Abstract
Nanotechnology involving manipulation of atoms and molecules at the nanoscale is one of the frontier areas of research in modern science. During the last few years, nanotechnology has witnessed breakthroughs in the fields of medicine, environment, therapeutics, drug development and biotechnology. This is due to the unique properties of nanomaterials (e.g. chemical, mechanical, optical, magnetic, and biological) which make them desirable for commercial and medical applications. Considering the theory and practice of using nanoparticles, nanotechnology has a great potential in improving treatment of various disorders and in vitro diagnostics. However, there is not much information available on the toxicity of nanoparticles in relation to human health. Toxic effect of nanomaterials on humans is the primary concern of the health industry. Nanomaterials are able to cross biological membranes and access cells, tissues and organs that larger-sized particles normally cannot. Nanomaterials can gain access to the blood stream via inhalation or ingestion. This may lead to both genotoxicity and biochemical toxicity. In this review we try to show which types, sizes and concentrations of nanoparticles are safe for human use and this will help in developing diagnostic, prognostic and therapeutic models using nanoparticles.
INTRODUCTION
Nanotechnology can be defined as the techniques aimed at characterizing and producing materials on the nanometer scale (< 100 nm) which exhibit specific physical/chemical properties and functions. The behavior of nanoparticles is a function of their size, shape and surface reactivity with the surrounding tissue. In principle, a large number of particles could overload the body’s phagocytes, cells that ingest and destroy foreign matter, thereby triggering stress reactions that lead to inflammation and weaken the body’s defense against other pathogens1. In addition to questions about what happens if non-degradable or slowly degradable nanoparticles accumulate in body organs, another concern is their potential interaction or interference with biological processes inside the body. Due to the importance of this size class of particles, the term nanotoxicology has been coined that aims to establish the relationship between nanoparticle physicochemical properties (e.g. size, surface properties and crystal phase) and their toxic potential2.
Nanotoxicology is a sub-specialty of particle toxicology. It addresses the toxicology of nanoparticles which appear to have toxicity effects that are unusual and not seen with larger particles. The smaller a particle, the greater it’s surface area to volume ratio and the higher its chemical reactivity and biological activity. Because of their large surface area, nanoparticles will, on exposure to tissue and fluids, immediately adsorb onto their surface some of the macromolecules they encounter. This may, for instance, affect the regulatory mechanisms of enzymes and other proteins.
Genotoxicity describes a deleterious action on a cell’s genetic material affecting its integrity. Genotoxic substances are known to be potentially mutagenic or carcinogenic, specifically those capable of causing genetic mutation. Genotoxins may be lethal and could cause various metabolic and developmental abnormalities. This can also be passed on to subsequent generations3.
Nanoparticles are molecules which are sub-100nm in range, which have specific physical, chemical and biological properties. These properties make them as potent molecules for diagnostics and therapeutics in modern medicine. Nanoparticles are widely used because of their desirable properties in industrial, medical and cosmetic fields4. These particles can be released into the human environment and then can be inhaled. Most exposure to airborne nanomaterials occurs in the work place5. The extremely small size of nanomaterials also means that they much more readily gain entry into the human body than larger sized particles. How these nanoparticles behave inside the body is still a major question that needs to be resolved.
Nanoscience and nanotechnology encompass a wide range of fields, including chemistry, physics, materials engineering, biology, medicine, and electronics. There is also a notable rise in the number of publications discussing their toxicity, particularly in the past two years. The total number of papers on toxicity, however, remains low compared to the total number of publications on nanomaterials. There are several reviews addressing nanotoxicology aspects; however, they are intended for a narrow, specialized audience. Several are comparatively general4,6 while others address selected aspects of nanoparticle toxicology, such as epidemiological reviews of exposure to particles and health affects;7 targeted drug delivery;8 particle characterization methods;9 screening strategies and future directions of research;10 and regulation of nanomaterials11.
While the tremendous positive impacts of nanotechnology are widely publicized, potential threats or risks to human health and the environment are just beginning to emerge. With limited information available for support, critics are presenting a number of concerns on the devastating effects of nanotoxicity on human health and the environment. Detailed studies on the long-term effects of NPs are the need of the day to overcome or reduce possible threats. Simultaneous agglomeration, sedimentation and diffusion at physiologically relevant concentrations should be taken into account while conducting quantitative studies on the uptake of NPs into biological systems, to assess the corresponding risks on human health12.
