clinical judgement and fear of litigation and policy and organization-related factors, such as test availability, institutional policies and clinical guidelines, and the use of structured test ordering forms (17).
FAILURE OF GUIDELINE IMPLEMENTATION
Despite the clear recommendations of the UK National Institute for Health and Care Excellence (NICE) guidelines for use of the CA125 antigen in detection of ovarian cancer (18), the data from the NHS Atlas of Variation (10) still demonstrates vast variation in the use of CA125 in UK primary care organizations. There is a need to target low-ordering areas, emphasising the importance of guideline implementation. It would be of great interest to determine if the areas with low CA125 ordering rates have higher morbidity and mortality for ovarian cancer. Schulenberg-Brand et al. (19) investigated the impact of local guidance on tumour marker ordering within a single surgical department in the UK through an audit process and found a significant rate of inappropriate ordering underpinned by an apparent lack of knowledge about the correct use of the test. For example, 33% of CA125 orders were made on male patients!
The use of faecal calprotectin to distinguish between irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) is well established (20). The test distinguishes patients with symptoms of functional IBS from those with organic symptoms of IBD with greater than 95% sensitivity and specificity. A normal faecal calprotectin result excludes IBD and removes the requirement for endoscopy. In our hospital, over the past 12 months, this has resulted in a 70% reduction in the number of endoscopy procedures. This does not only benefit patients but also provides a significant financial saving, since the cost of an endoscopy is $908 whereas a faecal calprotectin test costs $80.
In addition, calprotectin predicts clinical relapse in IBD with 90% sensitivity and 83% specificity. This again influences patient outcome by enabling treatment to be started earlier, thus resulting in improved outcomes. Despite this, calprotectin testing in the UK has not yet been implemented nationwide (10). For primary care organizations in England, the estimated annual rate of use for calprotectin tests ranges from 0.01 to 5.1 per thousand practice population, a 446-fold variation. Patchy uptake in primary care, despite the evidence of clinical utility, probably indicates a lack of understanding of the value of the test or its lack of availability from local laboratory services. This may be because secondary care providers are reluctant to lower the rates of endoscopy for financial reasons.
Failure of uptake of guidelines is a problem that spans all specialities and sectors of healthcare. A recent article by Misra et al. (21) confirmed the findings from Cabanagh et al. (22) in 1999, showing little change over 15 years. The barriers to guideline adherence include:
Lack of awareness of the existence of guidelines or unfamiliarity with the guideline content;
Lack of agreement with the specific guideline and/or lack of agreement with guidelines in principle;
Inertia of previous practice;
The guideline is contradictory to established practice or difficult to follow/use. There may be patient reluctance to comply with guideline;
External barriers such as resource availability, practice constraints and lack of time.
There have been several national initiatives to try to reduce over-diagnosis and change physician behaviour and adherence to guidance. In the UK, these include the NICE initiative of a ‘do not do’ recommendation database, comprising tests or procedures with limited or no value that should not be used (23). In the US, the ‘Choosing Wisely’ campaign (24) aims to help healthcare practitioners, patients and other stakeholders develop sustainable solutions to stop the overuse and misuse of medical tests and procedures that provide little or no benefit. In addition, a group from the Australian Government Department of Health identified potentially unsafe, ineffective or inappropriate services listed on the country’s Medicare Benefit Schedule (25).
The US National Physician Alliance (NPA) have created a project entitled ‘Promoting good stewardship in clinical practice’ that aimed to develop a list of the top five activities in family medicine, internal medicine and paediatrics where the quality of care can be improved. As part of the list for internal medicine, they recommended not obtaining blood chemistry panels or urinalysis for screening asymptomatic healthy adults, and only screening for type II diabetes mellitus in asymptomatic adults with hypertension (26).
Improving adherence to clinical guidelines requires targeting, proper dissemination and education. As we will see later, there is considerable overlap between successful implementation of guidelines or strategies and improving ordering behaviour. Guidelines should be written, published and disseminated, but it is essential that proper implementation strategies are devised and delivered, as implementation is crucial to ensuring a positive impact on patient outcome.
INAPPROPRIATE LABORATORY UTILIZATION
An analysis of 307 malpractice claims in the US (27) studied the principal areas of faulty processes which led to misdiagnosis in patients. The top cause, found in 55% of patients, was the failure to order the appropriate diagnostic/laboratory test. There is growing recognition that errors in test selection (inappropriate ordering) and result interpretation can have significant or adverse clinical consequences to patients and financial consequences to healthcare institutions (28).
As Moynihan et al. (29) have written, “Medicine’s much heralded ability to heal the sick is fast being challenged by its propensity to harm the healthy. Too many people are being over-dosed, over-treated and over-diagnosed.” They state that $200 billion may be wasted on unnecessary treatment every year in the US, for example screening programmes detecting early cancers that will never cause symptoms or death.
