Abstract
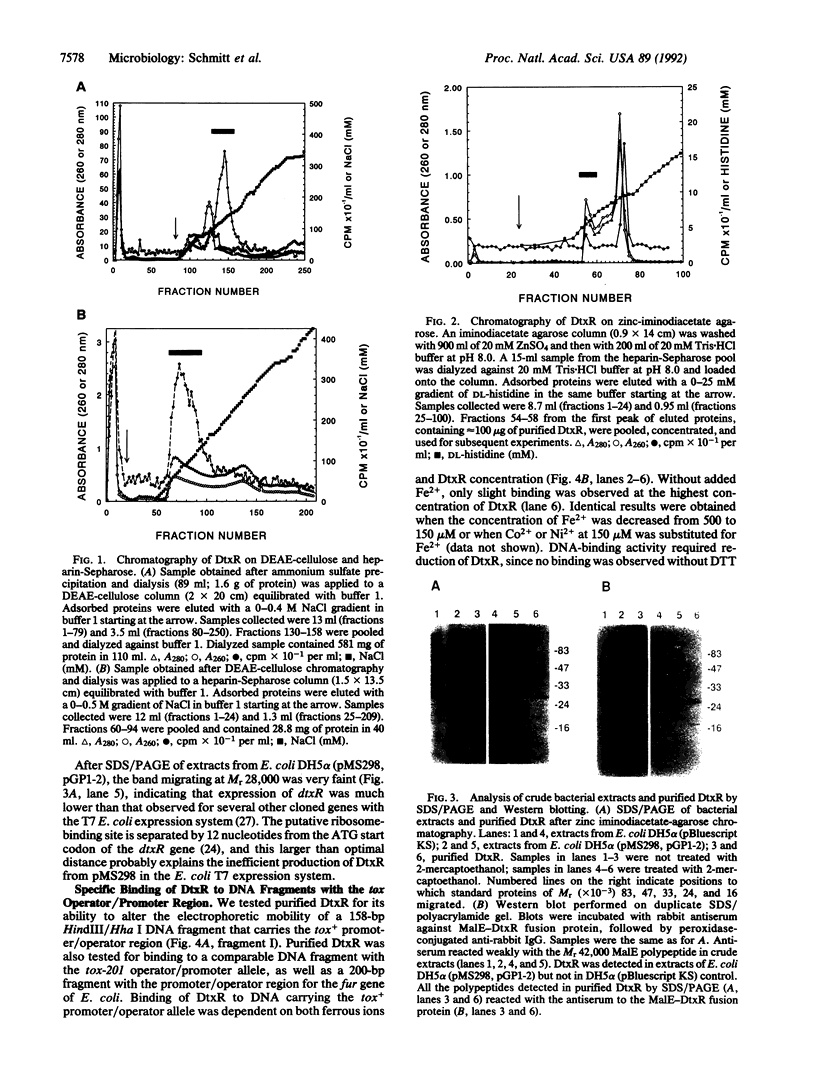
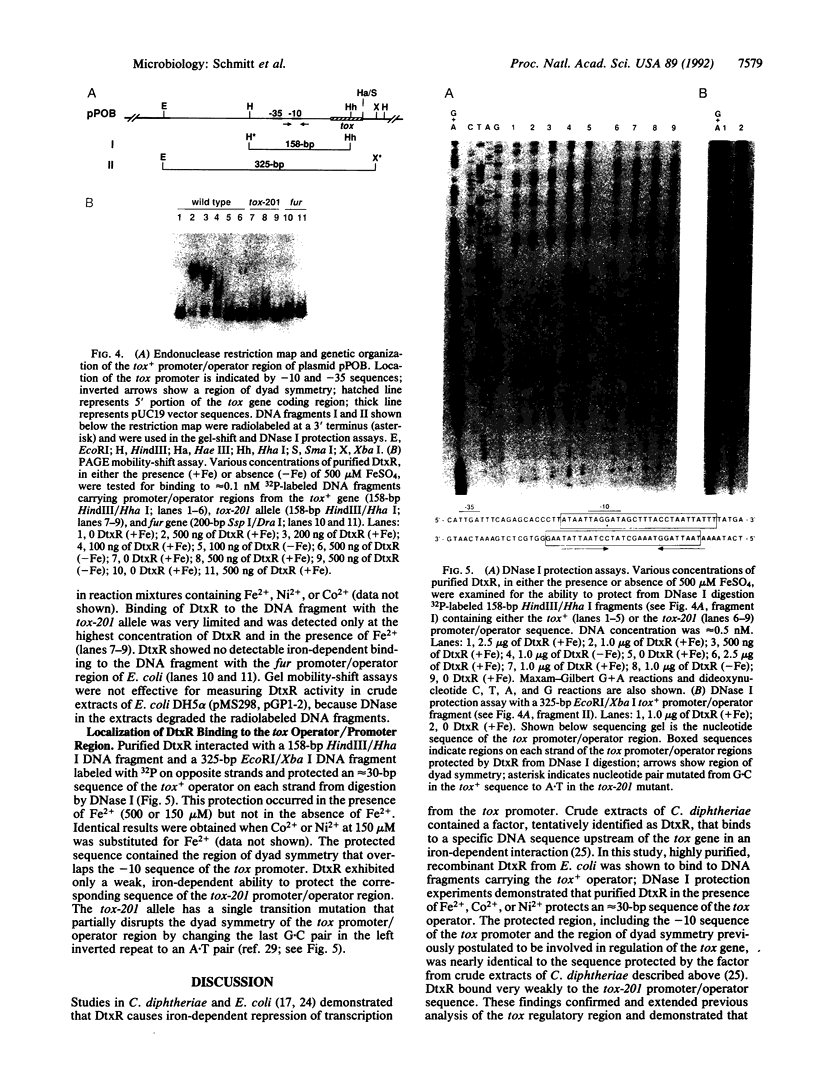
The diphtheria toxin repressor gene (dtxR) encodes a protein (DtxR) that regulates transcription of the diphtheria toxin gene (tox) by an iron-dependent mechanism. Cloned dtxR was expressed in Escherichia coli from the phage T7 gene 10 promoter, and DtxR was purified. Specific binding of DtxR to the tox+ operator was dependent on reduction of DtxR and the presence of ferrous ions. DtxR protected a sequence of approximately 30 nucleotide pairs, partially overlapping the tox promoter and containing a region of dyad symmetry, from digestion by DNase I. DtxR exhibited very little binding to the mutant tox-201 operator region and failed to bind to the promoter/operator region of the ferric uptake regulation (fur) gene of E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J., Murphy J. R. Analysis of the diphtheria tox promoter by site-directed mutagenesis. J Bacteriol. 1988 Dec;170(12):5949–5952. doi: 10.1128/jb.170.12.5949-5952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J., Oza M. N., Murphy J. R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramucci M. G., Twiddy E. M., Baine W. B., Holmes R. K. Isolation and characterization of hypertoxinogenic (htx) mutants of Escherichia coli KL320(pCG86). Infect Immun. 1981 Jun;32(3):1034–1044. doi: 10.1128/iai.32.3.1034-1044.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Russell L. M., Holmes R. K. Regulation of toxinogenesis in Corynebacterium diphtheriae: mutations in the bacterial genome that alter the effects of iron on toxin production. J Bacteriol. 1983 Apr;154(1):245–252. doi: 10.1128/jb.154.1.245-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- Fourel G., Phalipon A., Kaczorek M. Evidence for direct regulation of diphtheria toxin gene transcription by an Fe2+-dependent DNA-binding repressor, DtoxR, in Corynebacterium diphtheriae. Infect Immun. 1989 Oct;57(10):3221–3225. doi: 10.1128/iai.57.10.3221-3225.