Abstract
National and international cardiology guidelines have recommended a 1-hour turnaround time for reporting results of cardiac troponin to emergency department personnel, measured from the time of blood collection to reporting. Use of point-of-care testing (POCT) can reduce turnaround times for cardiac markers, but current devices are not as precise or sensitive as central laboratory assays. The gap is growing as manufacturers of mainframe immunoassay instruments have or will release troponin assays that are even higher than those currently available. These assays have analytical sensitivity that enables detection of nearly 100% of all healthy subjects which is not possible for current POCT assays. Use of high sensitivity troponin results in a lower value for the 99th percentile of a healthy population. Clinically, this enables for the detection of more cases of myocardial injury. In order to compete analytically, next generation POCT assays will to make technologic advancements, such as the use of microfluidic to better control sample delivery, nanoparticles or nanotubes to increase the surface-to-volume ratios for analytes and antibodies, and novel detection schemes such as chemiluminescence and electrochemical detectors to enhance analytical sensitivity. Multi-marker analysis using POCT is also on the horizon for tests that complement cardiac troponin.
Key words: Cardiac troponin, B-type natriuretic peptides, point-of-care testing, microfluidics
BACKGROUND AND CURRENT POCT DEVICES
The National Academy of Clinical Biochemistry has recommended a 1-hour turnaround (TAT) time for reporting of cardiac troponin (cTn) results, beginning with sample collection and ending with reporting (1). When troponin testing is conducted from the central laboratory, this goal is a challenge for most laboratories to meet. The sample must be labeled (1-2 min), put into an appropriate transportation container (2-5 min), sent to the laboratory (5-10 min), accessioned (5-10 min), centrifuged (10 min), delivered to the testing laboratory (1-5 min), loaded (1-5 min) and tested (20 min), results reviewed where appropriate (0-5 min), and finally released to the caregivers. Due to the difficulty of meeting this aggressive turnaround time goals, the in vitro diagnostics industry has been challenged to produce point-of-care testing (POCT) devices that have equivalent analytical sensitivity for measuring cardiac troponin.
POCT for troponin has been available for nearly 20 years. Among the earliest commercial point-of-care devices were qualitative lateral flow assays (e.g., Spectral Diagnostics for cardiac troponin I and Roche for troponin T). Whole blood samples containing the targeted analyte flow through a filter which separates plasma from erythrocytes. It is also mixed with a detecting antibody. This combination flows past an immobilized capture zone containing a second troponin antibody. The presence of troponin in the sample causes a line to be visualized. Shortly after the creation of qualitative assays, quantitative assays were constructed using small optical readers (Roche Cardiac Reader for troponin T and Alere Triage for troponin I). The iSTAT (Abbott) was among the first point-of-care device to make use of microfluidics to navigate sample through the various zones of the device. There have also been bench-level whole-blood analyzers that can be used for near-patient testing such as the Stratus CS (Siemens), AQT-90 (Radiometer), and Fastpath (Mitsubushi). These instruments have higher analytical sensitivity and precision than the hand-held POCT devices, and are equal to those obtained from the central laboratory.
There have been several studies that have documented the reduction of turnaround times when point-of-care testing has been implemented in the emergency department versus the central laboratory (Table 1) (2-6). With POCT, each of these studies demonstrated compliance with the 1-hour guideline. In contrast, none of the tests done in the corresponding central laboratory met the guideline. While many laboratories today are able to meet 1-h turnaround times, if 30 minutes is necessary, as desired by some ED physicians, then POCT becomes the only option.
Table 1.
Published reports on the reduction of turnaround times using point-of-care testing
| Study | POCT | Assay | Central lab | Δ,↓ |
|---|---|---|---|---|
| McCord, et al. 2001 | 24 | Triage | 71 | 66% |
| Caragher et al., 2002 | 38 | Stratus CS | 87 | 56% |
| Lee-Lewandrowski et al. 2003 | 17 | Spectral | 110 | 85% |
| Collinson et al. 2004 | 20 | Triage | 79 | 75% |
| Singer et al. 2005 | 15 | Stratus CS | 83 | 85% |
| Mean | 23 | 89 | 79% |
“HIGH SENSITIVITY” TROPONIN ASSAYS FROM THE CENTRAL LABORATORY
The performance of central laboratory analyzers for troponin continues to undergo improvements with regards to analytical sensitivity and precision. Figure 1 shows that a significant amount of myocardial necrosis was required for detection of troponin using troponin assays that were first released into the market. Current generation troponin assays have about 10 fold higher analytical sensitivity and can detect the initial onset of myocardial infarction earlier. High-sensitivity troponin assays can detect normal levels and the earliest increases of troponin after myocardial infarction, micro necrosis, and possibly reversible ischemia as well.