Five per cent of all healthy patients will get abnormal test results and false findings or trivial abnormalities can lead to unnecessary further testing and expensive and potentially risky interventions, leading to poor patient outcomes. Causes of over-utilization include patient pressure, duplicate ordering, lack of understanding of the diagnostic value of a test, ordering the wrong test, failure to understand the consequences of over-utilization, defensive testing, perverse financial incentives and ‘availability creates demand’ (where the key driver is technological advance). Some of the consequences of over-utilization include incorrect diagnosis and treatment, incorrect test ordering which delays the actual diagnosis, increased length of hospital stay, unnecessary blood loss, increased resource utilization and, most important, unnecessary patient alarm.
Moynihan et al. (29) point out that the concern about over-diagnosis does not preclude awareness that many people miss out on much needed healthcare. In fact resources wasted on unnecessary diagnoses and care can be much better spent treating and preventing genuine illness.
Van Walraven and Naylor, in their systematic review in 1998 (30), concluded that the frequency range of inappropriate testing was between 5% - 95% This was a review of North American studies, but similar non-American studies (UK, Netherlands, Australia, Canada, Egypt and Thailand) reported inappropriate testing rates between 10% - 50%. A study of hospitalized patients in our institution demonstrated that 34% of orders were inappropriate (unpublished data). Zhi et al. in their systematic review of the literature from 1997-2012 (31) found the overall mean rate of over-utilization of testing to be 20.6% (95% CI = 16.2-24.9%), with over-utilization of low volume tests higher at 32.2% (95% CI = 25.0-39.4%).
Laposata (28) has shown that the highest incidence of error in laboratory testing is in test selection by clinicians and interpretation of test results by clinicians. This confirms the work of Plebani (32) in his review of the literature: up to 68% of laboratory testing errors occur in the pre-pre-analytical phase which includes inappropriate test orders. Reviewing the diagnostic error and testing literature, Epner et al. (33) identified 5 causes of diagnostic error and harm relating to the testing process. They called this the ‘five cause taxonomy of testing-related diagnostic error’ and it includes both ordering an inappropriate test and not ordering an appropriate test.
There is often little thought given to the patient’s views and the non-clinical outcomes. A reduction in inappropriate ordering will reduce the need for some phlebotomy episodes and reduce the associated discomfort and inconvenience such as time off work, as well as minimizing potential patient anxiety. It must be recognised that inappropriate testing will impact on follow-up, by leading to false positive results, and unnecessary further interventions such as referral and further invasive investigations.
There is no point in ordering a test if no-one looks at the results and/or acts on them. The issue of failure to follow-up tests which have been ordered is addressed by Callen et al. elsewhere in this issue of eJIFCC.
In a very recent publication ‘Protecting Resources, Promoting Value: A doctors’ guide to cutting waste in clinical care’ (November 2014) from the UK Academy of Medical Royal Colleges (34) there is a guide/toolkit to help doctors and other clinicians to use resources in the most effective way to provide the best possible quality and quantity of care for patients. It promotes the identification of tests or procedures whose necessity should be questioned.
The emphasis of laboratory utilization programs should never be exclusively on reducing the number of tests. It is imperative to consider clinical outcomes and the changes to patient management. Zhi et al. (31) found the mean rate of under-utilization of testing in their systematic review to be 44.8% -more than twice the rate of over-utilization. Missed tests may have a significant impact on patient outcome. In a study looking at the effect of HbA1c ordering frequency, Fu and his colleagues showed lower frequency of HbA1c monitoring is significantly associated with poorer glycaemic control. To achieve HbA1c concentrations below a target of 53 mmol/mol the optimal testing frequency was 4 times per year (35).
STRATEGIES TO IMPROVE PHYSICIAN ORDERING
There have been several studies and audits published which describe initiatives to change ordering behaviour. An article in Bandolier (36) identified 49 studies reporting interventions which were designed to changed physicians’ ordering practice. The studies used a range of single or combined interventions which included: educational initiatives, guideline dissemination, Computerized Physician Order Entry (CPOE) design with algorithms, clinical pathway analysis, activity utilization and cost information, vetting of orders and restricting tests to ensure the appropriate test repertoire.
Successful intervention strategies included: 1) educational initiatives aimed at predisposing factors; 2) targeting re-enforcing factors by provision of activity and costing data; and 3) targeting enabling factors such as limiting the number of tests allowed by deleting tests from the laboratory repertoire or specialist vetting of orders. A summary of strategies can be found in Table 1.
Table 1.
