Abstract
Platelets show a substantial role in the maintenance of vascular integrity when these cells after a rapid activation adhere to the vessel wall lesion, aggregate with other platelets and leukocytes resulting in an arterial thrombosis. Analysis of in vivo platelet activation at an early time point is crucial in the detection of developing thrombotic events. In addition, the forecast of future complications as well as the evaluation of the efficacy of anti- platelet medication are also essential in a large group of patients. Changes in the levels of platelet receptors or alteration in other surface properties due to intra- and extracellular responses to a stimulus can be measurable primarily by flow cytometry with specific antibodies via the assessment of classical and alternative platelet activation markers. Some of these biomarkers have been already used in routine laboratory settings in many cases, while others still stand in the phase of research applications. Deficiency in platelet receptors is also accessible with this technique for the diagnosis of certain bleeding disorders. We here describe the most important types of platelet activation markers, and give an overview how the levels of these markers are altered in different diseases.
Keywords: P-selectin, heterotypic aggregates, microparticle, coated-platelets, flow cytometry, heparin-induced thrombocytopenia
INTRODUCTION
Platelets are involved in the regulation of hemostasis, as activated platelets normally adhere to the injured vessel wall. Thrombocytes form aggregates with each other, but also interact with leukocytes to avoid a substantial blood loss from the circulation. These cellular complexes also contribute to the development of local inflammatory events. In contrast, abnormal platelet function may result in thrombotic or bleeding complications.
In arterial thrombosis, the level of platelet reactivity increases, and the expression of several platelet activation proteins (markers) can be measured on the cell surface. In addition, distinct platelet subpopulations (e.g. coated-platelets) may be also investigated using such experiments. Discovery of novel biomarkers is still of interest to predict emergency thrombotic states, and to monitor the effects of anti-platelet therapy. The deficiency or lack of platelet receptors may generate a dysfunction in platelet aggregation and cause hemorrhage. Early detection of all these anomalies is demanding, and flow cytometry is a reliable laboratory method to analyze platelet function in ex vivo clinical samples. This tool is now getting available in more and more laboratories, and a combination of two or three antibodies against platelet receptors allows a sensitive and specific analysis of platelets. However, there are several preanalytical and methodological pitfalls, which may influence the measurement and the interpretation of these results. In this review, the classic and alternative platelet activation markers on flow cytometry are summarized, which have been assessed in a large number of studies to evaluate altered platelet function.
I. CLASSICAL PLATELET ACTIVATION MARKERS
I/1. CD62P
P-selectin (CD62P) is one of the most abundant proteins in the α-granules of platelets, which is exposed on the cell surface within seconds after platelet activation [1]. Flow cytometric analysis of surface-bound CD62P alone or in a combination with other markers (see below) has been used as the ‘gold standard’ marker for the assessment of platelet activation in ex vivo patient samples in the last 3 decades reviewed in [2].
Detection of elevated surface P-selectin was the subject of numerous clinical studies in acute coronary syndrome (ACS) [3-5], in type 1 and 2 diabetes mellitus (DM) [6-8], untreated hypertension [9], obesity with or without DM [10-11], peripheral artery disease (PAD) [12], acute ischemic stroke [13-16], essential thrombocythemia (ET) [17], and in those clinical conditions where platelet activation is one part of the disease pathomechanism such as in primary Raynaud’s disease [18]. Platelet-bound P-selectin values are typically determined in percent positivity (%), however, even a small change in mean fluorescence intensity (MFI) values may demonstrate a larger alteration in surface P-selectin measured on a logarithmic scale. P-selectin analysis in non-activated samples is for ‘baseline’ CD62P positivity in the current in vivo platelet activation status, while platelet reactivity can be evaluated with CD62P levels on stimulated platelets by using submaximal concentrations of classic agonists adenosine-diphosphate (ADP) (0.5-5 µM), collagen (1-2 μg/mL), or thrombin-receptor activating peptide (TRAP) (1-8 µM) [7,10,19,20]. Significantly higher P-selectin levels were independently correlated with the body mass index (BMI) [10], the atherosclerosis indicator carotid intima-media thickness (IMT), and the inflammation marker C-reactive protein (CRP) [11]. Stellos et al. further investigated surface-bound P-selectin as a prognostic marker in myocardial infarction (Ml), and they found a positive association between the extent of myocardial injury measured with the levels of troponin-I plus creatine kinase-MB and CD62P positivity independently of age, gender, and baseline medication [5]. CD62P values were significantly increased in ST-segment elevation Ml (STEMI) patients that reflected a greater degree of occlusive thrombus formation in these patients versus others with non- ST-segment elevation Ml (NSTEMI) or Troponin-I-positive unstable angina (UA). On the other hand, P-selectin positivity showed a limited sensitivity (57.5%) and specificity (69%) for detection of ACS and discrimination of chest pain of different origins [5]. In monitoring of anti-platelet medication, CD62P had a minor sensitivity to the effects of ADP-receptor blocker clopidogrel and acetylsalicylic acid (ASA) therapy in stroke [21]. Surprisingly, opposite findings were also reported when less CD62P positive platelets were measured in MI [20,22] and (convalescent) cerebral infarction as baseline values and in response to agonist stimulation compared to clinical control cohorts [19,23,24]. Likewise, CD62P expression rapidly declined after the onset of acute ischemic stroke [25]. These phenomena were explained to be due to the rapid shedding of P-selectin from circulating platelets, and the sequestration of these activated cells into heterotypic aggregates [26,27]. In fact, the plasma concentration of released/shed receptors (i.e. soluble P-selectin) was measured in parallel with immunoassays as an additional platelet marker in these studies [11,19,20,24]. It was also suggested that platelets were exhausted and failed to respond to thrombin in vitro after a substantial cellular activation during stroke [28]. Therefore, detection of CD62P by flow cytometry seems to be a more reliable tool for monitoring platelet function at acute but not chronic stimulus of platelets.