Figure 1.
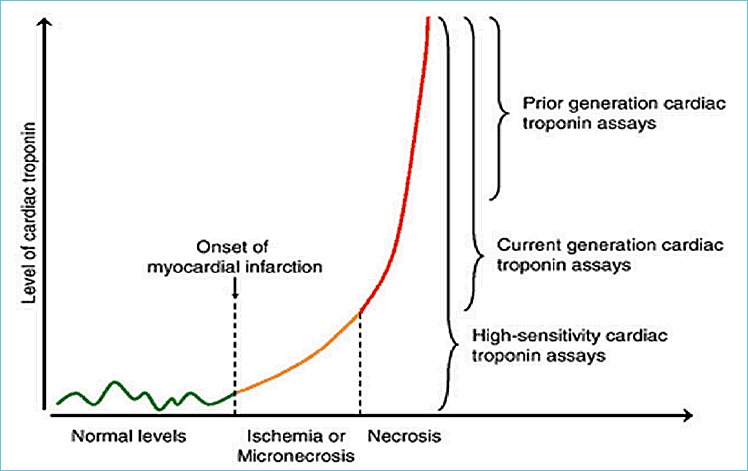
Analytical sensitivity improvements with different generations of troponin assays
There are several strategies that manufacturers have used to achieve the sensitivities needed to qualify as high sensitivity assays. These include use of longer incubation times, larger sample volumes, use of more than 2 antibodies for capture/detection, and use of chimeric antibodies to improve avidity towards the target protein. In terms of using longer incubation times, there to how long the analysis time can be practically extended. Increasing the overall analysis time from 20 to e.g. 40 minutes, is self-defeating, as the objective of POCT testing is reducing TATs. Increased sensitivity can be achieved by using additional antibodies and capturing troponin fragments where the epitope towards the primary antibodies have been lost. Troponin is known to degrade at both the C and N-terminus (7). Figure 2 illustrates the use of an additional antibody enables better detection of degraded fragments. Chimeric and humanized antibodies are formed by recombinant DNA techniques and are a mix of human and non-human antibody sequences. Use of these antibodies minimizes interference when human anti-mouse antibodies (HAMA) are present (8).
Figure 2.
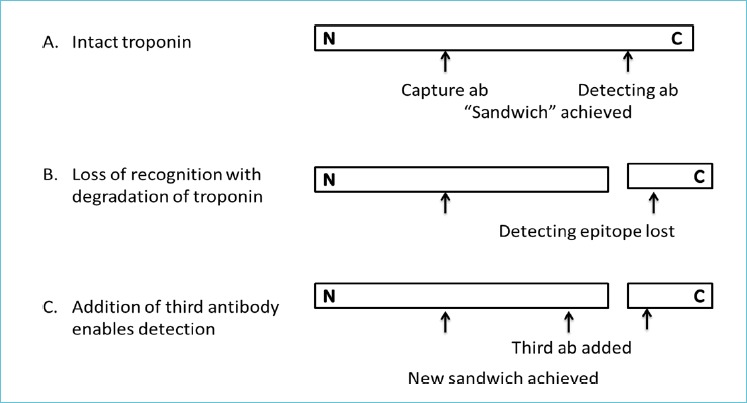
Increased sensitivity with the addition of a third antibody to the assay
GAP BETWEEN CURRENT POCT AND CENTRAL LAB ASSAYS FOR TROPONIN
Cardiac troponin is used for diagnosis of acute myocardial infarction (AMI) and risk stratification for future adverse cardiac events. Figure 3 illustrates the difference in analytical sensitivity between a POCT assay and the central laboratory (9). Singh et al. performed a direct comparison of a POCT assay versus the central lab (10). Out of 206 samples, there were 32 samples with discordant results. The majority of these discrepancies (82%) had a positive result on the central lab and negative result on the POCT assay. Improvements in the analytical sensitivity of troponin assays enable an early detection of AMI (11).