Summary of intervention strategies to improve physician ordering behaviour
| Pre laboratory | Laboratory | Post- laboratory |
|---|---|---|
| Educate and engage with users regarding testing, including presentations to clinical teams | Withdraw outdated tests | Perform clinical audit with adherence to local and national clinical guidelines |
| Make formal contributions to training, induction, undergraduate curriculae, guideline pathway development | Harmonize nomenclature | Perform audit of the impact of test results on patient pathways and outcomes, including whether results were reviewed, whether action was taken and the outcome |
| Develop laboratory formulary in conjunction with users | Standardize units | Include information on test appropriateness as part of report |
| Develop CPOE systems including test repertoire available to all who order tests. Use disease-specific or question-specific profiles | Standardize reference intervals | |
| Ensure targeting of clinical guidelines | Harmonize laboratory profiles | |
| Apply minimum re-testing intervals between repeat tests | ||
| Use reflex/reflective testing when appropriate | ||
| Introduce vetting of selected (esoteric) tests by senior laboratory staff |
Astion has described the factors he feels improve laboratory utilization (37). He describes both physician education and patient education as weak interventions in isolation. CPOE can improve laboratory utilization if thoughtfully implemented and education can be made more effective by combining it with other methods that make the desired behaviour more likely, including CPOE, use of formularies, implementing higher levels of approval for some tests and the use of physician utilization reports with performance feedback. As in many other studies, the best approach to improving laboratory utilization combines multiple interventions (17).
It is imperative that these interventions remain in place, or ordering behaviour will drift back to the initial condition. In a cluster randomized trial by Thomas et al. (38), the effect of enhanced feedback and brief educational reminder messages on 9 tests ordered in primary care over a 12 month period achieved a reduction of around 10% in the number of orders when used alone but when the initiatives were used together (in combination) they demonstrated a larger reduction - greater than 20% of total tests ordered.
Figure 2 shows the ‘test cycle’ and highlights the points at which the laboratory clinicians can become engaged in managing appropriate test utilization. The patient must always be the focus of all processes and outcomes.
Figure 2.
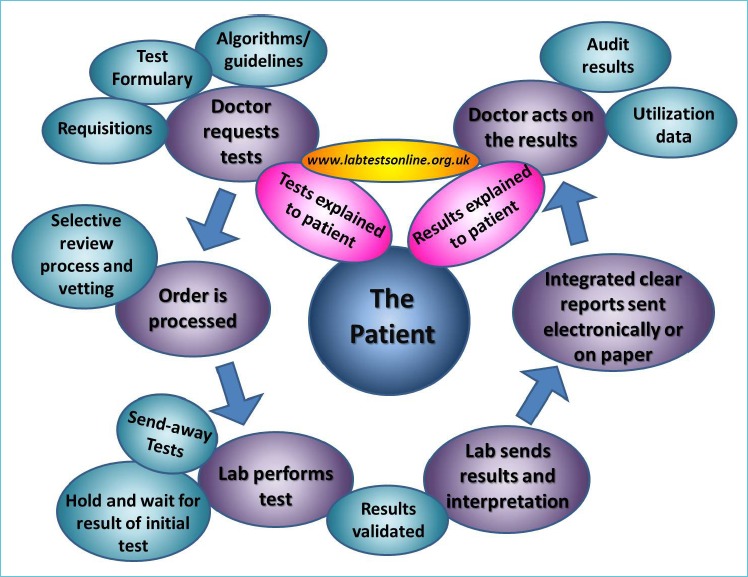
The ‘Test Cycle’
MEDICAL EDUCATION
One of the biggest areas of concern is the level of education of junior doctors (interns) about laboratory medicine, which has decreased in many countries. Khromova and Gray (39) surveyed junior medical staff in Sheffield and found they lacked confidence in both ordering and interpreting basic clinical chemistry tests, such as serum protein, magnesium and phosphate. Up to 75% of the junior doctors felt they needed further teaching in relation to investigations that they were not confident in ordering or interpreting. A study of interns in two teaching hospitals in Cape Town, South Africa (40) demonstrated similar results. The study concluded that junior doctors felt unprepared for their roles and needed more exposure to laboratory medicine in training, and more instruction on the basics of rational ordering of laboratory tests.
In a study of final year medical students at Oxford in 2010 (41), Clarke and Littlewood explored their attitudes and their competence in haematology Haematology was viewed as a particularly difficult speciality, but was nevertheless a popular and interesting career choice. A worrying lack of important clinical knowledge as the students began their internship was found. The study demonstrated the students’ relative lack of both confidence and competence in managing blood disorders, for example, only one third of the final year medical students knew the value of the International Normalized Ratio (INR) in deciding whether to administer vitamin K to a patient. As a result of this survey, the British Society of Haematology reviewed the curriculum for undergraduate haematology teaching.