I/2. CD40L
CD40L expression was first described on activated T-cells [29], and was later shown to be liberated to the platelet surface from α-granules, similarly to P-selectin [30]. It is now considered as an emerging platelet activation marker, and its level (CD154) was also increased when platelet activation was associated with endothelial dysfunction and inflammation in MI and UA [31]. Patients with UA who needed coronary angioplasty or had recurrent angina showed even higher CD40L expression on platelets compared with those without such complications [32]. Moreover, significant increase in CD40L on platelets was already detected in transient ischemic attack (TIA), not only complete stroke [33]. Especially in atherosclerotic ischemic stroke, CD40L positivity was enlarged compared to that in asymptomatic carotid stenosis [14]. Consequently, upregulated CD40L level was thought to initiate ischemic stroke from large artery atherosclerosis, and the concentration of this marker was correlated with worse clinical outcome after cerebral infarction [16,34].
I/3. CD63
CD63 (granulophysin, LAMP-3) is translocated from dense-granules and lysosomes to the plasma membrane after platelet activation [35]. CD63 expression was higher on day 1 in the stroke group versus control group, which remained significantly elevated until day 90 [25]. Similarly, Cha et al. found significantly higher CD63 platelet positivity in patients with atherosclerotic ischemic stroke than in normal subjects; however, no significant differences were seen between atherosclerotic ischemic stroke and asymptomatic carotid stenosis [14]. Additionally, increased CD63 level was predominantly detected in the acute stage of ischemic stroke compared with its convalescent stage and the control group [16,36]. In contrast, others found no elevation in CD63 positivity in either acute or convalescent stroke patients versus subjects without vascular disease [15]. Similarly to P-selectin, CD63 had an inferior role to detect the effects of clopidogrel and ASA in stroke patients [21]. Immunofluorescence analysis of CD63 by flow cytometry was a suitable method for the diagnosis of Hermansky-Pudlak syndrome accompanied with bruise and bleeding complications, where the significantly lower number of dense-granules and lysosomes in platelets was recognized by using anti-CD63 antibody versus a normal sample [35].
I/4. GPIIb/IIIa receptor (PAC-1 binding)
Fibrinogen receptors undergo a conformational change during platelet activation [37]. PAC-1 antibody was formerly developed by Shattil and his coworkers [37], and nowadays it is a commercially available monoclonal antibody, which specifically binds to the activated form of GPIIb/llla receptor complex induced by shear stress upon platelet aggregation. Increasing level of activated GPIIb/llla receptors was studied from clinically stable to unstable coronary artery diseases [38]. Also, constantly elevated PAC-1 binding at 3-month follow-up was associated with an increased incidence of recurrent stroke [36]. On the contrary, McCabe et al. did not find any difference in PAC-1 percent positivity between those with acute or convalescent cerebrovascular disease [15]. In manifest metabolic syndrome, higher expression of PAC-1 with augmented fibrinogen binding was observed compared to subjects with vascular disease [39]. PAC-1 was also found as a sensitive parameter in following clopidogrel effect along with decreased level of the intracellular vasodilator-stimulated phosphoprotein (VASP) [40].
II. ALTERNATIVE BIOMARKERS OF PLATELET ACTIVATION
II/1. PMPs
Platelet-derived microparticles (PMPs) has been employed as an alternative evaluation of platelet activation in recent years. These vesicles are formed during platelet ‘budding’, and thus contain several components from platelet cytoplasm and outer membrane. Consequently, PMPs were positive for CD62P and CD63 [41]. Moreover, those PMPs shed from phosphatidylserine (PS)-positive platelets were also positive for PS, and had 50- to 100-fold higher procoagulant activity than activated platelets [42]. Definition and analysis of PMPs are still a debated area of clinical flow cytometry. Due to the variable storage and preparation of samples, isolation of PMPs as well as differences in the settings of measurement, PMP numbers fairly varied even in the same disease causing potential inappropriate interpretations [43]. Yet, the need for standardized protocols is still demanding. The analysis of PMPs was first set by using fluorescent beads with standard size and amount for enumerating PMPs below 1 μm [44]. Beads were initially processed, and then clinical samples were measured within a standard collection time of 30 seconds. The numbers of PMPs were calculated based on the event count from the bead tube collected for the same time period. PMPs were gated into a restricted area by FSC and SSC parameters, and then identified by the presence of PS with Annexin V- FITC and their CD41 positivity. In addition, CD62P expression was also measured on these vesicles [44] (Figure 1). Previous studies described elevated number of PMPs in Ml, atrial fibrillation, and ischemic stroke with severe carotid atherosclerosis compared to healthy controls [23,41,45,46]. Others recently claimed that PMPs act as an independent marker of cardiovascular events in high-risk ACS patients, because atherosclerotic burden did not affect PMP number in stable angina subjects [47]. Furthermore, in ACS patients who underwent coronary stenting had even higher PMP numbers at 15 minutes after the intervention induced by the procedure-mediated trauma compared to those with diagnostic catheterization alone [44] (Figure 1). In terms of abnormal metabolic and inflammatory conditions, Csongrádi and her colleagues demonstrated significantly higher PMP concentrations in obese subjects and type 2 DM patients versus healthy individuals, where PMP levels were strongly associated with carotid IMT, BMI values, and CRP concentrations [11]. In agreement with this study, PMP levels were positively and independently correlated with carotid IMT in the convalescent phase of ischemic stroke as well [19]. Finally, PMPs could be also measured in the flow cytometric detection of heparin-induced thrombocytopenia (HIT) (see below). In summary, although increased PMP levels were documented even in the early phase of vascular diseases, it is still questionable whether flow cytometric analysis of PMPs is ready to be used as a biomarker for routine laboratory purposes due to relatively lower sensitivity and specificity, and the rather variable conditions of measurement [47].
Figure 1.

Representative dot plots of PMP analysis. PMPs were collected in patients after diagnostic catheterization without stenting (A) and clinical subjects after coronary stenting (B). PMPs were identified in R1 gate according to FSC and SSC parameters, and then by their Annexin V (PS) positivity (PMP number: 881 events [A] vs. 1117 events [B] in R2). During further analysis, PMPs were stained by anti-CD41-PECy5 and anti-CD62-PE antibodies to measure the activation status of PMPs (20.5% vs. 33.7%) (adapted after some minor modifications with permission from [44]).
