Abstract
B-type natriuretic peptide (BNP) and NT-proBNP are widely used plasma biomarkers for the diagnosis of acute decompensated heart failure and prognosis for future cardiac disease. The clinical performance of these tests for management of chronic heart failure is somewhat limited by the markers’ high biological variation. Biomarkers such as galectin-3 and soluble ST2 that reflect ongoing remodeling via cardiac fibrosis of the heart may provide complementary information to the natriuretic peptides in the management of chronic heart failure with regards to risk stratification for future adverse cardiac events (death, myocardial infarction, and need for heart transplantation). However, implementation of these biomarkers into routine clinical practice requires documentation that these tests will enable therapeutic decisions that can be made to improve clinical outcomes, and the availability of commercial assays. This report will discuss the need for novel heart failure biomarkers, ideal characteristics of assays, and review and compare the clinical performance of assays for galectin-3 and sST2 for chronic heart failure disease management.
Key words: B-type natriuretic peptides, adrenomedullin, ST2, galectin-3
BACKGROUND
B-type natriuretic peptide (BNP) and NT-proBNP originate from pro-BNP, and are released into the circulation following volume overload and myocardial stretching (1). The clinical value of B-type natriuretic peptide and NT-proBNP as biomarkers for patients who present to the emergency department with dyspnea and shortness of breath was first reported in the Breathing Not Properly (2) and PRIDE (3) studies, respectively. Since then, the natriuretic peptides have been incorporated into international guidelines on heart failure, including the 2012 update from the European Society of Cardiology (4). Although these biomarkers are widely used in clinical practice throughout the world, there are limitations that the affect the interpretation of results. In the absence of heart failure, there are differences between individuals regarding their age, gender and body mass index (5). Patients with renal failure have increased concentrations relative to those with normal renal function (6). BNP and NT-proBNP have high intra-individual biological variation resulting in relatively high values for reference change values (RCV) (7). The RCV is an important attribute for biomarkers where serial testing is needed as this value indicates the degree of change in test results needed to exceed variations observed in the blood of healthy individuals (8). Any change below the RCV could be due to normal variation and not disease progression or improvement. Ideally, biomarkers used for monitoring chronic heart failure should have low biological variation so that changes in serial results reflect disease progress or improvement. For use in managing chronic heart failure, data on long-term variation, i.e., weeks and months, is relevant. Using the standard statistical approach, Fokkema et al. reported week-to-week RCV of 113% and 98% for BNP and NT-proBNP, respectively, considerably higher variation than what is observed hour-by-hour or day-to-day (9) or for cardiac troponin I (10) or T (11). It should be noted that for markers that do not produce a parametric distribution, logarithmic conversion is needed resulting in the production of different RCV values depending on whether the next result in a series is increasing or decreasing.
Chronic heart failure is the consequence of patients suffering acute coronary syndrome and/or acute decompensated heart failure. Long-standing hypertension is also a common etiology. Chronic heart failure is a characterized by an insufficient delivery of blood from the heart to the body to meet metabolic needs. Systolic heart failure is caused by decreased ejection of blood the left ventricle of the heart. Diastolic heart failure is inadequate filling of the ventricle from the left atrium. Treatment for heart failure is focused on maintaining fluid balance, increasing ventricular output, maintenance of heart rhythm, inhibiting cardiac remodeling, and heart transplantation as a last resort. Due to the limitations as described above, the natriuretic peptides are not widely used in clinical practice and have not been adopted into clinical practice guidelines for monitoring the success of chronic heart failure treatment. There is therefore a need for identifying novel heart failure biomarkers. Previously in this Journal, Malinowski et al. reviewed 6 novel biomarkers of cardiac hypertrophy (12). The value of these markers in regards to therapeutic selection was not discussed however, and will be emphasized in this work specifically for galectin-3 and sST2.
IDEAL CHARACTERISTICS OF NOVEL BIOMARKER ASSAYS FOR CHRONIC HEART FAILURE
There are several ideal characteristics of a biomarker that are needed for use in managing patients with chronic heart failure. The marker should not be overly influenced by changes that occur during the acute phase of decompensated heart failure or acute coronary syndromes. Instead, changes in the concentrations of biomarkers should reflect chronic disease progression or improvement. Clinical research in chronic heart to date has focused on proteins that are released as part of the pathophysiologic process of chronic heart failure, especially remodeling by chronic inflammation, apoptosis, and cardiac fibrosis. Chronic inflammation plays a role in immobilizing leukocytes and macrophage to the site of damage and releasing myeloperoxidase and matrix metalloproteinases which subsequently degrade protective collagen layers. Significant conversion of viable myocardial cells to non-functioning apoptotic or fibrotic tissue is a predictor of decreased contractility and myocardial dysfunction. The success of novel biomarkers of heart failure will be linked to the early detection of these events. It is likely that increases in the levels of these markers during a non-acute phase of the disease would indicate disease progression.