There is very little hard evidence in the literature to demonstrate the impact of the knowledge of basic science by junior doctors on patient outcome (42), although there is no shortage of anecdotal and circumstantial evidence. A survey of 300 participants attending an AACC Annual Meeting in 2012 showed that the single most important issue identified as leading to ineffective test ordering was inadequate teaching about laboratory medicine in Medical School (unpublished data). 94% of respondents rated this as ‘highly important’ or ‘important’.
A survey by Laposata (28) showed that every US medical school teaches more than 100 hours of anatomic pathology, whilst only 9% have a separate and distinct course in laboratory medicine. The mean time spent teaching medical students on the appropriate selection of laboratory tests and the correct interpretation of results over the entire 4 year curriculum was 10 hours and it was less than 5 hours in many of the institutions. The survey showed that completion of anatomic pathology training required passing an examination, but there were no examinations for laboratory medicine, despite it forming a much greater part of the experience of most physicians.
A recent survey from the Clinical Laboratory Integration into Healthcare Collaborative (CLIHC) found that primary care physicians are uncertain about the right test to order 14.7% of diagnostic encounters and are uncertain about the correct interpretation of test results in 8.3% (1). With more than 500 million primary care visits per year in the US, the data indicates that approximately 23 million times per year, primary care physicians are not certain about the best use of the diagnostic test. Inadequate education in laboratory medicine must be seen as a patient safety issue.
LABORATORY FORMULARIES
A laboratory (test) formulary is analogous to the pharmaceutical formulary present in most institutions and can be used in many ways. It may simply outline what tests a clinician may order or what tests are permitted to be sent to outside (reference) laboratories. A test formulary requires an understanding of the clinical value of the test, the financial impact and whether or not there is a history of the test being poorly utilized. Many laboratories now have their own laboratory formulary to help the clinician to select the right test in specific situations. An example is Brigham and Womens’ Hospital, Boston, MA, USA (43). The trend is to use the laboratory formulary to reduce inappropriate ordering of expensive molecular and genomic tests. Effective laboratory formularies need to be developed with full involvement of laboratory staff, physicians and other stake holders.
HARMONIZATION OF NOMENCLATURE
CLIHC have also examined the issue of the wide inconsistencies in test nomenclature as a significant barrier to physicians ordering the correct test. For example, there are at least 18 different titles for vitamin D related tests in the US. In the UK, the National Laboratory Medicine Catalogue (NLMC) has the long-term objective that each test ‘name’ represents a single pathology test concept and each concept is represented by just one name. The NLMC aims to standardize ordering, reporting and analysing of pathology tests to ensure that the right patient gets the right test at the right time. There is a standardized list of pathology tests that have been validated for use within the UK NHS. This list is provided in an XML format and may be used within Laboratory Information Management Systems (LIMS), electronic patient records and pathology order communications (44).
HARMONIZING COMMON LABORATORY TEST PROFILES
A common source of physician confusion is that different laboratories provide different ‘profiles’ of tests to answer the same clinical question. This variation is often for historical reasons. In the UK, it has been revealed by a national pathology benchmarking initiative (45), which showed 12 different profiles for liver function tests among 50 laboratories subscribing to the initiative. As a consequence, the UK Association for Clinical Biochemistry and Laboratory Medicine, have produced proposals for a consensus view on profile composition, e.g. liver panel: bilirubin, alanine transaminase, alkaline phosphatase, albumin (46). As well as removing confusion, harmonizing profiles can save money and reduce further investigations instigated as a result of clinically irrelevant minor abnormalities in irrelevant tests.
Laboratories must increase their efforts to engage with the test user to provide the appropriate tests in any clinical situation, whatever the core profiles contain.
COMPUTERIZED PHYSICIAN ORDER ENTRY (CPOE)
CPOE can be a blessing or a curse, depending on how it is implemented. The worst case scenario is an electronic test order form in which the full menu of laboratory tests, from the most common to the most esoteric is made readily available to all practitioners, and repetitive interval-based testing (e.g. daily thyroid function testing) is easy to instigate. This is a recipe for laboratory mis-utilization, and the laboratory involved would have to bear responsibility for the resulting situation.
If CPOE is implemented with a strategy that prompts physicians with relevant information at the time of test ordering, it has been shown to decrease utilization of some commonly ordered tests in the in-patient setting. In one study, physicians were prompted electronically as to whether they wanted to continue their daily metabolic panel order after the patient had been in hospital for 72h (47). The effect of this was to reduce testing by 24% with no change in patient outcome. Design of the electronic order form is crucial: following literature searches showing that gamma-glutamyl transferase (GGT) need not form part of the routine liver panel, local experience of removing the GGT tick box for a 12 month period during 2010/2011 reduced GGT ordering by almost 50% (unpublished data).