II/2. Heterotypic aggregates
Platelet-leukocyte aggregates are generated in the blood stream when activated leukocytes and platelets rapidly produce cellular complexes with each other via exposed receptors, notably with CD62P through an interaction with P-selectin Glycoprotein Ligand-1 (PSGL-1) [48]. These heterotypic aggregates accumulate in the site of thrombus formation facilitating the development of variable vascular infarctions. Thus, therapeutic interference of these interactions may be a potential target of anti-platelet medication reviewed in [49]. The half-life of circulating interactions (platelet-monocytes) is much longer (about 30 minutes) compared to P-selectin expression [26]. Thus, the analysis of platelet-leukocyte aggregates may be a more consistent indicator of platelet activation than measuring the amount of P-selectin positive single platelets.
Patients with UA showed a significant increase in the level of neutrophil-platelet aggregates compared with patients with stable angina [50]. In ACS, not only the total level of platelet-monocyte complexes was augmented, but such tissue factor (TF)- positive population as well in contrast to stable angina or controls [51]. Significantly elevated levels of platelet-monocyte aggregates were published in the acute stage of cerebral infarction compared to control groups [15,16,33] that showed a good predictive value in early outcome and long-term prognosis after stroke in a recent study [34]. Yet, there were some contradictory data on the presence of platelet-leukocyte interactions in atrial fibrillation showing decreased levels versus control healthy individuals [20]. In terms of metabolic diseases, neutrophil-platelet aggregates were higher in type 1 DM patients with nephropathy compared to DM patients with normal renal function as well as non-diabetic persons [7]. There was a significant difference in the percentage of monocyte-platelet aggregates but not platelet-neutrophil or platelet-lymphocyte interactions between the diabetic especially with proliferative retinopathy and nephropathy and control groups [52]. Similarly to these data, enhanced leukocyte-platelet adhesion was correlated to platelet hyperreactivity among DM patients especially those with microangiopathy [53]. In chronic myeloproliferative diseases, the increased level of platelet-monocyte aggregates may also contribute to the vascular complications [54].
II/3. FXIII
Coagulation factor XIII (FXIII) is a protransglutaminase that is essential for maintaining hemostasis as a key regulator of fibrinolysis, and accelerates the fibrin cross-linking process [55]. FXIII is targeted and concentrated at the site where platelet-rich thrombi are formed. FXIII binds to activated platelets (Figure 2), and this interaction occurs via GPIIb/IIIa and αVβ3 receptors [56,57], and the surface-bound form was suggested to cross-link secreted α-granule proteins when coated-platelets are generated [58]. In a clinical study [59] in patients with PAD, platelet-associated FXIII was found significantly higher than in healthy controls, and the detection of FXIII on platelets was proposed as an alternative marker of platelet activation [59,60].
Figure 2.
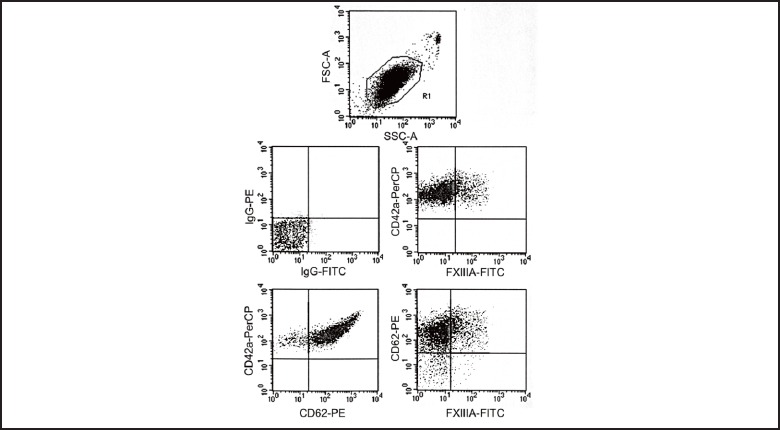
Representative dots plot series on TRAP- activated (20 µM) platelets (R1) in a normal whole blood sample analyzed by flow cytometry in three-color labeling experiments with anti-FXIIIA-FITC, anti- CD62-PE and anti-CD42a-PerCP antibodies. FXIII-A showed a co-expression with CD62P (23%). CD62% was 94% due to full platelet activation.
II/4. Phosphatidylserine
Phosphatidylserine (PS), a negatively charged lipid in the inner-leaflet of phospholipid membranes, is exposed to cell surface upon platelet activation to propagate coagulation events. Via cleaving FX and prothrombin into their active form, PS facilitates the assembly and activation of tenase and prothrombinase complexes. As a result, fibrin fibers are formed in the early phase of clot formation reviewed in [61]. PS exposure can be detected by the binding of Annexin V to platelets, which requires extracellular Ca2+, so it should be supported during such experiments [62]. Interestingly, this marker did not become a conventional platelet activation marker for ex vivo clinical samples, but was an available tool for studying in vitro procoagulant platelet responses, and identifying PMPs by flow cytometry.
Apart from these processes, PS expression also occurs during platelet apoptosis via caspase and calpain activation, when platelets undergo a cellular death pathway resulting in their clearance from the circulation by scavenger cells [63,64]. These events could be also induced in vitro by the classic platelet agonist, thrombin [65]. Aging, and stored platelets after several days were also positive for PS [66,67]. Overall, analysis of other biomarkers is necessary with PS to distinguish platelet activation and apoptosis-mediated changes from each other.