While prediction of adverse outcomes is important, there must be therapies that can be given to patients to reduce the rate of adverse events. Laboratory tests that decrease in levels in response to successful therapy over time might suggest therapeutic benefit. An ideal attribute is to find a “companion laboratory test” that is directly linked to a therapy and be predictive of therapeutic success. While there are tests to anticancer therapies that are co-approved by the US Food and Drug Administration (FDA), e.g., her2/neu for trastuzumab treatment of breast cancer (13), companion cardiac biomarkers do not exist for cardiovascular disease at the moment.
Given the widespread utilization of the natriuretic peptides today, the clinical performance of novel markers must complement or exceed the existing tests. It is unlikely that a test with equivalent performance will be sufficient for clinical laboratories to make a replacement. It is also advantageous to have new biomarkers that are approved by the either the FDA or the European CE Marking, or both. Finally, it is most useful if tests are available on automated immunoassay analyzers. While research enzyme-linked immunosorbent assays (ELISA) are acceptable and widely used for clinical trials, and the generation of research data and publications, ELISAs are not widely used by clinical laboratories. Alternatively, a test that is available on a point-of-care platform can be more readily acquired and put into use, even if the testing is conducted in the central laboratory by medical technologists.
NOVEL BIOMARKERS OF CHRONIC HEART FAILURE
Galectin-3
Galectin-3 is a 29-35 kDa protein that is part of a family of 15 galectins. It is widely distributed in epithelial cells, fibroblasts, dendritic and inflammatory cells. Studies in a rat model have suggested that there is a causal role of galectin-3 in heart failure. Sharma et al. injected galectin-3 into the pericardium and showed markedly increased left ventricular (LV) collagen and reduced LV dysfunction (14). In humans after acute myocardial infarction (AMI), increased galectin-3 promotes collagen deposition and fibrosis formation. Extensive ischemic damage to the myocardium following AMI is the leading cause of heart failure.
There have been numerous studies comparing the performance of galectin-3 against the natriuretic peptides in patients with chronic heart failure. In the study by Lok et al., 232 NYHA class III and IV heart failure patients were followed for 6.5 years (15).
The survival probability was under 40% in the highest galectin-3 quartile. Patients who had both a high galectin-3 and NT-proBNP had the worst outcomes. In the CORONA study, patients who exhibited an increase in galectin-3 had the worst outcomes than those whose levels were stable or decreased, irrespective of the initial galectin level (16). Since the RCV for galectin-3 at 63% is considerably lower than for BNP and NT-proBNP (17), these preliminary data suggest that serial testing may be more useful for monitoring disease status than the natriuretic peptides.
Studies on the role of galectin-3 in therapeutic monitoring have been published. In the CORONA, there was no benefit in treating chronic heart failure patients NYHA II-IV with rosuvastatin when the galectin-3 concentration was high (>19 ng/mL) (Figure 1), suggesting that the damage had already been done. However, for patients with a low galectin-3 (<19 ng/mL), there was improvement in clinical outcomes with use of this statin (18). Galectin-3 monitoring may be especially useful for heart failure treated with aldosterone antagonists, as these drugs block cardiac fibrosis, the stimulus for galectin-3 release. The galectin-3 assay has been approved by both the FDA and CE Mark and an ELISA is commercially available.
Figure 1.
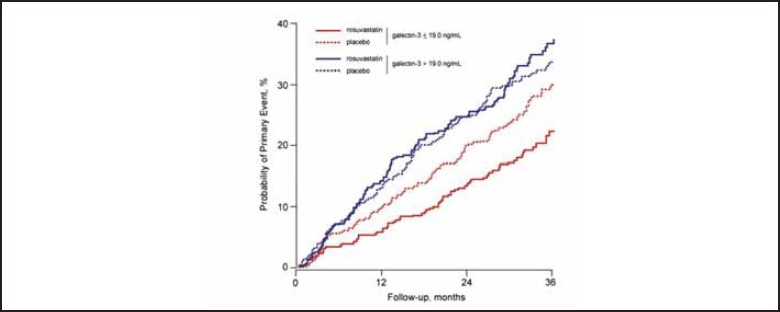
Probability of suffering an adverse event for patients with chronic heart failure according to galectin-3 levels and treated with rosuvastatin. Statistical difference was only observed for patients with low galectin-3. Used with permission from Oxford University Press. Gullestad et al. Eur Heart J 2012; doi:10.1093/eurheartj/ehs077.