Figure 3.
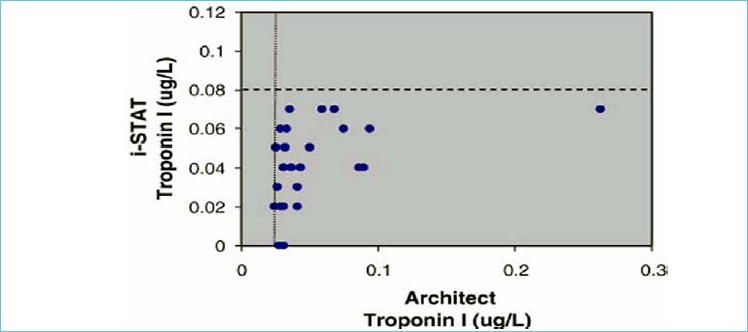
Discordant results on the iSTAT with positive results on the Architect
Improved sensitivity also allows the use of low cutoff concentration which improves the utility of troponin for risk stratification. Many studies have shown that patients who present with chest pain and have a minor increase in cardiac troponin have a higher incidence of adverse events (death, AMI) at 30 days and 1 year. James et al. compared the performance of a POCT cTnl assay against a central lab cTnT assay (12). The odds ratio for 30-day death or Ml was 1.64 (95% CI: 1.31-2.06) for the POCT assay and 4.29 (CI: 3.02-6.09) for the central lab assay. These reports suggest that there will be a compromise in clinical performance when POCT assays are used in lieu of the central laboratory. To overcome these limitations, some clinical laboratories send samples tested by POCT devices to the central laboratory for repeat testing. This can lead to confusion by the physicians, while increasing the cost for testing. An alternative approach is to develop POCT assays that are as sensitive as the central laboratory.
GOALS FOR NEXT GENERATION POCT ASSAYS
The definition of myocardial infarction underwent a dramatic change in 1999 with the adoption of troponin as the preferred biomarker (13), replacing the WHO definitions (14). This definition has undergone refinements. The Third Redefinition of Myocardial Infarction affirmed the concept that the cutoff concentration is established at the 99th percentile of a healthy population with assay imprecision of 10% or less (15). For the best assays currently available in the U.S., this equates to a cutoff of between 25-40 ng/L. Current POCT devices cannot meet this sensitivity. Near-patient instruments for troponin are able to match the sensitivity limit of these existing assays.
Next-generation high sensitivity (hs) troponin assays have been developed and are available outside the U.S. and from CLIA-certified reference laboratories as a lab developed test. These assays have a 99th percentile cutoff of about 10 ng/L and a limit of detection of <1 ng/L. High-sensitivity assays are able to detect troponin in the majority of healthy subjects (16). The analytical sensitivity of these assays may now be sufficient to meet current and future clinical needs, and further improvements in analytic sensitivity will be unnecessary. This is especially true because there is no clinical value for detecting troponin concentrations that are below the normal range. For POCT, these specifications for hs-cTn central laboratory assays are the goals for next-generation devices.
APPROACHES TOWARDS NOVEL POCT ASSAYS
Commercial interest in novel POCT assays stems from the fact that there are large numbers of patients who present to an emergency department each day with chest pain requiring troponin testing, and the existence of international guidelines that recommend a rapid turnaround time for reporting results. Unfortunately, developing POCT assays that meet these needs has been challenging. Described below are approaches that have been taken to improve POCT troponin testing (names of companies developing these prototype assays have been purposely omitted).
Microfluidics
Passive lateral flow technology is an inadequate means to deliver sample to the measurement zones, as this has been associated with high analytical imprecision. Differences in the viscosity of real blood samples due to variances in hemoglobin and protein content can limit precision, which impacts on analytical sensitivity. Next-generation POCT assays will most likely make use of microfluidic technology. Through the use of built-in pumps and valves, this advance enables precise movement of fluids and reagents from the point of sample application to the measurement zones within a POCT device. Washing of unbound antibodies or reagents can also be better controlled. Improved quality control schemes is possible including the testing of specific analytes that can be incorporated with each device instead of a simple check of fluid flow as is the case with lateral flow POCT. Due to its increased complexity however, the costs of microfluidic devices are higher than later flow technology.