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. G., Crothers D. M. CAP and RNA polymerase interactions with the lac promoter: binding stoichiometry and long range effects. Nucleic Acids Res. 1983 Jan 11;11(1):141–158. doi: 10.1093/nar/11.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N. B. Conversion by corynephages and its role in the natural history of diphtheria. J Hyg (Lond) 1984 Dec;93(3):405–417. doi: 10.1017/s0022172400065001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C., Aggarwal A. K. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. doi: 10.1146/annurev.bi.59.070190.004441. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Twiddy E. M. Characterization of monoclonal antibodies that react with unique and cross-reacting determinants of cholera enterotoxin and its subunits. Infect Immun. 1983 Dec;42(3):914–923. doi: 10.1128/iai.42.3.914-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorek M., Delpeyroux F., Chenciner N., Streeck R. E., Murphy J. R., Boquet P., Tiollais P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science. 1983 Aug 26;221(4613):855–858. doi: 10.1126/science.6348945. [DOI] [PubMed] [Google Scholar]
- Kanei C., Uchida T., Yoneda M. Isolation from corynebacterium diphtheriae C7(beta) of bacterial mutants that produce toxin in medium with excess iron. Infect Immun. 1977 Oct;18(1):203–209. doi: 10.1128/iai.18.1.203-209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Murphy J. R. Characterization of the diphtheria tox transcript in Corynebacterium diphtheriae and Escherichia coli. J Bacteriol. 1985 Sep;163(3):1114–1119. doi: 10.1128/jb.163.3.1114-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Skiver J., McBride G. Isolation and partial characterization of a corynebacteriophage beta, tox operator constitutive-like mutant lysogen of Corynebacterium diphtheriae. J Virol. 1976 Apr;18(1):235–244. doi: 10.1128/jvi.18.1.235-244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Ratti G., Rappuoli R., Giannini G. The complete nucleotide sequence of the gene coding for diphtheria toxin in the corynephage omega (tox+) genome. Nucleic Acids Res. 1983 Oct 11;11(19):6589–6595. doi: 10.1093/nar/11.19.