Studies on the effect of NPs on human health have also gained momentum recently. A study suggests that inhaled carbon NPs are capable of rapid translocation into the circulation10. On the contrary, a conflicting report suggests that inhaled carbon NPs remain within the lung up to 6 h after inhalation, without passing to systemic circulation4. In a recent study13, suitability of mouse spermatogonial stem cell line as a model system to assess nanotoxicity was evaluated in the male germline in vitro. As nanomaterial-based products enter the market, there is an urgent need for related research in order to prevent dramatic consequences of any health-oriented issues caused by nanotechnology-driven products. Recent research has brought to light concerns over the safety of use of nanomaterials and also the long-term adverse effect of their use. Hence it is essential for us to establish the toxicity, safety and risks involved in the usage of these nanoparticles.
During the last years engineered nanoparticles (NPs) have been extensively used in different technologies and consequently many questions have arisen about the risk and the impact on human health following exposure to nanoparticles14. Nevertheless, at present knowledge about the cytotoxicity induced by NPs is still largely incomplete. In this context, we try to show which types, sizes and concentrations of nanoparticles are safe for human use and this will help in developing diagnostic, prognostic and therapeutic models using nanoparticles.
Different classes of nanoparticles and their toxicity
Colloidal gold, also known as “nanogold”, is a suspension of sub-micrometre-sized particles of gold in a fluid - usually water. The liquid is usually either an intense red colour (for particles less than 100 nm), or a dirty yellowish colour (for larger particles)15. In recent days gold nanoparticles are widely using in all the fields, particularly in medicine.
The toxicity of GNPs has been investigated at the cellular level. GNPs enter cells in a size- and shape-dependent manner16,17. Uptake of GNPs reaches a maximum when the size nears 50 nm and when the aspect ratio approaches unity. The transport efficiency reaches a plateau 30 min after incubation. The uptake of GNPs is consistent with receptor-mediated endocytosis. Nevertheless, most GNPs can enter cells efficiently, and most studies indicate that they are nearly harmless to cultured cells18,19,20,21
In all, gold nanoparticles may not affect cell viability in short-term but affect cell proliferation and cause DNA damage. Its mechanism of action and involvement are yet to be elucidated. Currently gold nanoparticles (AuNPs) are used in different biomedical applications, such as intracellular gene regulation22, chemotherapy23 and drug delivery24,25, as well as in optical and electronic applications26.
A previous study revealed that gold-silica nanoshells release significant heat when exposed to near-infrared (NIR) light (650–950 nm) and have been used to produce thermal cytotoxicity in vitro 27. Unfortunately, this treatment approach is mechanistically limited to use in superficial malignant tumors because of the minimal tissue penetration (< 2–3 cm depth) by NIR wavelength light28. However, the gold-silica nanoshell study demonstrated that nanogold has potential clinical use as a thermal conductor of non-invasive energy sources. Gold, like most metals, is an excellent conductor of electrical and thermal energy.
Silver nanoparticles are used as antibacterial/antifungal agents in a diverse range of applications: air sanitizer sprays, socks, pillows, slippers, face masks, wet wipes, detergent, soap, shampoo, toothpaste, air filters, coatings of refrigerators, vacuum cleaners, washing machines, food storage containers, cellular phones, and even in liquid condoms (Peter D, 2009).
Ingestion of Ag can cause argyria, the benign condition characterized by the bluish-graying of the skin that occurs through the preferential deposition of Ag in the basal lamina of soft tissues such as the skin, liver, and spleen29 and blood vessels, gastrointestinal tract, liver, and kidney30 (Fig 1, Table 1).