As yet, there is limited published evidence on the impact of CPOE on clinical outcomes. However, the potential of the approach has been investigated in the context of imaging (48). It was proposed that a system linking electronic ordering of imaging orders to best practice diagnostic pathways represented the way to maximize appropriate referrals.
A systematic review in 2006 identified 19 studies of the impact of CPOE on laboratory testing (49). Eleven of these compared CPOE (with and without decision support) to no CPOE for laboratory testing in a range of countries (South Korea, USA, UK, Canada, Norway), and eight studies compared CPOE with and without specific decision support (all in USA). Eight of the first group of studies and all of the second group considered outcomes that could be specifically related to appropriateness issues such as clinical indicators, length of stay or appropriateness of stay. The CPOE systems (both with and without decision support) showed an overall trend towards reduced test volume and cost, when compared to no CPOE. Overall, fewer tests and (when measured) fewer inappropriate tests were performed in the decision support group.
In addition, the decision support group showed a significant reduction in the median time to appropriate treatment for critical results reported in one of the randomized controlled trials.
Four of the studies found that CPOE systems combined with decision support improved adherence to guidelines provided on the system. One of the advantages of CPOE is the ability to link electronically to relevant knowledge resources, but care is needed not to make the ordering process unwieldy.
In a UK study of implementation of CPOE (50), it was shown to be associated with a reduction in the proportion of outpatient appointments at which full blood count, urea and electrolytes and urine culture tests were ordered and at which full blood count tests were repeated. However, the system was associated with an almost 4-fold increase in the use of urea and electrolytes testing amongst day case patients.
A recent publication from Turner et al. (51) looked at pre-analytical errors from primary care during two six-month periods pre- and post-implementation of electronic ordering. Outcomes measured included whether there was correct information on the sample, whether the correct sample was received and whether clinical history was provided. There was a marked decrease in the number of pre-analytical errors following the introduction of electronic ordering (2764 pre-implementation versus 498 post-implementation). The error rate dropped across all general practices: pre-implementation error rates ranged up to 5.7% of orders, post-implementation error rates were less than 0.6%
In 2014, the Association for Clinical Biochemistry and Laboratory Medicine and the Royal College of Pathologists in the UK proposed ‘National Minimum Retesting Intervals in Pathology’ (52). The recommendations cover minimum intervals before retesting for common tests in clinical biochemistry, therapeutic drug monitoring, haematology and immunology in specified clinical situations, supported by an evidence base. In this context, electronic ordering has an advantage over laboratory-based interventions as it can prevent inappropriate repeat orders at the source prior to phlebotomy, minimising the inconvenience for patients and the burden on phlebotomy staff and laboratory reception staff. Electronic orders can also provide links to external sources such as diagnostic algorithms and other resource sites such as Lab Tests Online (www.labtestsonline. org.uk), which help the primary care physician choose the correct test and explain the result to the patient.
Epner and Astion have reported on the use of CPOE to reduce diagnostic errors, particularly: the use of CPOE templates in a specific care testing environment, e.g. diabetes care; the incorporation of reflex testing strategies, e.g. autoantibody panel after positive ANA test; decreasing the number of synonyms for the same test; and restriction of ordering of specific tests to a defined set of physicians or specialists, e.g. medical geneticists (53).
VETTING (RESTRICTION) OF TESTS
Laboratories have the option to vet high cost, low volume tests, often those referred to specialist laboratories, on a individual basis. Fryer et al. reduced the number of urine toxicology screens from 30 to less than 5 orders per month (54), resulting in an annual saving of around $48,000. In another study, consultant level restriction of a specific test, C-reactive protein (CRP), led to an 85% reduction in test volume (55).
In our experience, restriction of CRP and ESR ordering with the use of computerized decision support led to a 17% reduction of orders (unpublished data). Application of knowledge-based rules significantly improves the appropriateness of test ordering. Sometimes it may be appropriate to use a ‘send and hold’ process in which a specimen is sent to the laboratory but the test is not performed until another initial test result comes back. Flow cytometry is a good example, along with molecular assays and genetics studies in haematological diseases, which can be held until the bone marrow aspirate and biopsy is viewed by the pathologist and then sent for testing in appropriate cases. Using fixed rules as a form of vetting, Srivastava et al. prospectively measured the efficiency and effectiveness of reflex and reflective testing in specific clinical scenarios (56). These approaches improved the diagnosis of hypovitaminosis D, hypomagnesaemia, hypothyroidism, hyperthyroidism and haemochromatosis, improving both the clinical utility of the laboratory service and the patient outcome.
PROVIDING COST INFORMATION ON LABORATORY TEST ORDERING
Healthcare budgets worldwide are facing increasing pressure to reduce costs and improve efficiency whilst maintaining quality. Pathology investigations cost the UK National Health Service £2.5 billion per year. A review commissioned by the UK Department of Health estimated that 20% of this could be saved by improving utilization of pathology services, despite the annual increase of 8-10% in workload.
