Abstract
The goal of standardization in Laboratory Medicine is to achieve comparable results in human samples, independent of the reagent kits, instruments, and laboratory where the assay is carried out. To pursue this objective in clinical enzymology, the IFCC has established reference measurement systems for the most important clinical enzymes. These systems are based on the following requirements: a) reference methods, well described in procedures that are extensively evaluated; b) suitable reference materials; and c) reference laboratories operating in a highly controlled manner. Using these reference systems and the manufacturer’s standing procedures, industry can assign traceable values to commercial calibrators. Clinical laboratories, which use routine procedures with validated calibrators to measure enzymes in human specimens, can finally obtain values which are traceable to higher-order reference procedures. These reference systems constitute the structure of the traceability chain to which the enzyme routine methods can be linked via an appropriate calibration process, provided that they have a comparable specificity (i.e. they are measuring the same quantity).
Achieving interlaboratory agreement of enzyme activity measurements represents one of the most important standardization efforts in Laboratory Medicine (1). The fact that the determinations of some enzymes are among the most frequently ordered tests in clinical laboratories emphasizes the importance of standardised measurement results in practice. These enzymatic determinations are indeed important biochemical parameters for the diagnosis and monitoring of diseases of liver, pancreas, skeletal muscle, bone, etc. (2).
Measuring enzymes, variability of the results among laboratories is often observed. In 2002, the Institute for Reference Materials and Measurements (IRMM) surveyed approximately 900 global laboratories in an International Measurement Evaluation Program for two commonly measured enzymes in human serum [γ-glutamyltransferase (GGT) and α-amylase (AMY)]. Results for GGT showed biases of -60% to +30% and results for AMY showed a deviation from the enzyme certified value ranging from -50% to >250%! This large variation of results among laboratories may easily lead to a loss of information for clinicians.
The catalytic activity of an enzyme is a property measured by the catalyzed rate of reaction, produced in a specific assay system, and is not an amount of substance. If the components of the reaction system (e.g., pH and buffer, temperature, presence of activators and inhibitors, substrate nature and concentration) are changed, the magnitude of the measured activity will also change (3). Therefore, the numerical results of catalytic activity measurements depend entirely on the experimental conditions under which the measurements are made. Consequently, two procedures that measure the catalytic activity of the same enzyme but under different analytical conditions may produce different results for a given sample.
One way to improve the comparability of enzyme results can therefore be through the widespread use of standardized analytical methods (“method globalization”) (4). With this goal in mind, from the 1970s national and international expert panels have carried out fundamental research work to determine the optimized conditions for measurement of the catalytic activity of several enzymes in human serum. Recommended measurement procedures were defined, which gave optimum reaction conditions, such as substrate concentration, pH and buffer concentration. However, this approach for enzyme standardization has shown insurmountable limitations and the goal of a single, universal method to measure the catalytic concentration of a given enzyme in daily practice has not be achieved (5).
Therefore, the efforts to standardize enzyme measurements should obtain result comparability for human serum samples independent of the test kits and instruments used. To achieve this goal one approach is needed for a reliable transfer of the trueness of measurement values from a higher-order method to methods which are routinely used in the laboratories. Such a “reference measurement system” (RMS) approach is based on the concepts of metrological traceability and a hierarchy of measurement procedures (6,7). In applying the RMS theory, enzymes represent a special class of analytes. They are defined in terms of the so-called “catalytic amount”, which is the amount of an agree-upon substrate converted to product in an agreed-upon measurement system. Compared with other analytes, the numerical results of catalytic activity measurements depend entirely on the experimental conditions under which the measurements are made. In the standardization of enzyme assays, therefore, a reference measurement procedure, which defines the conditions under which a given enzyme activity is measured, occupies the highest level of the traceability chain (8). In addition to the reference measurement procedure the system also requires reference materials for the intermediate transfer of values from the reference procedure to the routine laboratory assays. Once the reference material is certified, this material and the manufacturer’s standing procedure can be used by industry to assign values to commercial calibrators. Routine clinical laboratories that use commercial methods and validated calibrators to measure patient samples in turn obtain values traceable to the reference measurement procedure and independent of the particular method or instrument, finally permitting the globalization of the enzyme results (Figure 1) (9).
Figure 1.