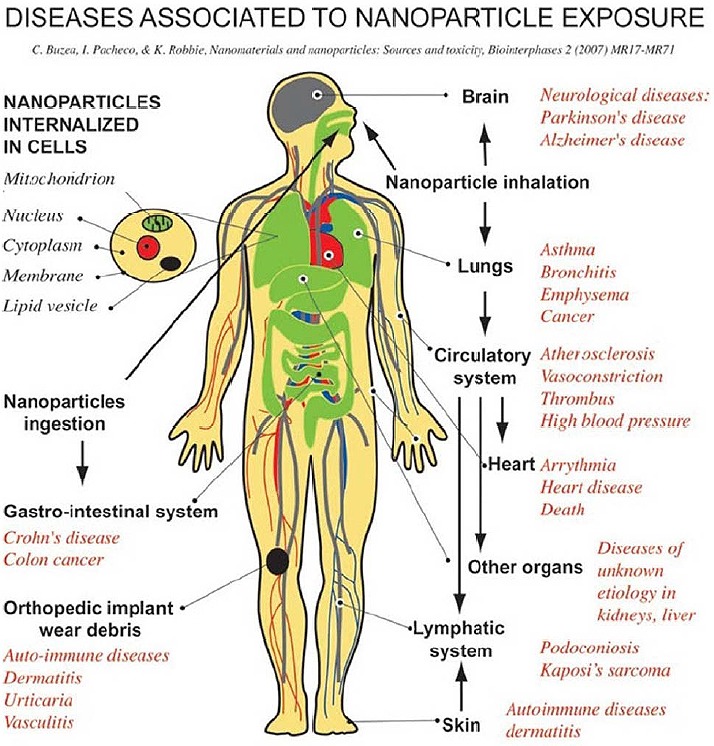
Table 1:
Involvement of nanoparticles toxicity in various pathways.
| Membrane damage/leakage/thinning | Cationic NPs |
|---|---|
| Protein binding/unfolding responses/loss of function/fibrillation | TiO2, Carbon NPS |
| DNA cleavage and mutation | Ag NPs |
| Mitochondrial damage: electron transfer/ATP/Apoptosis | Ag and Gold NPs |
| Lysosomal damage: proton pump activity/lysis/frustrated phagocytosis | Ag, Gold NPs and Carbon Nanotubes (CNTs) |
| Inflammation: signaling cascade/cytokines/chemokines/adhesion | Metal Oxide NPs (eg. TiO2) and CNTs |
| Fibrogenesis and tissue remodeling injury | CNTs |
| Blood platelet, vascular endothelial and clotting abnormalities | SiO2 |
| Oxidative stress injury, radical production, GSH (Glutathione) depletion, lipid peroxidation, membrane oxidation, protein oxidation. | CNTs, Metal Oxide NPs, Cationic NPs |
Carbon nanotubes (CNTs; also known as buckytubes) are allotropes of carbon with a cylindrical nanostructure. These cylindrical carbon molecules have novel properties that make them potentially useful in many applications in nanotechnology. Determining the toxicity of carbon nanotubes has been one of the most pressing questions in nanotechnology. Unfortunately such research has only just begun and the data are still fragmentary and subject to criticism. Under some conditions, nanotubes can cross membrane barriers, which suggest that if raw materials reach the organs they can induce harmful effects such as inflammatory and fibrotic reactions (Jelena K et al. 2007) (Fig 1, Table 1).
Nanotechnology is a global cross-disciplinary undertaking that has extraordinary potential to change our lives by improving existing products and enabling new ones. Like most new technologies, the emphasis on benefits has been offset by considerable debate about the uses and safety of nanotechnologies31,32,33,34. Occupational medicine is replete with examples of respirable dust particle exposures causing disease, and so matters of safety have been focused largely on nanoparticles (NP), which represent only one aspect of nanotechnology. NP, generally defined as particles less than 100 nm in any dimension and previously called ultrafine particles by inhalation toxicologists, are a concern because they may enter the body through the lungs, skin, or gut depending on the type of exposure. Several recent papers have highlighted this area of toxicology, its novelty, the gaps in research, and possible testing strategies for NP and nanotubes2,35,36. There is sufficient “ultrafine particle” toxicology literature to consider that the large and diverse group of manufactured NP arising from nanotechnology comprises an inhalation hazard of unknown potential. Most of the concern has flowed from this perception, enhanced by limited studies indicating that they may also be taken up through the skin or gut. The notion, for which some limited support exists, that NP may gain access to the blood poses a relatively new particle toxicological problem, i.e., particle effects at sites other than the lungs (Fig 1, Table 1).