The review estimated that 25% of pathology tests were unnecessary, representing a huge potential waste of resource (57).
A controlled clinical trial at John Hopkins’ Hospital displayed ‘fees’ for 61 random laboratory tests in their CPOE. In the ‘active arm’, there was an 8.59% decrease in the number of tests per patient. In the ‘control arm’, there was a 5.64% increase (58).
In a similar study involving 215 primary care physicians in Massachusetts, Medicare reimbursement rate for 27 laboratory tests was displayed. In the intervention group there was a significant decrease of 19% in ordering rates compared to control physicians for 5 tests. In addition, the majority (81%) of physicians reported that the intervention improved their knowledge of the relative cost of laboratory tests (59).
CONCLUSION
Successful management of laboratory test utilization requires the entire laboratory team to use their skills and knowledge to identify utilization issues, implement a programme that will achieve more effective laboratory testing and establish appropriate processes from the beginning to the end of the test cycle. This is not easy and requires interactions with our clinical colleagues that some laboratory workers may find uncomfortable - questioning clinicians, and advising that they should not order a particular test but another test is more appropriate. Generally, clinicians have few direct incentives to restrict laboratory utilization, and are not being trained to do so. It is disappointing that there is so little literature on the effectiveness of appropriate test utilization on patient outcomes, as well as on cost effectiveness across the whole patient pathway.
“We need to recognise that the target of requesting of the test and of the results should be the patient. It is the person who actually, in the end, is going to have to change their lives and start adopting new behaviours....” (Goetz [60], adapted).
REFERENCES
- 1.Hickner J, Thompson PJ, Wilkinson T Epner P, Shaheen M, Pollock AM, et al. Primary care physicians’ challenges in ordering in clinical laboratory tests and interpreting results. J Am Board Fam Med 2014; 27: 268-274. [DOI] [PubMed] [Google Scholar]
- 2.Research and Markets. In vitro diagnostics (IVD) market, techniques and applications. Dublin: Research and Markets, 2013. http://www.researchandmarkets.com/publication/pfdniq/in_vitro_diagnostic_ivd_market_technique. Accessed November 2014. [Google Scholar]
- 3.Alexander B. Reducing healthcare costs through appropriate test utilization. Critical Values 2012; 5: 6-8. [Google Scholar]
- 4.Holladay EB. Test right. Critical Values 2012; 5: 3. [Google Scholar]
- 5.The Lewin Group. The value of laboratory screening and diagnostic tests for prevention and health care improvement, 2009. http://www.lewin.com/publications/publication/403/ Accessed November 2014. [Google Scholar]
- 6.Mogyorosy Z, Mogyorosy G. Practice pattern and geographic variation in test ordering: a literature review. Orvosi Hetilap 2006; 147: 25-31. [PubMed] [Google Scholar]
- 7.Larson A, Palmer M, Hulten G, Tryding N. Large differences in laboratory utilization between hospitals in Sweden. Clin Chem Lab Med 2000; 38: 383-389. [DOI] [PubMed] [Google Scholar]
- 8.Smellie WS, Galloway MJ, Chinn D. Benchmarking practice use of pathology services: a model for monitoring change. J Clin Pathol 2000; 53: 476-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smellie WS, Clark G, Nulty CA. Inequalities of primary care microbiology testing between hospital catchment areas. J Clin Pathol 2000; 56: 199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health England. NHS Atlas of Variation in diagnostics services. November 2013. http://www.riehtcare.nhs.uk/index.php/atlas/diagenostics-the-nhsatlas-of-variation-in-diaenostics-services/. Accessed November 2014. [Google Scholar]
- 11.Clinical Guideline 108: Chronic heart failure: management of chronic heart failure in adults in primary and secondary care. National Institute for Health and Care Excellence, 2010. https://www.nice.ore.uk/euidance/ce108. Accessed November 2014. [Google Scholar]
- 12.Kanzaria HK, Hoffman JR, Probst MA, Berry S, Brook RH. Emergency physician perceptions of medically unnecessary advanced diagnostic imaging, (abstract) Acad Emerg Med 2014; 21 (Suppl. 1). [DOI] [PubMed] [Google Scholar]
- 13.Studdert DM, Mello MM, Sage WM, DesRoches CM, Peugh J, Zapert K, Brennan TA. Defensive medicine among high risk specialist physicians in a volatile malpractice environment. JAMA 2005; 293: 2609-2617. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman JR, Kanzaria HK. Intolerance of error and culture of blame drive medical excess. BMJ 2014; 349: g5702. [DOI] [PubMed] [Google Scholar]
- 15.Whiting P, Toerien M, de Sallis I, Sterne AC, Dieppe P, Egger M, Fahey T. A review identifies and classifies reasons for ordering diagnostic tests. J Clin Epidemiol 2007; 60: 918-919. [DOI] [PubMed] [Google Scholar]
- 16.Sood R, Sood A, Ghosh AK. Non-evidence-based variables affecting physicians test-ordering tendencies: a systematic review. Neth J Med 2007; 65: 167-177. [PubMed] [Google Scholar]