III. DEFICIENCY IN PLATELET GLYCOPROTEINS
Inherited platelet disorders are characterized by abnormalities of platelet function and production causing mucocutaneous bleeding symptoms with distinct intensity reviewed in [68]. When platelets show defects with an absence or malfunction of receptor(s) in adhesion receptors (GPIb/V/IX complex; Bernard-Soulier syndrome [BS]), or aggregation receptors (GPIIb/GPIIIa complex; Glanzmann-thrombasthenia [GT]), platelets fail to bind to the main ligands von Willebrand factor (vWF) and fibrinogen, respectively. BS was characterized with lacking ristocetin-induced aggregation, prolonged bleeding time, large platelets and thrombocytopenia resulting in epistaxis, gingival and cutaneous bleeding in an adult female patient [69]. GT platelets showed impaired aggregation to natural agonists (ADP, collagen, arachidonic acid) causing mucosal bleeding or epistaxis in a young male subject [70]. In both diseases, flow cytometric analysis of surface glycoprotein expression was essential for the final diagnosis when surface properties of patient platelets were compared to those from a healthy age-matched sample. In the type II GT patient, platelets were identified by anti- CD42a (GPIX) with no difference between patient and control in this term, while GPIIb receptors (CD41) were hardly detectable (Figure 3A). In addition, GPIIIa receptors were also absent by using anti-CD61 antibody (data not shown) [70]. In case of the BS person, GPIX by anti-CD42a showed a significantly lower expression, and GPIb receptors with anti-CD42b antibodies demonstrated a null level versus those of a healthy individual. Platelets were identified by their CD41 positivity [69] (Figure 3B).
Figure 3.

Representative dot plots of a complex flow cytometry analysis of platelet glycoproteins in a GT(A) and a BS(B) patient. Platelets were identified by anti-CD42a (GPIX), and GPIIb receptors (CD41; 1.5%) were hardly detectable in GT (A). In contrast, in a BS individual, markedly less GPIX (CD42a) receptors could be detected (67%), and there was no GPIb receptors (2%; CD42b) compared to a healthy person (CD42a: 99%; CD42b: 91%). Results from these patients were depicted with “P”, while data of healthy controls were marked with “C”. These dot plots were adapted with permission after some minor modifications from [69,70].
IV. Reticulated platelets
Percent of reticulated/immature platelets was suggested as a useful marker of augmented production or turnover of platelets in subjects with increased platelet activation long ago [71]. These platelets have large size with higher density compared to normal platelets. They also demonstrate an enhanced reactivity as they secrete more granule contents upon activation than smaller platelets [72]. Elevated level of reticulated platelets was measured in increased thrombopoiesis such as in ET, or when a compensatory mechanism occurs due to a large platelet loss (e.g. immune thrombocytopenic purpura) [73]. Flow cytometric analysis of reticulated platelets was formerly set using thiazole orange staining to detect their mRNA content and a platelet-specific (e.g. anti-GPIb) antibody for platelet gating [71]. ACS patients had significantly higher level of reticulated platelets versus healthy individuals [74]. Moreover, reticulated platelet percent was increased in both early and late phase of ischemic stroke/TIA after adjustment for age [75]. In terms of monitoring of anti-platelet drugs, larger immature platelet fraction was observed in aspirin treatment in those after stent thrombosis showing an increased platelet turnover [76]; however, it was not confirmed in subjects with stroke [75].
V. HEPARIN-INDUCED THROMBOCYTOPENIA (HIT)
HIT is one of the most common immune-mediated reactions caused by platelet activating IgG antibodies, which usually bind to heparin/PF4 complexes after heparin administration. Heparin/PF4/IgG complexes may induce platelet aggregation with increased thrombin generation resulting in a prothrombotic state [77]. Three types of HIT can be distinguished according to the onset of thrombocytopenia. Typically, thrombocytopenia begins between 4 and 15 days after the start of heparin therapy. Sometimes HIT develops within the first 24 hours of heparin administration (rapid-onset), or several days after the discontinuation of heparin (delayed-onset) [77]. Functional test for HIT laboratory diagnosis is available on flow cytometers as well [78,79]. Oláh and co-workers recently analyzed a patient sample from a rapid-onset HIT with the following methodology [80]. Normal platelets were incubated with the serum of a HIT patient and the therapeutic concentration of heparin (0.3 IU/mL). Annexin V binding on the surface of platelets and microparticle release were measured, and platelets were identified by CD41 positivity. For instance, in a negative control, PRP were incubated with heparin alone, while Ca-ionophore-stimulated sample (10 µM) was used as a positive control. Then, PRP with the patient plasma was studied, and finally PRP with plasma plus heparin (0.3 lU/ml). Due to the presence of HIT, a significantly increased Annexin V positivity could be measured compared to samples with heparin or plasma alone (Figure 4).
Figure 4.

Representative dot plots of HIT investigation by flow cytometry. Normal platelets were incubated with the serum of a HIT patient and heparin (0.3 IU/mL).
VI. COATED-PLATELETS
Coated-platelets are produced by a simultaneous activation of collagen and α-thrombin, and represent a subpopulation of activated platelets with high PS exposure and a substantial prothombinase activity [58]. In addition, coated-platelets are characterized by the retention of several α-granule-derived coagulation factors e.g. factor V, vWF, thrombospondin, and fibrinogen on their surface [58], which are covalently bound together via serotonin creating a potentially procoagulant surface matrix [81]. Elevated levels of coated-platelets were measured in patients with TIA and ischemic stroke compared to healthy subjects [82,83]. In contrast, significantly lower levels of coated- platelets were also shown in spontaneous cerebral bleeding, severe hemophilia A, and asymptomatic ET versus healthy cohorts [17,84,85]. Dale and his coworkers previously set a standardized methodology [58]. Accordingly, subsequent immunostaining and platelet activation were assessed in gel-filtered platelets by biotinylated-fibrinogen and anti-CD41 antibody with convulxin and α-thrombin. Coated-platelets were then indirectly labeled with streptavidin-PE to detect enhanced fibrinogen binding compared to the rest of platelets. Detection of P-selectin percent positivity was simultaneously performed (Figure 5).
Figure 5.
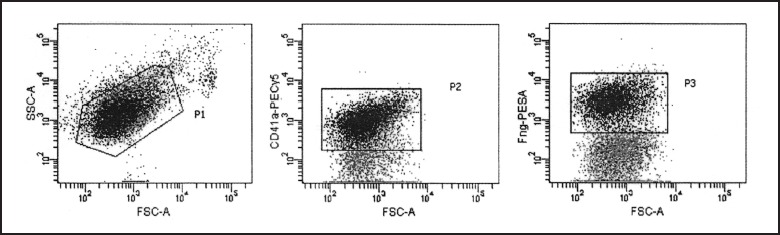
Representative dot plots of coated-platelet measurement in a normal sample on flow cytometer. Platelets were gated (P1) based on FSC-SSC parameters, and then these events were further analyzed in P2 gate where only CD41-positive cells were counted (92%). Finally, coated-platelets were separated from the rest of platelets according to their increased fibrinogen binding detected by biotinylated- fibrinogen and streptavidin-PE (38%; P3).