This paper examines the toxicology issues relating to carbon nanotubes (CNT), one of the major new NP that are set to be produced in bulk for many diverse purposes. CNTare discussed here exclusively as an inhalation hazard. Global revenues from CNT in 2006 are estimated at ~ $230 million with a growth rate of ~ 170%. This provides potential for workplace and even eventual general exposure, if there is attrition of materials that contain them. Concern has been raised over the safety of CNT because they have three properties that are clearly associated with pathogenicity in particles.
They are NP and so could have more toxicity than larger sized particles.
They are fiber shaped and so might behave like asbestos and other pathogenic fibers which have toxicity associated with their needle-like shape.
They are essentially graphitic and so are expected to be biologically biopersistent.
Titanium dioxide (TiO2) particles with diameter larger than 100 nm are considered biologically inert in both humans and animals37. Based on this understanding, titanium dioxide nanoparticles have been widely used in many products, such as white pigment, food colorant, sunscreens, and cosmetic creams13. However, adverse effects of TiO2 nanoparticles have recently been uncovered38,39. New research is exploring the potential use of nanostructured TiO2 photocatalyst materials for sterilizing equipment of environmental microorganisms in the health care facility40.
Exposure to nano-titanium dioxide showed DNA and chromosome damage to a degree “linked to all the big killers of man, namely cancer, heart disease, neurological disease and aging41. Warheit and co-workers recently dosed the lungs of rats with 3 sizes of titanium dioxide nanoparticles and suggested that toxicity is not dependent upon particle size or surface area42.
A clear conclusion regarding particle size could not be reached as only 3 sizes were tested, of which one was of a different crystal phase, and the two of the same crystal phase (rutile) were of different shapes (rods, ~200 nm × 40 nm, and dots, ~10-20 nm). In a follow up study, these authors tested the pulmonary toxicity of 3 sizes (hydrodynamic diameter: 136 nm, 149 nm and 382 nm) of the rutile phase with alumina surface coating, and compared it to 25 nm TiO2 particles (of a different crystal phase)43. In a third study, they examined the toxic potential of 3 samples of different crystal phases, however, the sizes were also different44. Surface properties were proposed to account for the differences in biological responses. Oberdörster et al2,45 conducted pulmonary toxicity tests with 20 nm (80% anatase) and 250 nm (100% anatase) titanium dioxide particles and found that the total surface area was a metric that related to neutrophil lung inflammation in rats. No clear trends with regard to the influence of TiO2 crystallinity and particle size on biological activity could be seen in these different studies and contrasting concepts were proposed. One major reason was the paucity of different well-characterized TiO2 samples (in terms of size and crystallinity) that were used, thus precluding the development of convincing conclusions about the function of parameters such as particle size or crystal phase.
In 1990, the lipid nanoparticles were invented in the laboratories, the first patent filings took place in 1991. The lipid nanoparticles were developed as alternative to traditional carriers such as polymeric nanoparticles and leptosomes46. Meanwhile many research groups are active worldwide, their results are reviewed which cover many different administration routes: dermal and mucosal, oral, intravenous/parenteral, pulmonary but also ocular (Fig 1, Table 1). The lipid nanoparticles are also used for peptide/protein delivery, in gene therapy and various miscellaneous applications (e.g. vaccines)29,47,48. Although lipid nanoparticles represent potent drug carriers, for many formulations toxicity data are rare.
Quantum dots are nanoparticles made of semiconductor materials with fluorescent properties. Crucial for biological applications quantum dots must be covered with other materials allowing dispersion and preventing leaking of the toxic heavy metals49.
Polymers such as polysaccharide chitosan nanoparticles have been used for some time now as drug delivery systems 50. Recently, water-soluble polymer hybrid constructs have been developed. These are polymer–protein conjugates or polymer–drug conjugates. Polymer conjugation to proteins reduces immunogenicity, prolongs plasma half-life and enhances protein stability. Polymer–drug conjugation promotes tumour targeting through the enhanced permeability and retention effect and, at the cellular level following endocytic capture, allows lysosomotropic drug delivery 51 (Fig 1, Table 1).