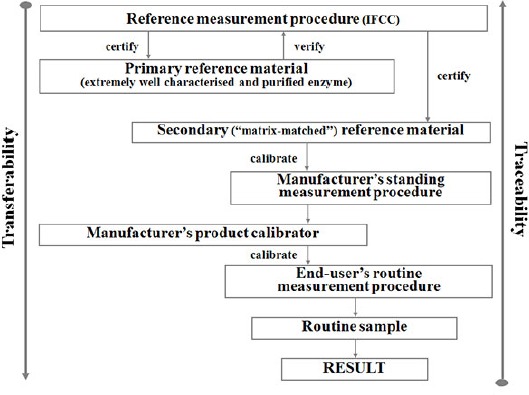
The reference measurement system for enzyme measurement.
The applicability of the enzyme RMS concept is possible only if the reference materials used to transfer trueness to the field methods are commutable and if the reference measurement procedure and corresponding lower-order routine methods have identical, or at least very similar, specificities for the measured enzyme. Commutability in enzymology has been defined as “the ability of an enzyme [reference or control] material to show interassay activity changes similar to those of the same enzyme in human serum” (10). To assure complete traceability, the reference material used to align manufacturers’ measurement procedures must be commutable by the reference measurement procedure and each of the commercial methods for which this common reference material is to be used as the master calibrator. Calibration of commercial methods with non-commutable reference materials can cause worse, rather than improved, agreement of results among methods for clinical samples. Commutability of reference materials can be affected by many factors including the source of material, purification procedure, matrix of the solution, lyophilisation, and addition of stabilizers or other additives (5). If commutable reference materials suitable for the direct calibration of field methods are lacking, a panel of native human sera, with values certified by the reference measurement procedure and acting as a secondary reference material, represents the only possible alternative for establishing traceability to the RMS (9). Calibration of the commercial system must be in accordance with correlation results obtained using the value-assigned human samples (11).
Apart from the need for commutability of secondary reference materials, the practical implementation of standardization in enzymology through a RMS also requires that the commercial methods have similar analytical specificities toward the specific enzyme when compared to the reference procedure. For example, it will not be possible to definitively align procedures for transaminases (ALT or AST) that do not incorporate pyridoxal-5′-phosphate to a procedure that does, such as the IFCC reference procedure, because the ratio of preformed holoenzyme to apoenzyme differs among human serum samples.
To specifically promote the establishment of RMSs in clinical enzymology in 1997 the IFCC created a Working Group that few years later was upgraded to a Committee. The first objective of the group was to select suitable primary reference measurement procedures to underpin trueness of the system and traceability of measurement results. A decision was taken to modify, when existing, the original IFCC methods recommended in 1980s for the measurement of catalytic concentrations of enzymes at 30 °C, avoiding, however, sample blanking and changing the reaction temperature to 37 °C, with a thorough re-evaluation of incubation times and linearity. The measurement conditions should be described in the form of detailed standard operating procedures (SOPs) and, in order to achieve low levels of measurement uncertainty, careful control of all metrological aspects related to gravimetry, volumetry, pH, reaction temperature, and photometry has to be promoted (Table 1).
Table 1.
Aspects to be carefully controlled in performing reference measurement procedures for enzymes
|
The resulting uncertainty for all relevant steps of the analytical procedures can therefore be known and this permits to keep under control all major components of the uncertainty budget. IFCC reference methods for the measurement of creatine kinase (CK), lactate dehydrogenase (LDH), ALT, AST, GGT, and AMY are now available (12-17). The IFCC Committee is also working on a candidate reference procedure for alkaline phosphatase (ALP), which is ready for approval and publication, and on a concept for the development of a reference method for pancreatic lipase.
From the beginning, reference procedures have been validated and tested for transferability in a network of reference laboratories. Under the patronage of the IFCC, a worldwide group of laboratories was selected to provide the necessary skill and equipment for performing measurements following the established SOPs. Some of these laboratories are now listed in the database of the Joint Committee for Traceability in Laboratory Medicine (JCTLM) and are able to deliver a reference measurement service to interested customers according to the specified analytical requirements for reference procedures, thus providing results within specified narrow limits of uncertainty (18).