Platelets were identified by their CD41 positivity. In a negative control, PRP were incubated with heparin alone (2%; A), and Ca-ionophore-stimulated sample (99%; 10 µM) was used as a positive control (B). PRP with the patient plasma was studied (12%; C), and PRP with plasma plus heparin (0.3 lU/ml) (25%; D). Due to the presence of HIT, a significantly increased Annexin V positivity could be measured compared to samples with heparin or plasma alone.
CONCLUSIONS
Investigation of platelet biomarkers has not been only an approach to study platelet reactivity in variable diseases, but also provided new insights for a better understanding of the complexity of platelet physiology. For instance, modulating the activity of intracellular proteins (e.g. protein phosphatases) via activation signaling with potential anti-platelet drugs could be easily tested through platelet biomarkers by flow cytometry [86]. Novel aspects can be also studied, i.e. two distinct subpopulations of procoagulant platelets have been recently described after high concentrations of thrombin or collagen- related peptide based on the quantification of PS positivity, PAC-1 binding, and intracellular Ca2+ concentration [87,88]. The relative function of these platelet subpopulations needs further analysis.
In summary, platelet activation markers generally show good sensitivity and specificity even in the detection of lower degree of change in platelet reactivity, and except for PMP analysis, these biomarkers provide a good reproducibility as well.
ACKNOWLEDGEMENTS
This work was supported by a Mecenatura grant (Mec-10/2011) of the Medical and Health Science Center, University of Debrecen (B.N.Jr).
References
- 1.McEver RP. Selectins. Curr Opin Immunol 1994; 6:75-84. [DOI] [PubMed] [Google Scholar]
- 2.Kappelmayer J, Nagy B, Jr, Miszti-Blasius K, Hevessy Z, Setiadi H. The emerging value of P-selectin as a disease marker. Clin Chem Lab Med 2004; 42:475-486. [DOI] [PubMed] [Google Scholar]
- 3.Gurbel PA, O’Connor CM, Dalesandro MR, Serebruany VL. Relation of soluble and platelet P-selectin to early outcome in patients with acute myocardial infarction after thrombolytic therapy. Am J Cardiol 2001; 87:774-777. [DOI] [PubMed] [Google Scholar]
- 4.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 2003; 24:2166-2179. [DOI] [PubMed] [Google Scholar]
- 5.Stellos K, Bigalke B, Stakos D, Henkelmann N, Gawaz M. Platelet-bound P- selectin expression in patients with coronary artery disease: impact on clinical presentation and myocardial necrosis, and effect of diabetes mellitus and anti- platelet medication. J Thromb Haemost 2010; 8:205-207. [DOI] [PubMed] [Google Scholar]
- 6.Tschoepe D, Driesch E, Schwippert B, Nieuwenhuis HK, Gries FA. Exposure of adhesion molecules on activated platelets in patients with newly diagnosed IDDM is not normalized by near-normoglycemia. Diabetes 1995; 44:890-894. [DOI] [PubMed] [Google Scholar]
- 7.Tarnow I, Michelson AD, Barnard MR, Frelinger AL, 3rd, Aasted B, Jensen BR, Parving HH, Rossing P, Tarnow L. Nephropathy in type 1 diabetes is associated with increased circulating activated platelets and platelet hyperreactivity. Platelets 2009; 20:513-519. [DOI] [PubMed] [Google Scholar]
- 8.Nagy B, Jr, Csongrádi É, Bhattoa HP, Balogh I, Blaskó G, Paragh G, Kappelmayer J, Káplár M. Investigation of Thr715Pro P-selectin gene polymorphism and soluble P-selectin levels in type 2 diabetes mellitus. Thromb Haemost 2007; 98:186-191. [PubMed] [Google Scholar]
- 9.Preston RA, Coffey JO, Materson BJ, Ledford M, Alonso AB. Elevated platelet P-selectin expression and platelet activation in high risk patients with uncontrolled severe hypertension. Atherosclerosis 2007; 192:148-154. [DOI] [PubMed] [Google Scholar]
- 10.Schneider DJ, Hardison RM, Lopes N, Sobel BE, Brooks MM, Pro-Thrombosis Ancillary Study Group Association between increased platelet P-selectin expression and obesity in patients with type 2 diabetes: a BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) substudy. Diabetes Care 2009; 32:944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csongrádi É, Nagy B, Jr, Fülöp T, Varga Z, Karányi Z, Magyar MT, Oláh L, Papp M, Facskó A, Kappelmayer J, Paragh G, Káplár M. Increased levels of platelet activation markers are positively associated with carotid wall thickness and other atherosclerotic risk factors in obese patients. Thromb Haemost 2011; 106:683-692. [DOI] [PubMed] [Google Scholar]
- 12.Zeiger F, Stephan S, Hoheisel G, Pfeiffer D, Ruehlmann C, Koksch M. P-selectin expression, platelet aggregates, and platelet-derived microparticle formation are increased in peripheral arterial disease. Blood Coagul Fibrinolysis 2000; 11:723–728. [DOI] [PubMed] [Google Scholar]
- 13.Zeller JA, Tschoepe D, Kessler C. Circulating platelets show increased activation in patients with acute cerebral ischemia. Thromb Haemost 1999; 81:373-377. [PubMed] [Google Scholar]
- 14.Cha JK, Jeong MH, Jang JY, Bae HR, Lim YJ, Kim JS, Kim SH, Kim JW. Serial measurement of surface expressions of CD63, P-selectin and CD40 ligand on platelets in atherosclerotic ischemic stroke. A possible role of CD40 ligand on platelets in atherosclerotic ischemic stroke. Cerebrovasc Dis 2003; 16:376-382. [DOI] [PubMed] [Google Scholar]