Liposomes are nanoparticles comprising lipid bilayer membranes surrounding an aqueous interior. The amphilic molecules used for the preparation of these compounds have similarities with biological membranes and have been used for improving the efficacy and safety of different drugs52. Usually, liposomes are classified into three categories on the basis of their size and lamellarity (number of bilayers): small unilamellar vesicles or oligolamellar, large unilamellar vesicles and multilamellar vesicles. The active compound can be located either in the aqueous spaces, if it is water-soluble, or in the lipid membrane, if it is lipid-soluble.
Recently, a new generation of liposomes called ‘stealth liposomes’ have been developed. Stealth liposomes have the ability to evade the interception by the immune systems, and therefore, have longer half-life53.
Cellular interaction with nanoparticles
Like nanoorganisms (viruses), nanoparticles are able to enter cells and interact with subcellular structures. Cellular uptake, subcellular localization, and ability to catalyze oxidative products depend on nanoparticle chemistry, size, and shape13. The mechanism by which nanoparticles penetrate cells without specific receptors on their outer surface is assumed to be a passive uptake or adhesive interaction. This uptake may be initiated by van der Waals forces, electrostatic charges, steric interactions, or interfacial tension effects, and does not result in the formation of vesicles41,54. (Steric interactions occur when nanoparticles have molecules with size, geometries, bonding, and charges optimized for the interaction with the receptors). After this type of uptake, the nanoparticles are not necessarily located within a phagosome (which offers some protection to the rest of the cellular organelles from the chemical interaction with the nanoparticle). For example, C60 molecules enter cells and can be found along the nuclear membrane and within the nucleus. Very small nanoparticles, such as C60 molecules with a diameter of 0.7 nm, are able to penetrate cells via a different mechanism than phagocytosis, probably through ion channels or via pores in the cell membrane55. This type of uptake and free movement within the cell makes them very dangerous by having direct access to cytoplasm proteins and organelles. Uptake location is likely to depend on material type; however, current research does not provide sufficient information to drawing conclusions on this subject. Upon nonphagocytic uptake, nano-particles can be found in various locations inside the cell, such as the outer-cell membrane, cytoplasm, mitochondria, lipid vesicles, along the nuclear membrane, or within the nucleus56. Depending on their localization inside the cell, the nanoparticles can damage organelles or DNA, or ultimately cause cell death. Recent studies have used a composite phenotype (psychosis) that includes BPD, SCZ, psychosis not otherwise specified, and schizoaffective disorder, to identify shared susceptibility loci. Several chromosomal regions are reported to be shared between these syndromes (18p, 6q, 10p, 13q, 22q)57.
Nanoparticles are internalized not only by professional phagocytes such as alveolar macrophages,4 but by various types of cells, including endothelial cells, pulmonary epithelium,58 gastrointestinal epithelium, red blood cells, platelets, and nerve cells10 (Fig 1). Particle internalization location depends on nanoparticle size. For example, environmental particles with size between 2.5 and 10 μm were found to collect in large cytoplasmic vacuoles, while smaller nanoparticles (100 nm) localize in organelles, such as mitochondria, leading to disruption of mitochondrial architecture59.
Biomarkers to assay the toxicity
With very few exceptions, previous nanotoxicity studies implicitly involved the assumption that the techniques developed for risk assessment of hazardous chemical substances can be applied in unchanged form to explore cell response in NP laden media. This misleading approach has the consequence that the actual dose of exposure is ill defined or, more often, completely unknown48.
Pro-inflammatory cytokines such as interleukin- 6 (IL-6) and tumor necrosis factor alpha (TNF- alpha) are involved in the formation of toxic peroxynitrite by increasing the activity of nitric oxide synthase (NOS) enzyme. Nitric oxide (NO) is potent inflammatory mediator because of its strong reactivity with oxygen, superoxide and iron-containing compounds. Prostaglandins are well known as proinflammatory mediators, and inhibition of cyclooxygenase (COX) has long been used in the management of inflammation. Levels of prostaglandin E2 (PGE2) are increased in various states of inflammation. Unlike larger particles, nanomaterials may be taken up by cell mitochondria and the cell nucleus. Studies demonstrate the potential for nanomaterials to cause DNA mutation and induce major structural damage to mitochondria, even resulting in cell death. Size is therefore a key factor in determining the potential toxicity of a particle.