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Holmes R. K. Characterization of a defective diphtheria toxin repressor (dtxR) allele and analysis of dtxR transcription in wild-type and mutant strains of Corynebacterium diphtheriae. Infect Immun. 1991 Nov;59(11):3903–3908. doi: 10.1128/iai.59.11.3903-3908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Holmes R. K. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect Immun. 1991 Jun;59(6):1899–1904. doi: 10.1128/iai.59.6.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer S., Hantke K., Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200(1):110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S. P., Holmes R. K. Iron regulation of the cloned diphtheria toxin promoter in Escherichia coli. Infect Immun. 1988 Sep;56(9):2430–2436. doi: 10.1128/iai.56.9.2430-2436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S. P., Krafft A. E., Nootheti P., Holmes R. K. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb Pathog. 1990 Oct;9(4):267–273. doi: 10.1016/0882-4010(90)90015-i. [DOI] [PubMed] [Google Scholar]
- Uchida T., Gill D. M., Pappenheimer A. M., Jr Mutation in the structural gene for diphtheria toxin carried by temperate phage . Nat New Biol. 1971 Sep 1;233(35):8–11. doi: 10.1038/newbio233008a0. [DOI] [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E., GLADSTONE G. P. The neurotoxin of Shigella shigae. III. The effect of iron on production of the toxin. Br J Exp Pathol. 1953 Apr;34(2):221–229. [PMC free article] [PubMed] [Google Scholar]
- Wee S., Neilands J. B., Bittner M. L., Hemming B. C., Haymore B. L., Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Met. 1988;1(1):62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Holmes R. K. Regulation of toxinogenesis in Corynebacterium diphtheriae. I. Mutations in bacteriophage beta that alter the effects of iron on toxin production. J Virol. 1981 Mar;37(3):936–945. doi: 10.1128/jvi.37.3.936-945.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]