Increased surface area-to-volume ratio for antibody-antigen reactions
All troponin assays are based on the reaction of the analyte with antibodies. Within the finite limits of the detection zone, the analytical sensitivity is a direct function of the ability of the assay to capture as much as the antigen as possible. The manufacturing of nanoparticles and nanotubes has become consistent and reliable. Their use in lieu of micro particles can greatly increase the surface-to-volume ratio over the micro particles used in central laboratory immunoassays. This enables the immobilization of a higher density of capture antibodies thereby retaining and detecting as much of the target analyte as possible.
Novel detection schemes
Visual detection of labeled gold micro particles has inherent sensitivity limitations. For the same number of troponin molecules captures, the use of other detection schemes can substantially increase the analytical sensitivity of troponin assays. Among the novel signal technologies used include fluorescence, chemiluminescence, and electrochemical detection. This migration to more sensitive technologies is consistent with the advancement made in the central laboratory where spectrophotometric measurements of immunoassays labeled with enzymes have given way towards chemiluminescence and electrochemistry. The challenge is to make miniaturize detectors so that they can be applicable to POCT. Relative to visual detection, these advanced detectors can increase the assay sensitivity 10-100 fold.
Connectivity advances
A disadvantage of current POCT assays is connectivity between measuring device and the patient’s medical records. The documentation and dissemination of test results is of critical importance to the effective delivery of testing. Central laboratory and bench-top satellite laboratory instruments can be directly interfaced to a laboratory or hospital information system. Hand-held devices require wireless transmission of data to the appropriate portals. The worldwide advance in telecommunications will allow caregivers direct access to secure medical information through pagers and smart phones. This will be a requirement for next-generation POCT devices.
Regulatory issues
In the U.S., all clinical assays must be approved by the Food and Drug Administration prior to routine clinical use or be validated as a “Laboratory Developed Test.” The FDA lists levels of complexity and requirements for utilization of tests. All central laboratory and POCT troponin assays are currently listed as “moderately complex tests.” This requires a certain degree of training and supervision of the testing personnel. “Waived test have less stringent requirements for testing personnel. Manufacturers of next-generation POCT troponin assays should consider seeking waived status for their devices. This will accelerate adoption of POCT in the ED. There is one POCT assay for BNP that is FDA cleared as a waived test. The history of its submission and approval could be a model for getting a next-generation POCT troponin assay cleared as a waived test.
On-vitro analysis
A unique concept for POCT could be “on-vitro” analysis. While in vitro refers to the testing environment outside the body and in vivo refers to studies within the body, the term “on vitro” could refer to a term whereby testing is conducted outside the body, but the device is placed on the skin of the patient. Blood is automatically sampled and tested within the device on demand or at regular intervals while worn. There are diagnostic companies on vitro devices for painless collection of blood, particularly for neonates. Samples contained within the device could be directed by microfluidics to test areas. On vitro diagnostic tests may be convenient and ideal for cardiac markers as serial testing is required for accurate diagnosis and rule out.
SUMMARY
The analytical sensitivity gap between central laboratory testing platforms and POCT assays for cardiac troponin is significant and has hindered the adoption of POCT for many hospitals. Although not discussed, there may also be a need for POCT platforms that can undergo multi-marker analysis. While troponin is the main analyte for AMI diagnosis, B-type natriuretic peptide (BNP) and NT-proBNP have shown to be useful for short-term risk stratification. There are also other biomarkers that can be used for the early rule out of AMI such as competing (17).
High sensitivity troponin might also be useful as a risk stratification marker in primary care, i.e., for patients who are asymptomatic (18). This is based on observations that increased troponin is associated with high risk for adverse cardiac outcomes in the absence of acute coronary syndromes (19). If this becomes adopted as part of routine medical care for high risk patients, then POCT for hs-cTn may be useful and convenient when tested in physician offices and clinics. Therapeutic measures such as the administration of statins, beta blockers or an angiotensin converting enzyme inhibitor can be prescribed before the patient leaves the office.