SOLUBLE ST2
Soluble sST2 is part of the interleukin-1 receptor family and consists of two isoforms. The gene that encodes sST2 was found to be up-regulated following a cardiac cell culture model that was subjected to mechanical overload (19). Subsequently, the role of ST2 was discovered. The ST2L is a receptor with interleukin-33 (IL-33) as it’s ligand. Binding of IL-33 to the ST2L has cardio-protective effects, such as reduced fibrosis, reduced hypertrophy, and preserved ventricular function. Soluble ST2 circulates in blood and is a decoy receptor. Binding of IL-33 to sST2 reduces the availability of binding to ST2L thereby reducing the benefits of ST2L. Like galectin-3, increased concentrations of sST2 are associated with cardiac fibrosis and remodeling and adverse cardiac outcomes.
In patients with chronic heart failure, sST2 provides synergistic incremental value to BNP/NT-proBNP. Ky et al., examined soluble sST2 predicted death or heart transplantation among 1141 chronic heart failure patients (20). The combination of sST2 and NT-proBNP provided the highest area under the receiver operating characteristic curve while patients with an increase in both markers had the highest incidence of terminal heart failure requiring a cardiac transplant (Figure 2). As observed with galectin-3, serial changes in sST-2 is predictive of outcomes (Figure 3) (21). Tobias et al. measured sST2 levels at presentation and two days after a presentation of acute heart failure (22). Patients who died during the follow-up period had lower decrease in sST2 levels on day of their presentation than survivors (median change -18% vs. -37%, respectively). These investigators showed that a decrease in sST2 of >50% was indicative of therapeutic success with diuretics and beta blockers resulting in better survival than the group of heart failure patients who either had a decline of between 25-50% or who had a marginal decline or increase in serial sST2 results.
Figure 2.
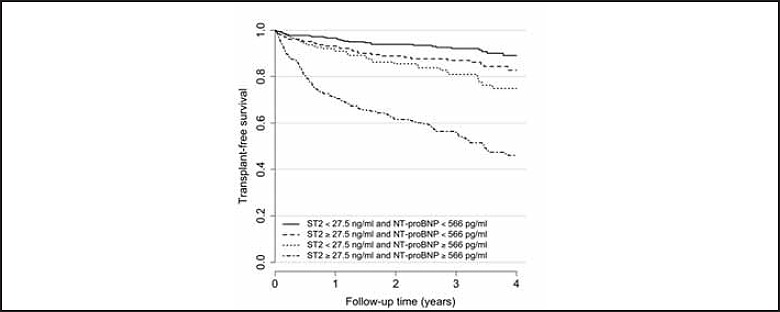
Comparison of sST2 and NT-proBNP for transplant-free survival of patients with chronic heart failure. Used with permission from Ky et al. Circ Heart Fail 2011;4:180-7.
Figure 3.
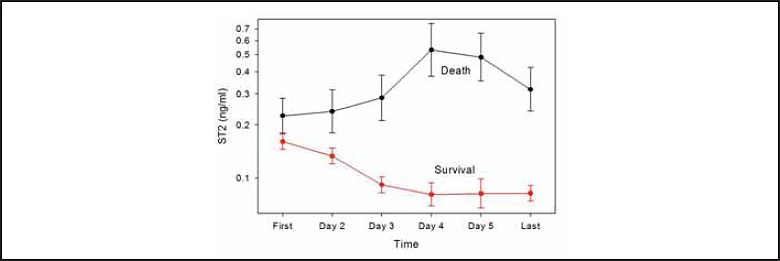
Change in sST2 predicts clinical outcomes. Used with permission from Elsevier Science Boisot et al. J Cardiac Fail 2008;14:732-8.
The RCV for sST2 at 29.8% is very low among all of the cardiac biomarkers studied to date (23), justifying the serial cutoffs used in the various clinical trials. The low RCV of sST2 increases the utility of serial measurements relative to markers that have a higher RCV (e.g., BNP). sST2, unlike the natriuretic peptides, has also been shown to be minimally influenced by renal dysfunction (24). The sST2 assay on an ELISA format has been cleared by the FDA and CE Mark.
DISCUSSION
There was a debate some twenty years ago about the advantages and disadvantages of cardiac troponin T vs. I for diagnosis of acute coronary syndromes and risk stratification for future adverse cardiac events. The availability of these tests was limited according to the access of the clinical laboratory to manufacturers of automated immunoassay analyzers. While there were some differences in the analytical sensitivity and specificity, and absolute biomarker results are not interchangeable between the biomarkers, the clinical value of both tests are largely equivalent. One decade ago, another debate was launched regarding the attributes of BNP versus NT-proBNP. It is largely accepted that the results of these biomarkers are largely equivalent for diagnosis of acute decompensated heart failure and risk stratification for future adverse cardiac events. For both the troponins and the natriuretic peptides, it is unnecessary for a clinical laboratory to offer both tests on the same patient population.