- 15.McCabe DJ, Harrison P, Mackie IJ, Sidhu PS, Purdy G, Lawrie AS, Watt H, Brown MM, Machin SJ. Platelet degranulation and monocyte-platelet complex formation are increased in the acute and convalescent phases after ischaemic stroke or transient ischaemic attack. Br J Haematol 2004; 125:777-787. [DOI] [PubMed] [Google Scholar]
- 16.Tsai NW, Chang WN, Shaw CF, Jan CR, Chang HW, Huang CR, Chen SD, Chuang YC, Lee LH, Wang HC, Lee TH, Lu CH. Levels and value of platelet activation markers in different subtypes of acute non-cardio-embolic ischemic stroke. Thromb Res 2009; 124:213-218. [DOI] [PubMed] [Google Scholar]
- 17.Reményi G, Szász R, Debreceni IB, Szarvas M, Batár P, Nagy B, Jr, Kappelmayer J, Udvardy M. Comparison of coated-platelet levels in patients with essential thrombocythemia with and without hydroxyurea treatment. Platelets. 2012, DOI:10.3109/09537104.2012.731112. [DOI] [PubMed] [Google Scholar]
- 18.Shemirani AH, Nagy B, Jr, Takáts AT, Zsóri KS, András C, Kappelmayer J, Csiki Z. Increased mean platelet volume in primary Raynaud’s phenomenon. Platelets 2012; 23:312-316. [DOI] [PubMed] [Google Scholar]
- 19.Lukasik M, Dworacki G, Michalak S, Kufel-Grabowska J, Watala C, Kozubski W. Chronic hyper-reactivity of platelets resulting in enhanced monocyte recruitment in patients after ischaemic stroke. Platelets 2012; 23:132-142. [DOI] [PubMed] [Google Scholar]
- 20.Alberti S, Angeloni G, Tamburrelli C, Pampuch A, Izzi B, Messano L, Parisi Q, Santamaria M, Donati MB, de Gaetano G, Cerletti C. Platelet-leukocyte mixed conjugates in patients with atrial fibrillation. Platelets 2009; 20:235-241. [DOI] [PubMed] [Google Scholar]
- 21.Grau AJ, Reiners S, Lichy C, Buggle F, Ruf A. Platelet function under aspirin, clopidogrel, and both after ischemic stroke: a case-crossover study. Stroke 2003;34:849-854. [DOI] [PubMed] [Google Scholar]
- 22.Serebruany VL, Gurbel PA, Shustov AR, Ohman EM, Topol EJ. Heterogeneity of platelet aggregation and major surface receptor expression in patients with acute myocardial infarction. Am Heart J 1998;136:398-405. [DOI] [PubMed] [Google Scholar]
- 23.Lukasik M, Rozalski M, Luzak B, Michalak S, Kozubski W, Watala C. Platelet activation and reactivity in the convalescent phase of ischaemic stroke. Thromb Haemost 2010; 103:644-650. [DOI] [PubMed] [Google Scholar]
- 24.Järemo P, Eriksson M, Lindahl TL, Nilsson S, Milovanovic M. Platelets and acute cerebral infarction. Platelets 2012; DOI: 10.3109/09537104.2012.712168. [DOI] [PubMed] [Google Scholar]
- 25.Marquardt L, Ruf A, Mansmann U, Winter R, Schuler M, Buggle F, Mayer H, Grau AJ. Course of platelet activation markers after ischemic stroke. Stroke 2002;33:2570-2574. [DOI] [PubMed] [Google Scholar]
- 26.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA, 1996; 93:11877-11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001; 104:1533-1537. [DOI] [PubMed] [Google Scholar]
- 28.Jurk K, Jahn UR, Van Aken H, Schriek C, Droste DW, Ritter MA, Bernd Ringelstein E, Kehrel BE. Platelets in patients with acute ischemic stroke are exhausted and refractory to thrombin, due to cleavage of the seven- transmembrane thrombin receptor (PAR-1). Thromb Haemost 2004; 91:334-344. [DOI] [PubMed] [Google Scholar]
- 29.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help). J Exp Med 1992; 175:1091-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998; 391:591-594. [DOI] [PubMed] [Google Scholar]
- 31.Abuel-Makrem MA, Mahmoud YZ, Sayed D, Nassef NM, Abdel-Kader SS, Zakhary M, Ghazaly T, Matta R. The role of platelets CD40 ligand (CD154) in acute coronary syndromes. Thromb Res 2009; 124:683-688. [DOI] [PubMed] [Google Scholar]
- 32.Garlichs CD, Eskafi S, Raaz D, Schmidt A, Ludwig J, Herrmann M, Klinghammer L, Daniel WG, Schmeisser A. Patients with acute coronary syndromes express enhanced CD40 ligand/CD154 on platelets. Heart 2001;86:649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garlichs CD, Kozina S, Fateh-Moghadam S, Handschu R, Tomandl B, Stumpf C, Eskafi S, Raaz D, Schmeisser A, Yilmaz A, Ludwig J, Neundörfer B, Daniel WG. Upregulation of CD40-CD40 ligand (CD154) in patients with acute cerebral ischemia. Stroke 2003; 34:1412-1418. [DOI] [PubMed] [Google Scholar]
- 34.Lukasik M, Dworacki G, Kufel-Grabowska J, Watala C, Kozubski W. Upregulation of CD40 ligand and enhanced monocyte-platelet aggregate formation are associated with worse clinical outcome after ischaemic stroke. Thromb Haemost 2012; 107:346-355. [DOI] [PubMed] [Google Scholar]
- 35.Nishibori M, Cham B, McNicol A, Shalev A, Jain N, Gerrard JM. The protein CD63 is in platelet dense granules, is deficient in a patient with Hermansky- Pudlak syndrome, and appears identical to granulophysin. J Clin Invest 1993;91:1775-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fateh-Moghadam S, Htun P, Tomandl B, Sander D, Stellos K, Geisler T, Langer H, Walton K, Handschu R, Garlichs C, Daniel WG, Gawaz M. Hyperresponsiveness of platelets in ischemic stroke. Thromb Haemost 2007;97:974-978. [PubMed] [Google Scholar]