The greater chemical reactivity of nanomaterials results in increased production of reactive oxygen species (ROS), including free radicals and it is one of the primary mechanisms of nanoparticle toxicity; it may result in oxidative stress, inflammatory cytokines production and consequent damage to proteins, membranes, DNA and cell death6.
Humanized NPs, termed nano-proresolving medicines, are mimetics of endogenous resolving mechanisms, possess potent beneficial bioactions, can reduce nanotoxicity, and offer new therapeutic approaches60.
Summary
Nanotoxicology is an emerging new multidisciplinary field of science, and therefore there is a risk of change in its rapid development in the near future. Therefore, development of novel nanoparticles for pharmacology, therapeutics and diagnostics must proceed in tandem with assessment of any toxicological and environmental side effects of these particles. As the bioenvironment is already polluted with nanoparticulates of particulate matter caution should be taken to prevent and contain any environmental effects of intentionally generated nanomaterials. In this review we tried to put our effort to show the toxicity of various nanoparticles, which will be a very valuable reference source to students and investigators in this research field to guide them in their future work.
Author’s Disclosures
The authors declare that they have no competing interests.
ACKNOWLEDGMENT
I am very grateful to Dr. Kamineni Shashidhar for his constant support and encouragement. I am also thankful to Kamineni Institute of Medical Sciences, providing all the support during this review drafting.
Bibliography
- 1.Kim I., Chu X. Y., Kim S., Provoda C. J., Lee K. D., Amidon G. L. Identification of a Human Valacyclovirase: Biphenyl Hydrolase-Like Protein As Valacyclovir Hydrolase. J. Biol. Chem. 2003, 278, 25348-25356. [DOI] [PubMed] [Google Scholar]
- 2.Oberdorster G., Maynard A., Donaldson K., Castranova V., Fitzpatrick J., Ausman K., Carter J., Karn B., Kreyling W., Lai D., Olin S., Monteiro-Riviere N., Warheit D., Yang H. Principles for Characterizing the Potential Human Health Effects From Exposure to Nanomaterials: Elements of a Screening Strategy. Part Fibre. Toxicol. 2005, 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahamed M., Siddiqui M. A., Akhtar M. J., Ahmad I., Pant A. B., Alhadlaq H. A. Genotoxic Potential of Copper Oxide Nanoparticles in Human Lung Epithelial Cells. Biochem. Biophys. Res. Commun. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Hoet P. H., Bruske-Hohlfeld I., Salata O. V. Nanoparticles - Known and Unknown Health Risks. J. Nanobiotechnology. 2004, 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Totsuka Y., Higuchi T., Imai T., Nishikawa A., Nohmi T., Kato T., Masuda S., Kinae N., Hiyoshi K., Ogo S., Kawanishi M., Yagi T., Ichinose T., Fukumori N., Watanabe M., Sugimura T., Wakabayashi K. Genotoxicity of Nano/Microparticles in in Vitro Micronuclei, in Vivo Comet and Mutation Assay Systems. Part Fibre. Toxicol. 2009, 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nel A., Xia T., Madler L., Li N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622-627. [DOI] [PubMed] [Google Scholar]
- 7.Englert N. Fine Particles and Human Health--a Review of Epidemiological Studies. Toxicol. Lett. 2004, 149, 235-242. [DOI] [PubMed] [Google Scholar]
- 8.Moghimi S. M., Hunter A. C., Murray J. C. Nanomedicine: Current Status and Future Prospects. FASEB J. 2005, 19, 311-330. [DOI] [PubMed] [Google Scholar]