The next generation of debate among cardiac biomarkers may be between galectin-3 and sST2 for chronic heart failure disease management. Both markers are indicative of fibrosis formation and remodeling and are indicators of adverse cardiac events independent to the natriuretic peptides. Their potential utility has been described in a several applications within heart disease including diagnosis and risk stratification for acute coronary syndromes, acute decompensated heart failure, and chronic heart failure. Given the existing prominence of troponin and the natriuretic peptide in the first two of these categories, it is likely that galectin-3 and sST2 will be most useful in chronic heart failure disease management. Therefore head-to-head comparisons between the two tests in this scenario will need to occur in this population now that both tests are cleared by the FDA and CE Mark. In the end, it is likely that the availability within a clinical laboratory of one marker over the other will be determined by vendor and instrumentation access and not clinical performance because the two provide equivalent value.
References
- 1.Hall C. Essential biochemistry and physiology of (NT-pro) BNP. Eur J Heart Failure 2004;6:257-260. [DOI] [PubMed] [Google Scholar]
- 2.Maisel AS, Krishnaswamy K, Novak R, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161-167. [DOI] [PubMed] [Google Scholar]
- 3.Januzzi J, Camargo CA, Anwaruddin S, et al. The N-terminal pro-BNP Investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005;95:948-954. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J 2012;33:1787-1847. [DOI] [PubMed] [Google Scholar]
- 5.Mehra MR, Uber PA, Park MH, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol 2004;43:1590-1595. [DOI] [PubMed] [Google Scholar]
- 6.McCullough PA, Due P, Omland T, et al. B-Type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kid Diseases. 2003, 41:571-579. [DOI] [PubMed] [Google Scholar]
- 7.Wu AHB. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biological variation in interpretation of results. Am Heart J. 2006, 152:828-834. [DOI] [PubMed] [Google Scholar]
- 8.Fraser CG. Biological variation. From principles to practice. AACC Press, Washington DC: 2001. [Google Scholar]
- 9.Fokkema MR, Hermann Z, Muskiet FAJ, Moecks J. Reference change values for brain natriuretic peptides revisited. Clin Chem 2006;52:1602-1603. [DOI] [PubMed] [Google Scholar]
- 10.Wu AHB, Lu A, Todd J, Moecks J, Wians F. Short- and long-term biological variation in cardiac troponin I with a high-sensitivity assay: implications for clinical practice. Clin Chem 2009;55:52-58. [DOI] [PubMed] [Google Scholar]
- 11.Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:1086-1090. [DOI] [PubMed] [Google Scholar]
- 12.Malinowski B, Fulgheri G, Wicinski M, Grzesk E, Odrowaz-Sypniewska G, Grzesk G, Darwish D. Potential markers in cardiac hypertrophy? eJIFCC 2012;23:1-6. [PMC free article] [PubMed] [Google Scholar]
- 13.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Sem Oncol 1999;26(4 Suppl 12):78-83. [PubMed] [Google Scholar]
- 14.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121-3128. [DOI] [PubMed] [Google Scholar]
- 15.Lok DJA, Van Der Meer P, Bruggink PW, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data form the DEAL-HF study. Clin Res Cardiol 2010;99:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deBoer RA, van Veldhuisen DJ, Gullestad L, et al. Changes in galectin-3 levels over time predict mortality and morbidity in chronic HF. J Cardiac Fail 2011;17:S92. [Google Scholar]
- 17.Wu AHB, Wians FH. unpublished observations, 2012. [Google Scholar]
- 18.Gullestad L, Ueland T, Kjekshus J, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure. (CORONA). Eur Heart J 2012; doi:10.1093/eurheartj/ehs077 [DOI] [PubMed] [Google Scholar]
- 19.Weinberg EO, Shimpo M, De Keulenaer G, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002;106:2961-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail 2011;4:180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boisot S, Beede J, Isakson S, et al. Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J Cardiac Fail 2008;14:732-738. [DOI] [PubMed] [Google Scholar]
- 22.Briedthardt T, Socrates T, Drexler B, et al. Interleukin family member ST2 rapidly responds to heart failure treatment and its changes predict one-year mortality. Eur Heart J 2011;32:125. Abstract #P805.21239420 [Google Scholar]
- 23.Bieplinger B, Januzzi JL, Steinmair M, et al. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma. Clin Chim Acta 2009;409:33-40. [DOI] [PubMed] [Google Scholar]
- 24.Bayes-Genis A, De Antonio M, Gala A, et al. Relationship between high sensitivity ST2 levels and renal function in heart failure. Eur Heart J 2011;32:1092. Abstract #P5788. [Google Scholar]