- 17.Smellie W. Demand management and test request rationalisation. Ann Clin Biochem 2012; 49: 323-336. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Guideline 122: The recognition and initial management of ovarian cancer. National Institute for Health and Care Excellence, 2011. https://www.nice.ore.uk/euidance/ce122 Accessed November 2014. [Google Scholar]
- 19.Schulenburg-Brand D, Kumar N, Zouwali S. The impact of local guidelines on the tumour marker requesting patterns of a general surgery department. Ann Clin Biochem 2013; 50: 438-442. [DOI] [PubMed] [Google Scholar]
- 20.Diagnostics Guideline 11: Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel. National Institute for Health and Care Excellence, 2013. https://www.nice.ore.uk/euidance/DGll. Accessed November 2014. [Google Scholar]
- 21.Misra S, Barth JH. Guidelines are written, but are they followed? Ann Clin Biochem 2013; 50: 400-402. [DOI] [PubMed] [Google Scholar]
- 22.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PC, Rubin HR. Why don’t physicians follow clinical practice guidelines? JAMA 1999; 282: 1458-1465. [DOI] [PubMed] [Google Scholar]
- 23.‘Do not do’ database. National Institute for Health and Care Excellence, http://www.nice.ore.uk/savings-andproductivity/collection. Accessed November 2014. [Google Scholar]
- 24.Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing Wisely - the politics and economics of labelling low-value services. N Engl J Med 2014; 370: 589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elshaug AG, Watt AM, Mundy L, Wills CD. Over 150 potentially low value healthcare practices: an Australian study. Med J Aust 2012; 197: 556-560. [DOI] [PubMed] [Google Scholar]
- 26.The “Top 5” lists in primary care: meeting the responsibility of professionalism. Arch Intern Med 2011; 171: 1385-1390. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi TK, Kachalia A, Thomas EJ, Puopolo AL, Yoon C, Brennan TA, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med 2006; 145: 488-496. [DOI] [PubMed] [Google Scholar]
- 28.Laposata M. Putting the patient first - using the expertise of laboratory professionals to produce rapid and accurate diagnoses. LabMedicine 2014; 45: 4-5. [DOI] [PubMed] [Google Scholar]
- 29.Moynihan R, Doust J, Henry D. Preventing overdiagnosis - how to stop harming the healthy. BMJ 2012; 344: e3502. [DOI] [PubMed] [Google Scholar]
- 30.Van Walraven C, Naylor D. Do we know what inappropriate laboratory utilization is? JAMA 1998; 280: 550-558. [DOI] [PubMed] [Google Scholar]
- 31.Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: A 15-year meta-analysis. PLoS ONE 2013; 8(11): e78962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 2010; 47: 101-110. [DOI] [PubMed] [Google Scholar]
- 33.Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ Qual Saf 2013; 22: ii6-ii10. doi 10.1136/bmjqs-2012-001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Protecting resources, promoting value: a doctors’ guide to cutting waste in clinical care. Academy of Medical Royal Colleges, 2014. http://www.aomrc.ore.uk/dmdocuments/Promoting%20value%20FINAL.pdf Accessed November 2014. [Google Scholar]
- 35.Fu C, Ji L, Wang W, Luan R, Chen W, Zhan S, Xu B. Frequency of glycated haemoglobin monitoring was inversely associated with glycemic control of patients with type 2 diabetes mellitus. J Endocrinol Invest 2012; 35: 269-273. [DOI] [PubMed] [Google Scholar]
- 36.Using labs best. Bandolier 1999. 61: 4 http://www.medicine.ox.ac.uk/bandolier/band61/b61-4.html. Accessed November 2014. [Google Scholar]
- 37.Astion M. Interventions that improve laboratory utilization: from gentle guidance to strong restrictions. Lab Errors Patient Saf 2006; 2: 2. [Google Scholar]
- 38.Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: a cluster randomised trial. Lancet 2006; 367: 1990-1996. [DOI] [PubMed] [Google Scholar]
- 39.Khromova V, Gray T. Learning needs in clinical biochemistry for doctors in foundation years. Ann Clin Biochem 2008; 45: 33-38. [DOI] [PubMed] [Google Scholar]
- 40.Stanfliet JC., Macauley J, Pillay TS. Quality of teaching in chemical pathology: ability of interns to order and interpret laboratory tests. J Clin Pathol 2009; 62: 664-666. [DOI] [PubMed] [Google Scholar]
- 41.Clarke R, Littlewood T. ‘Haematophobia?’ Attitudes of Oxford medical students towards haematology. Bull Royal Coll Pathol 2010; 150: 118-122. [Google Scholar]
- 42.Freedman DB. Is the medical undergraduate curriculum fit for purpose? Ann Clin Biochem 2008; 45: 1-2. [DOI] [PubMed] [Google Scholar]
- 43.List of available tests. Brigham and Women’s Hospital Department of Pathology, Boston, MA, USA: http://bwhpatholoev.partners.org/labmanual.aspx. Accessed November 2014 [Google Scholar]
- 44.National laboratory medicine catalogue. Royal College of Pathologists; http//www.rcpath.org/clinical-effectiveness/patholoev-cataloeues/national-laboratory-medicine-cataloeue/national-laboratory-medicine-cata-loeue.htm. Accessed November 2014. [Google Scholar]
- 45.Keele University Benchmarking Service. www.keele.ac.uk/benchmarkine Accessed November 2014.