REFERENCES
- 1.Apple FS, Jesse RL, Newby LK, Wu AHB, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biomarkers of acute coronary syndromes. Clin Chem 2007;53:547-551. [DOI] [PubMed] [Google Scholar]
- 2.McCord J, Nowak RM, McCullough PA, Foregack C, Borzak S, Tokarski G, Tomlanovich MC, Jacobsen G, Weaver WD. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 2001;104:1483-1488. [DOI] [PubMed] [Google Scholar]
- 3.Caragher TE, Fernandez BB, Jacogs FL, Barr LA. Evaluation of quantitative cardiac biomarker point-of-care testing in the emergency department. J Emerg Med 2002;22:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Lee-Lewandrowski E, Corboy D, Lewandrowski K, Sinclair J, McDermot S, Benzer TL. Implementation of a point-of-care satellite laboratory in the emergency department of an academic medical center. Impact on test turnaround time and patient emergency department length of stay. Arch Pathol Lab Med 2003;127:456-460. [DOI] [PubMed] [Google Scholar]
- 5.Collinson PO, John C, Lynch S, Rao A, Canepa-Anson R, Carson E, Cramp D. A prospective randomized controlled trial of point-of-care testing on the coronary care unit. Ann Clin Biochem 2004;41:397-404. [DOI] [PubMed] [Google Scholar]
- 6.Singer AJ, Ardise J, Gulla J, Cangro J. Point-of-care testing reduces length of stay in emergency department chest pain patients. Ann Emerg Med 2005;45:587-591. [DOI] [PubMed] [Google Scholar]
- 7.Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation 2000;102:1221-1226. [DOI] [PubMed] [Google Scholar]
- 8.Hosono M, Endo K, Sakahara H, Wantanabe Y, Saga T, Nakai X, et al. Human/mouse chimeric antibodies show low reactivity with human anti-murine antibodies (HAMA). Br J Cancer 1992;65:197-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochholzer W, Morrow DA, Giugliano RP. Novel biomarkers in cardiovascular disease: update 2010. Am Heart J 2010;160:583-564. [DOI] [PubMed] [Google Scholar]
- 10.Singh J, Akbar MS, Adabag S. Discordance of cardiac troponin I assays on the point-of-care i-STAT and Architech assays from Abbott Diagnostics. Clin Chim Acta 2009;403:359-360. [DOI] [PubMed] [Google Scholar]
- 11.Melanson SEF, Morrow DA, Jarolim P. Earlier detection of myocardial injury in a preliminary evaluation using a new troponin I assay with improved sensitivity. Am J Clin Pathol 2007;128:282-286. [DOI] [PubMed] [Google Scholar]
- 12.James SK, Lindahl B, Armstrong P, Califf R, Simoons ML, Venge P, Wallentin L. A rapid troponin I assay is not optimal for determination of troponin status and prediction of subsequent cardiac events at suspicion of unstable coronary syndromes. Int J Cardiol 2004;93:113-120. [DOI] [PubMed] [Google Scholar]
- 13.The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined – A consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959-969. [DOI] [PubMed] [Google Scholar]
- 14.Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization Task Force on standardization of clinical nomenclature. Circulation 1979;59:607-609. [DOI] [PubMed] [Google Scholar]
- 15.Thygessen K, Alpert JS, Jaffe A, Simoons ML, Chait-man BR, White HD, et al. Third universal redefinition of myocardial infarction. Euro Heart J. 2012;33:2551-2567. [DOI] [PubMed] [Google Scholar]
- 16.Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem 2009;55:303-306. [DOI] [PubMed] [Google Scholar]
- 17.Maisel A, Mueller C, Neath SX, Christenson RH, Mor-genthaler NG, McCord J, et al. Copeptin Helps in the Early Detection of Patients With Acute Myocardial Infarction: Primary Results of the CHOPIN Trial (Copeptin Helps in the early detection Of Patients with acute myocardial INfarction). J Am Coll Cardiol. 2013;62:150-160. [DOI] [PubMed] [Google Scholar]
- 18.Wu AHB, Christenson RH. Analytical and assay issues for use of cardiac troponin testing for risk stratification in primary care. Clin Biochem 2013;46:969-978. [DOI] [PubMed] [Google Scholar]
- 19.deFilippi CR, deLemos JA, Christenson RH, Gottdiener JS, Kop WJ, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2492-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]