- 9.Powers K. W., Brown S. C., Krishna V. B., Wasdo S. C., Moudgil B. M., Roberts S. M. Research Strategies for Safety Evaluation of Nanomaterials. Part VI. Characterization of Nanoscale Particles for Toxicological Evaluation. Toxicol. Sci. 2006, 90, 296-303. [DOI] [PubMed] [Google Scholar]
- 10.Oberdorster G., Sharp Z., Atudorei V., Elder A., Gelein R., Lunts A., Kreyling W., Cox C. Extrapulmonary Translocation of Ultrafine Carbon Particles Following Whole-Body Inhalation Exposure of Rats. J. Toxicol. Environ. Health A 2002, 65, 1531-1543. [DOI] [PubMed] [Google Scholar]
- 11.Thomas T., Thomas K., Sadrieh N., Savage N., Adair P., Bronaugh R. Research Strategies for Safety Evaluation of Nanomaterials, Part VII: Evaluating Consumer Exposure to Nanoscale Materials. Toxicol. Sci. 2006, 91, 14-19. [DOI] [PubMed] [Google Scholar]
- 12.Hoet P., Lison D. A Nonoccupational Source of Mercury Intoxication. Clin. Chem. 1997, 43, 1248. [PubMed] [Google Scholar]
- 13.Donaldson K., Stone V., Tran C. L, Kreyling W., Borm P. J. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uboldi C., Bonacchi D., Lorenzi G., Hermanns M. I., Pohl C., Baldi G., Unger R. E., Kirkpatrick C. J. Gold Nanoparticles Induce Cytotoxicity in the Alveolar Type-II Cell Lines A549 and NCIH441. Part Fibre. Toxicol. 2009, 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tkachenko A., Xie H., Franzen S., Feldheim D. L. Assembly and Characterization of Biomolecule-Gold Nanoparticle Conjugates and Their Use in Intracellular Imaging. Methods Mol. Biol. 2005, 303, 85-99. [DOI] [PubMed] [Google Scholar]
- 16.Chithrani B. D., Ghazani A. A., Chan W. C. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano. Lett. 2006, 6, 662-668. [DOI] [PubMed] [Google Scholar]
- 17.Chithrani B. D., Chan W. C. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano. Lett. 2007, 7, 1542-1550. [DOI] [PubMed] [Google Scholar]
- 18.Becker M. L., Bailey L. O., Wooley K. L. Peptide-Derivatized Shell-Cross-Linked Nanoparticles. 2. Biocompatibility Evaluation. Bioconjug. Chem. 2004, 15, 710-717. [DOI] [PubMed] [Google Scholar]
- 19.Connor E. E., Mwamuka J., Gole A., Murphy C. J., Wyatt M. D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325-327. [DOI] [PubMed] [Google Scholar]
- 20.Hauck T. S., Ghazani A. A., Chan W. C. Assessing the Effect of Surface Chemistry on Gold Nanorod Uptake, Toxicity, and Gene Expression in Mammalian Cells. Small 2008, 4, 153-159. [DOI] [PubMed] [Google Scholar]
- 21.Paciotti G. F., Myer L., Weinreich D., Goia D., Pavel N., McLaughlin R. E., Tamarkin L. Colloidal Gold: a Novel Nanoparticle Vector for Tumor Directed Drug Delivery. Drug Deliv. 2004, 11, 169-183. [DOI] [PubMed] [Google Scholar]
- 22.Rosi N. L., Giljohann D. A., Thaxton C. S., Lytton-Jean A. K., Han M. S., Mirkin C. A. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science 2006, 312, 1027-1030. [DOI] [PubMed] [Google Scholar]
- 23.Podsiadlo P., Sinani V. A., Bahng J. H., Kam N. W., Lee J., Kotov N. A. Gold Nanoparticles Enhance the Anti-Leukemia Action of a 6-Mercaptopurine Chemotherapeutic Agent. Langmuir 2008, 24, 568-574. [DOI] [PubMed] [Google Scholar]
- 24.Han G., Ghosh P., Rotello V. M. Multi-Functional Gold Nanoparticles for Drug Delivery. Adv. Exp. Med. Biol. 2007, 620, 48-56. [DOI] [PubMed] [Google Scholar]
- 25.Han G., Ghosh P., Rotello V. M. Functionalized Gold Nanoparticles for Drug Delivery. Nanomedicine. (Lond) 2007, 2, 113-123. [DOI] [PubMed] [Google Scholar]