- 37.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem 1985; 260:11107-11114. [PubMed] [Google Scholar]
- 38.Tantry US, Bliden KP, Suarez TA, Kreutz RP, Dichiara J, Gurbel PA. Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the Thrombotic Risk Progression (TRIP) study. Platelets 2010;21:360-367. [DOI] [PubMed] [Google Scholar]
- 39.Serebruany VL, Malinin A, Ong S, Atar D. Patients with metabolic syndrome exhibit higher platelet activity than those with conventional risk factors for vascular disease. J Thromb Thrombolysis 2008; 25:207-213. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz UR, Geiger J, Walter U, Eigenthaler M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thromb Haemost 1999; 82:1145-1152. [PubMed] [Google Scholar]
- 41.van der Zee PM, Biró É, Ko Y, de Winter RJ, Hack CE, Sturk A, Nieuwland R. P- selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem 2006; 52:657-664. [DOI] [PubMed] [Google Scholar]
- 42.Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost 2007; 97:425-434. [PubMed] [Google Scholar]
- 43.Shah MD, Bergeron AL, Dong JF, López JA. Flow cytometric measurement of microparticles: pitfalls and protocol modifications. Platelets 2008; 19:365-372. [DOI] [PubMed] [Google Scholar]
- 44.Nagy B, Jr, SzükT Debreceni IB, Kappelmayer J. Platelet-derived microparticle levels are significantly elevated in patients treated by elective stenting compared to subjects with diagnostic catheterization alone. Platelets. 2010, 21:147-151. [DOI] [PubMed] [Google Scholar]
- 45.Katopodis JN, Kolodny L, Jy W, Horstman LL, De Marchena EJ, Tao JG, Haynes DH, Ahn YS. Platelet microparticles and calcium homeostasis in acute coronary ischemias. Am J Hematol 1997; 54:95-101. [DOI] [PubMed] [Google Scholar]
- 46.Choudhury A, Chung I, Blann AD, Lip GY. Elevated platelet microparticle levels in nonvalvular atrial fibrillation: relationship to p-selectin and antithrombotic therapy. Chest 2007; 131:809-815. [DOI] [PubMed] [Google Scholar]
- 47.Biasucci LM, Porto I, Di Vito L, De Maria GL, Leone AM, Tinelli G, Tritarelli A, Di Rocco G, Snider F, Capogrossi MC, Crea F. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ J 2012;76:2174-2182. [DOI] [PubMed] [Google Scholar]
- 48.Li N, Hu H, Lindqvist M, Wikström-Jonsson E, Goodall AH, Hjemdahl P. Platelet-leukocyte cross talk in whole blood. Arterioscler Thromb Vasc Biol 2000; 20:2702-2708. [DOI] [PubMed] [Google Scholar]
- 49.Nagy B, Jr, Miszti-Blasius K, Kerényi A, Clemetson KJ, Kappelmayer J. Potential therapeutic targeting of platelet-mediated cellular interactions in atherosclerosis and inflammation. Curr Med Chem 2012; 19:518-531. [DOI] [PubMed] [Google Scholar]
- 50.Ott I, Neumann FJ, Gawaz M, Schmitt M, Schömig A. Increased neutrophil- platelet adhesion in patients with unstable angina. Circulation 1996; 94:1239-1246. [DOI] [PubMed] [Google Scholar]
- 51.Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet- leukocyte aggregates. Arterioscler Thromb Vasc Biol 2008; 28:947-953. [DOI] [PubMed] [Google Scholar]
- 52.Káplár M, Kappelmayer J, Veszprémi A, Szabó K, Udvardy M. The possible association of in vivo leukocyte-platelet heterophilic aggregate formation and the development of diabetic angiopathy. Platelets 2001; 12:419-422. [DOI] [PubMed] [Google Scholar]
- 53.Hu H, Li N, Yngen M, Ostenson CG, Wallén NH, Hjemdahl P. Enhanced leukocyte-platelet cross-talk in Type 1 diabetes mellitus: relationship to microangiopathy. J Thromb Haemost 2004; 2:58-64. [DOI] [PubMed] [Google Scholar]
- 54.Káplár M, Kappelmayer J, Kiss A, Szabó K, Udvardy M. Increased leukocyte- platelet adhesion in chronic myeloproliferative disorders with high platelet counts. Platelets 2000; 11:183-184. [DOI] [PubMed] [Google Scholar]
- 55.Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev 2011; 91:931-972. [DOI] [PubMed] [Google Scholar]
- 56.Nagy B, Jr, Simon Z, Bagoly Z, Muszbek L, Kappelmayer J. Binding of plasma factor XIII to thrombin-receptor activated human platelets. Thromb Haemost 2009; 102:83-89. [DOI] [PubMed] [Google Scholar]
- 57.Magwenzi SG, Ajjan RA, Standeven KF, Parapia LA, Naseem KM. Factor XIII supports platelet activation and enhances thrombus formation by matrix proteins under flow conditions. J Thromb Haemost 2011; 9:820-833. [DOI] [PubMed] [Google Scholar]
- 58.Dale GL, Friese P, Batár P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 2002; 415:175-179. [DOI] [PubMed] [Google Scholar]
- 59.Devine DV, Andestad G, Nugent D, Carter CJ. Platelet-associated factor XIII as a marker of platelet activation in patients with peripheral vascular disease. Arterioscler Thromb Vasc Biol 1993; 13:857-862. [DOI] [PubMed] [Google Scholar]
- 60.Devine DV, Bishop PD. Platelet-associated factor XIII in platelet activation, adhesion, and clot stabilization. Semin Thromb Haemost 1996; 22:409-413. [DOI] [PubMed] [Google Scholar]
- 61.Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost 2002; 88:186-193. [PubMed] [Google Scholar]
- 62.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995;184:39-51. [DOI] [PubMed] [Google Scholar]
- 63.Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MT, Piccoli A, Totani L, Cianflone D, Maseri A, Manfredi AA. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood 2009;113:5254-5265. [DOI] [PubMed] [Google Scholar]