After the first step of enzyme standardization consisting of the development of new primary reference measurement procedures and establishment of reference laboratories, the improvement of consistency of results may be reached using appropriate reference materials for trueness transfer to lower hierarchical level. Initially, the IFCC and IRMM cooperated to certify the reference materials for GGT, LDH, ALT, CK and AMY, previously prepared by the Community Bureau of Reference of the European Union (19). The results obtained from different reference laboratories agreed within very narrow limits, so that the values assigned to the five reference materials carried a very low uncertainty (from 1.4% for LDH to approximately 4% for CK). More recently, a reference material for AST has been released, after a certification campaign involving 12 reference laboratories. The characteristics of the IFCC/IRMM enzyme reference materials are shown in Table 2.
Table 2.
Characteristics of the enzyme reference materials certified by the IFCC in cooperation with the Institute for Reference Materials and Measurements (IRMM)
| Enzyme | Code | Origin | Form | Certified concentration | Uncertainty |
|---|---|---|---|---|---|
| GGT | ERM-AD452 | Pig kidney | Light subunit | 114.1 U/L | ±2.4 U/L |
| LDH | ERM-AD453 | Human erythrocytes | LDH1 isoenzyme | 502.0 U/L | ±7.0 U/L |
| ALT | ERM-AD454 | Pig heart | - | 186.0 U/L | ±4.0 U/L |
| CK | ERM-AD455 | Human heart | MB isoenzyme | 101.0 U/L | ±4.0 U/L |
| AMY | IRMM/IFCC 456 | Human pancreas | Pancreatic isoenzyme | 546.0 U/L | ±18.0 U/L |
| AST | ERM-AD457 | Recombinant | Liver cytosolic isoenzyme | 104.6 U/L | ±2.7 U/L |
As said before, the commutability of the materials intended to be used for calibration of commercial systems is a key issue and the main criterion required for the transfer of trueness to routine enzyme methods. For the reported reference materials, commutability has only been shown for a restricted number of methods and additional information is still needed before to use them to ensure result comparability for certain methodologies, for instance procedures using dry chemistry. Furthermore, these monoenzyme reference materials are available in limited amounts, are relatively expensive to purchase, and therefore cannot be used routinely to calibrate enzyme assays. Additional multienzyme materials that behave in a similar manner to human samples would, therefore, be very useful (20).
A further issue associated with the standardization efforts is the need to develop scientifically sound and globally useful reference intervals for serum enzyme catalytic concentrations. Lack of proper reference intervals may indeed hamper the implementation of standardization in enzymology as: a) the implementation of standardization can modify the enzyme results, b) without adequate reference intervals this situation can impair the interpretation of the results and, paradoxically, worsen the patient’s outcome, c) the absence of reliable reference intervals for the newly standardized commercial methods may hamper their adoption, and d) usually, a single clinical laboratory or manufacturer have not enough means to adequately produce reference limits. Reference intervals obtained with analytical procedures producing results traceable to the corresponding RMS can actually be transferred among laboratories (becoming “common”), providing that they use commercial assays giving results traceable to the same RMS and populations have the same characteristics or, alternatively, it is known that the specific enzyme is not influenced by ethnicity or environment (21). The definition of common reference intervals should hopefully cause disappearance of different intervals employed for the same enzyme, providing to clinicians more congruent and effective information. Some preliminary examples of common reference intervals for enzymes can already be found in literature. In Caucasian subjects, the reference interval for CK was found to be 46 to 171 U/L for males and 34 to 145 U/L for females when measured with an assay traceable to the IFCC 37 °C reference procedure (22). In another study, the reference interval for GGT activity in adult men was 11-49 U/L, when data were produced with three assays traceable to the IFCC reference procedure (23). Finally, the reference interval for LDH activity in adult Caucasian subjects, determined at 37 °C with a procedure traceable to the IFCC reference method, was found to be 125 to 220 U/L (24). Large multicentre studies are, however, needed for the robust definition of common reference intervals using a protocol for collaborative experiments including well defined prerequisites (21). Particularly, in the production of reference intervals, the employed methods must produce results traceable to the RMS for that specific enzyme. For this reason, the trueness of participating laboratories should be verified and, if necessary, experimental results corrected in accordance with correlation results with the reference procedure. Alternatively, the samples from reference individuals can be collected in the different centers, frozen and shipped to a central laboratory, where all the enzymatic analyses are performed. The latter approach is simpler, allows a better control of the analytical phase, but uses frozen samples, thus introducing a variable not typical for the clinical laboratories that may pose some doubts especially for more unstable enzymes, e.g. ALT.