- 46.Consultation on standardized test order sets. Association for Clinical Biochemistry and Laboratory Medicine; www.acb.org.uk/whatwedo/science/bestpractice/test profiles.aspx. Accessed November 2014. [Google Scholar]
- 47.Neilsen EG, Johnson KB, Rosenbloom ST, Dupont WD, Talbert D, Giuse DA, et al. The impact of peer management on testing ordering behaviour. Ann Intern Med 2004; 141: 196-204. [DOI] [PubMed] [Google Scholar]
- 48.Bairstow PJ, Mendelson R, Dhillon R, Valton F. Diagnostic imaging pathways: development, dissemination, implementation, and evaluation. Int J Qual Health Care 2006; 18: 51-57. [DOI] [PubMed] [Google Scholar]
- 49.Georgiou A, Williamson M, Westbrook JI, Ray S. The impact of computerised physician order entry systems on pathology services: a systematic review. Int J Med Inform 2007; 76: 514-529. [DOI] [PubMed] [Google Scholar]
- 50.Collin S, Reeves BC, Hendy J, Fulop N, Hutchings A, Priedaner E. Implementation of computerised physician order entry (CPOE) and picture archiving and communication systems (PACS) in the NHS: quantitative before and after study. BMJ 2008; 337: a939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner HE, Deans KA, Kite A, Croal BL. The effect of electronic ordering on pre-analytical errors in primary care. Ann Clin Biochem 2013; 50: 485-488. [DOI] [PubMed] [Google Scholar]
- 52.Final report of the national minimum retesting intervals project. Association for Clinical Biochemistry and Laboratory Medicine; http://www.acb.ore.uk/docs/default-source/guidelines/acb-mri-recommendations-a4-computer.pdf?sfvrsn=2 Accessed November 2014. [Google Scholar]
- 53.Epner P, Astion M. Focusing on test ordering practices to cut diagnostic errors. Clin Lab News 2012; 38 (No 7) 17-18. [Google Scholar]
- 54.Fryer AA, Smellie WS. Managing demand for laboratory tests: a laboratory toolkit. J Clin Pathol 2013; 66: 62-72. [DOI] [PubMed] [Google Scholar]
- 55.Hutton HD, Drummond HS, Fryer AA. The rise and fall of C-reactive protein: managing demand within clinical biochemistry. Ann Clin Biochem 2009; 46: 155-158. [DOI] [PubMed] [Google Scholar]
- 56.Srivastava R, Bartlett WA, Kennedy IM, Hiney A, Fletcher C, Murphy MJ. Reflex and reflective testing: efficiency and effectiveness of adding on laboratory tests. Ann Clin Biochem 2010; 47: 223-227. [DOI] [PubMed] [Google Scholar]
- 57.Report of the second phase of the review of NHS pathology services in England. Chaired by Lord Carter of Coles. December 2008. http://webarchive.national-archives.gov.uk/20130107105354/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicvAndGuidance/DH091985. Accessed November 2014. [Google Scholar]
- 58.Feldman LS, Shihab HM, Thiemann D, Yeh HC, Ardolino M, Mandell S, Brotman DJ. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013; 173: 903-908. [DOI] [PubMed] [Google Scholar]
- 59.Horn DM, Koplan KE, Senese MD, Orav EJ, Sequist TD. The impact of cost displays on primary care physician laboratory test ordering. J Gen Intern Med 2014; 29: 708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goetz T. The decision tree: taking control of your health in the new era of personalized medicine. Emmaus, PA: Rodale Books, 2010. [Google Scholar]