- 64.Jackson SP, Schoenwaelder SM. Procoagulant platelets: are they necrotic? Blood 2010; 116:2011-2018. [DOI] [PubMed] [Google Scholar]
- 65.Leytin V, Allen DJ, Mykhaylov S, Lyubimov E, Freedman J. Thrombin-triggered platelet apoptosis. J Thromb Haemost 2006; 4:2656-2663. [DOI] [PubMed] [Google Scholar]
- 66.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128:1173-1186. [DOI] [PubMed] [Google Scholar]
- 67.Albanyan AM, Harrison P, Murphy MF. Markers of platelet activation and apoptosis during storage of apheresis- and buffy coat-derived platelet concentrates for 7 days. Transfusion 2009; 49:108-117. [DOI] [PubMed] [Google Scholar]
- 68.Nurden AT, Freson K, Seligsohn U. Inherited platelet disorders. Haemophilia. 2012; 18 Suppl 4:154-160. [DOI] [PubMed] [Google Scholar]
- 69.Schlammadinger A, Tóth J, Nagy B, Jr, Fazakas F, Hársfalvi J, Kappelmayer J, Muszbek L, Radványi G, Boda Z. Bernard-Soulier szindróma: a herediter thrombocytopéniák ritka oka. Hemat Transzf 2007; 40:40-46. [Google Scholar]
- 70.Kerényi A, Szegedi I, Sarudi S, Kappelmayer J, Kiss C, Muszbek L. Glanzmann thrombasthenia II. típusa. Esetleírás. Klin Kisérl Lab Med 1999; 25:162-168. [Google Scholar]
- 71.Ault KA, Rinder HM, Mitchell J, Carmody MB, Vary CP, Hillman RS. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am J Clin Pathol 1992; 98:637-646. [DOI] [PubMed] [Google Scholar]
- 72.Matic GB, Chapman ES, Zaiss M, Rothe G, Schmitz G. Whole blood analysis of reticulated platelets: improvements of detection and assay stability. Cytometry 1998; 34:229-234. [DOI] [PubMed] [Google Scholar]
- 73.Harrison P, Robinson MS, Mackie IJ, Machin SJ. Reticulated platelets. Platelets 1997; 8:379-383. [DOI] [PubMed] [Google Scholar]
- 74.Lakkis N, Dokainish H, Abuzahra M, Tsyboulev V, Jorgensen J, De Leon AP, Saleem A. Reticulated platelets in acute coronary syndrome: a marker of platelet activity. J Am Coll Cardiol 2004; 44:2091-2093. [DOI] [PubMed] [Google Scholar]
- 75.McCabe DJ, Harrison P, Sidhu PS, Brown MM, Machin SJ. Circulating reticulated platelets in the early and late phases after ischaemic stroke and transient ischaemic attack. Br J Haematol 2004; 126:861-869. [DOI] [PubMed] [Google Scholar]
- 76.Würtz M, Grove EL, Wulff LN, Kaltoft AK, Tilsted HH, Jensen LO, Hvas AM, Kristensen SD. Patients with previous definite stent thrombosis have a reduced antiplatelet effect of aspirin and a larger fraction of immature platelets. JACC Cardiovasc Interv 2010; 3:828-835. [DOI] [PubMed] [Google Scholar]
- 77.Haas S, Walenga JM, Jeske WP, Fareed J. Heparin-induced thrombocytopenia: the role of platelet activation and therapeutic implications. Semin Thromb Hemost. 1999; 25 Suppl 1:67-75. [PubMed] [Google Scholar]
- 78.Lee DH, Warkentin TE, Denomme GA, Hayward CP, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia: detection of platelet microparticles using flow cytometry. Br J Haematol 1996; 95:724-731. [DOI] [PubMed] [Google Scholar]
- 79.Tomer A, Masalunga C, Abshire TC. Determination of heparin-induced thrombocytopenia: a rapid flow cytometric assay for direct demonstration of antibody-mediated platelet activation. Am J Hematol 1999; 61:53-61. [DOI] [PubMed] [Google Scholar]
- 80.Oláh Z, Kerényi A, Kappelmayer J, Schlammadinger A, Rázsó K, Boda Z. Rapid- onset heparin-induced thrombocytopenia without previous heparin exposure. Platelets 2012; 23:495-498. [DOI] [PubMed] [Google Scholar]
- 81.Szász R, Dale GL. Thrombospondin and fibrinogen bind serotonin-derivatized proteins on COAT-platelets. Blood 2002; 100:2827-2831. [DOI] [PubMed] [Google Scholar]
- 82.Prodan CI, Joseph PM, Vincent AS, Dale GL. Coated-platelets in ischemic stroke: Differences between lacunar and cortical stroke. J Thromb Haemost 2008; 6:609-614. [DOI] [PubMed] [Google Scholar]
- 83.Prodan CI, Vincent AS, Dale GL. Coated-platelet levels are elevated in patients with transient ischemic attack. Transl Res 2011; 158:71-75. [DOI] [PubMed] [Google Scholar]
- 84.Prodan CI, Vincent As, Dale GL. Coated platelet levels correlate with bleed volume in patients with spontaneous intracerebral hemorrhage. Stroke 2010;41:1301-1303. [DOI] [PubMed] [Google Scholar]
- 85.Saxena K, Pethe K, Dale GL. Coated-platelet levels may explain some variability in clinical phenotypes observed with severe hemophilia. J Thromb Haemost 2010;8:1140-1142. [DOI] [PubMed] [Google Scholar]
- 86.Simon Z, Kiss A, Erdödi F, Setiadi H, Debreceni IB, Nagy B, Jr, Kappelmayer J. Protein phosphatase inhibitor calyculin-A modulates activation markers in TRAP- stimulated human platelets. Platelets 2010; 21:555-562. [DOI] [PubMed] [Google Scholar]
- 87.Topalov NN, Yakimenko AO, Canault M, Artemenko EO, Zakharova NV, Abaeva AA, Loosveld M, Ataullakhanov FI, Nurden AT, Alessi MC, Panteleev MA. Two types of procoagulant platelets are formed upon physiological activation and are controlled by integrin α(IIb)β(3). Arterioscler Thromb Vasc Biol 2012; 32:2475-2483. [DOI] [PubMed] [Google Scholar]
- 88.Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost 2012; DOI:10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]