In the European Union the implementation of result traceability in Laboratory Medicine to available RMSs is mandatory by law (25). However, the introduction of correctly standardized assays in enzymology is a complicated task. Overall, it appears that in many cases method bias can be reduced by better calibration to the internationally accepted reference systems, even if commercial assays using methodological principles that differ in analytical specificity when compared with the internationally recommended RMSs should be replaced by analytical procedures in which the traceability of calibration to the corresponding IFCC reference measurement procedure has been experimentally proven (26). In 2006, a study involving 70 European laboratories assessed enzyme assays from six major manufacturers for traceability to IFCC RMSs through a commutable serum-based material targeted with ALT, AST, CK, GGT, LDH, and AMY reference procedures (27). Results from commercial methods were assessed by a system using a maximum allowable error derived from the desirable analytical performance that is based on the biological variation model. Of these enzyme measurements, CK and ALT results were very good. For AST and GGT, only two company systems would fully comply. Finally, LDH and AMY measurements had still major drawbacks, suggesting need of major improvement. This was mainly the result of using methods with different analytical specificity for these enzymes, obtaining results that were not traceable to the internationally accepted RMS. In some cases, diagnostic companies may still prefer to produce enzyme methods with non-IFCC traceable calibrations and permit laboratories to choose between the different marketed assays and/or calibrations, of which some are clearly not traceable to the corresponding RMS.
Although a more correct implementation of the RMS concept by the manufacturers is required, it is responsibility of our profession to verify the accuracy and comparability of the commercially available enzyme methods (Table 3).
Table 3.
Major steps in the achievement of standardization of enzyme measurements
|
A major role must be accomplished by the External Quality Assessment Scheme (EQAS) organisers through the use of commutable materials with values assigned by IFCC reference procedures. True value assignment to EQAS materials allows objective evaluation of the performance of enzyme measurements, through an accuracy-based (instead of inferior consensus-based) grading of the competency of participating clinical laboratories by applying clinically allowable total error limits derived from biological variability data (Table 4).
Table 4.
Clinically allowable total errors for measurements of diagnostically important enzymes.
Total error goals were calculated as: bias goal + (1.96 x imprecision goal). Bias and imprecision goals (desirable and optimum) were derived from intraindividual and interindividual biological variabilities (available at http://www.westgard.com/biodatabase1.htm) of the respective enzymes, according to (28).
| Quality level | ||
|---|---|---|
| Desirable | Optimum | |
| AST | ±17.2% | ±8.5% |
| ALT | ±35.9% | ±17.9% |
| GGT | ±24.3% | ±12.2% |
| LDH | ±12.7% | ±6.3% |
| CK | ±33.8% | ±17.0% |
| AMY | ±16.0% | ±8.0% |
In conclusion, the establishment of traceability to enzyme RMSs may provide a means of ensuring result comparability that can be obtained without disruptive changes to existing working methods or to an individual laboratory’s preference for an analytical system.
References
- 1.Panteghini M, Forest JC. Standardization in laboratory medicine: new challenges. Clin Chim Acta 2005;355:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Panteghini M, Bais R, van Solinge WW. Enzymes. Burtis CA, Ashwood ER, Bruns DE, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. Philadelphia, PA: Elsevier Saunders, 2006:597-643. [Google Scholar]
- 3.Bais R, Panteghini M. Principles of clinical enzymology. Burtis CA, Ashwood ER, Bruns DE, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. Philadelphia, PA: Elsevier Saunders, 2006:191-218. [Google Scholar]
- 4.Schmidt ES, Schmidt FW. Standardization by reference methods: a German viewpoint. Clin Chim Acta 1988;173:9-18. [DOI] [PubMed] [Google Scholar]
- 5.Panteghini M, Ceriotti F, Schumann G, Siekmann L. Establishing a reference system in clinical enzymology. Clin Chem Lab Med 2001;39:795-800. [DOI] [PubMed] [Google Scholar]
- 6.Panteghini M. Traceability, reference systems and result comparability. Clin Biochem Rev 2007;28:97-104. [PMC free article] [PubMed] [Google Scholar]
- 7.Panteghini M. Traceability as a unique tool to improve standardization in laboratory medicine. Clin Biochem 2009;42:236-240. [DOI] [PubMed] [Google Scholar]
- 8.ISO 18153:2003. In vitro diagnostic medical devices — Measurement of quantities in biological samples — Metrological traceability of values for catalytic concentration of enzymes assigned to calibrators and control materials. ISO, Geneva, Switzerland. [Google Scholar]
- 9.Infusino I, Bonora R, Panteghini M. Traceability in clinical enzymology. Clin Biochem Rev 2007;28:155-161. [PMC free article] [PubMed] [Google Scholar]
- 10.Rej R. Accurate enzyme activity measurements. Two decades of development in the commutability of enzyme quality control materials. Arch Pathol Lab Med 1993;117:352-364. [PubMed] [Google Scholar]
- 11.Lasky FD. Achieving accuracy for routine clinical chemistry methods by using patient specimen correlations to assign calibrator values. A means to managing matrix effects. Arch Pathol Lab Med 1993;117:412-419. [PubMed] [Google Scholar]
- 12.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 2. Reference procedure for the measurement of catalytic concentration of creatine kinase. Clin Chem Lab Med 2002;40:635-642. [DOI] [PubMed] [Google Scholar]
- 13.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 3. Reference procedure for the measurement of catalytic concentration of lactate dehydrogenase. Clin Chem Lab Med 2002;40:643-648. [DOI] [PubMed] [Google Scholar]
- 14.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin Chem Lab Med 2002;40:718-724. [DOI] [PubMed] [Google Scholar]
- 15.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin Chem Lab Med 2002;40:725-733. [DOI] [PubMed] [Google Scholar]
- 16.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 6. Reference procedure for the measurement of catalytic concentration of γ-glutamyltransferase. Clin Chem Lab Med 2002;40:734-738. [DOI] [PubMed] [Google Scholar]
- 17.Schumann G, Aoki R, Ferrero CA, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 8. Reference procedure for the measurement of catalytic concentration of α-amylase. Clin Chem Lab Med 2006;44:1146-1155. [DOI] [PubMed] [Google Scholar]
- 18. http://www.bipm.org/en/committees/jc/jctlm/jctlm-db/
- 19.Siekmann L, Bonora R, Burtis CA, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 7. Certification of four reference materials for the determination of enzymatic activity of γ-glutamyltransferase, lactate dehydrogenase, alanine aminotransferase and creatine kinase according to IFCC reference procedures at 37 °C. Clin Chem Lab Med 2002;40:739-745. [DOI] [PubMed] [Google Scholar]
- 20.Scharnhorst V, Apperloo J, Baadenhuijsen H, Vader HL. Multicenter evaluation of the commutability of a potential reference material for harmonization of enzyme activities. Clin Chem Lab Med 2004;42:1401-1407. [DOI] [PubMed] [Google Scholar]
- 21.Ceriotti F, Hinzmann R, Panteghini M. Reference intervals: the way forward. Ann Clin Biochem 2009;46:8-17. [DOI] [PubMed] [Google Scholar]
- 22.Schumann G, Klauke R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta 2003;327:69-79. [DOI] [PubMed] [Google Scholar]
- 23.Steinmetz J, Schiele F, Gueguen R, et al. Standardization of γ-glutamyltransferase assays by intermethod calibration. Effect on determining common reference limits. Clin Chem Lab Med 2007;45:1373-1380. [DOI] [PubMed] [Google Scholar]
- 24.Pagani F, Bonora R, Panteghini M. Reference interval for lactate dehydrogenase catalytic activity in serum measured according to the new IFCC recommendation. Clin Chem Lab Med 2003;41:970-971. [DOI] [PubMed] [Google Scholar]
- 25.Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Official Journal of the European Communities 1998(December 7);L331:1-37. [Google Scholar]
- 26.Cattozzo G, Guerra E, Ceriotti F, Franzini C. Commutable calibrator with value assigned by the IFCC reference procedure to harmonize serum lactate dehydrogenase activity results measured by 2 different methods. Clin Chem 2008;54:1349-1355. [DOI] [PubMed] [Google Scholar]
- 27.Jansen R, Schumann G, Baadenhuijsen H, et al. Trueness verification and traceability assessment of results from commercial systems for measurement of six enzyme activities in serum. An international study in the EC4 framework of the Calibration 2000 project. Clin Chim Acta 2006;368:160-167. [DOI] [PubMed] [Google Scholar]
- 28.Fraser CG, Petersen PH, Libeer JC, Ricos C. Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem 1997;34:8-12. [DOI] [PubMed] [Google Scholar]