- 26.Daniel M. C., Astruc D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293-346. [DOI] [PubMed] [Google Scholar]
- 27.Gobin A. M., Lee M. H., Halas N. J., James W. D., Drezek R. A., West J. L. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano. Lett. 2007, 7, 1929-1934. [DOI] [PubMed] [Google Scholar]
- 28.Huang X., El-Sayed I. H., Qian W., El-Sayed M. A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115-2120. [DOI] [PubMed] [Google Scholar]
- 29.Fung M. C., Bowen D. L. Silver Products for Medical Indications: Risk-Benefit Assessment. J. Toxicol. Clin. Toxicol. 1996, 34, 119-126. [DOI] [PubMed] [Google Scholar]
- 30.Danscher G. Light and Electron Microscopic Localization of Silver in Biological Tissue. Histochemistry 1981, 71, 177-186. [DOI] [PubMed] [Google Scholar]
- 31.Brumfiel G. Nanotechnology: A Little Knowledge. Nature 2003, 424, 246-248. [DOI] [PubMed] [Google Scholar]
- 32.Dreher K. L. Health and Environmental Impact of Nanotechnology: Toxicological Assessment of Manufactured Nanoparticles. Toxicol. Sci. 2004, 77, 3-5. [DOI] [PubMed] [Google Scholar]
- 33.Giles J. Nanotechnology: What Is There to Fear From Something So Small? Nature 2003, 426, 750. [DOI] [PubMed] [Google Scholar]
- 34.Hood E. Nanotechnology: Looking As We Leap. Environ. Health Perspect. 2004, 112, A740-A749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donaldson K., Stone V., Tran C. L., Kreyling W., Borm P. J. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaton A., Donaldson K. Nanoscience, Nanotoxicology, and the Need to Think Small. Lancet 2005, 365, 923-924. [DOI] [PubMed] [Google Scholar]
- 37.Gurr J. R., Wang A. S., Chen C. H., Jan K. Y. Ultrafine Titanium Dioxide Particles in the Absence of Photoactivation Can Induce Oxidative Damage to Human Bronchial Epithelial Cells. Toxicology 2005, 213, 66-73. [DOI] [PubMed] [Google Scholar]
- 38.Hussain S., Thomassen L. C., Ferecatu I., Borot M. C., Andreau K., Martens J. A., Fleury J., Baeza-Squiban A., Marano F., Boland S. Carbon Black and Titanium Dioxide Nanoparticles Elicit Distinct Apoptotic Pathways in Bronchial Epithelial Cells. Part Fibre. Toxicol. 2010, 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N., Duan Y., Hong M., Zheng L, Fei M., Zhao X., Wang J., Cui Y., Liu H., Cai J., Gong S., Wang H., Hong F. Spleen Injury and Apoptotic Pathway in Mice Caused by Titanium Dioxide Nanoparticules. Toxicol. Lett. 2010, 195, 161-168. [DOI] [PubMed] [Google Scholar]
- 40.Shintani H., Kurosu S., Miki A., Hayashi F., Kato S. Sterilization Efficiency of the Photocatalyst Against Environmental Microorganisms in a Health Care Facility. Biocontrol. Sci. 2006, 11, 17-26. [DOI] [PubMed] [Google Scholar]
- 41.Donaldson K., Tran L., Jimenez L. A., Duffin R., Newby D. E., Mills N., MacNee W., Stone V. Combustion-Derived Nanoparticles: a Review of Their Toxicology Following Inhalation Exposure. Part Fibre. Toxicol. 2005, 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warheit D. B., Webb T. R., Sayes C. M., Colvin V. L., Reed K. L. Pulmonary Instillation Studies With Nanoscale TiO2 Rods and Dots in Rats: Toxicity Is Not Dependent Upon Particle Size and Surface Area. Toxicol. Sci. 2006, 91, 227-236. [DOI] [PubMed] [Google Scholar]
- 43.Warheit D. B., Webb T. R., Reed K. L., Frerichs S., Sayes C. M. Pulmonary Toxicity Study in Rats With Three Forms of Ultrafine-TiO2 Particles: Differential Responses Related to Surface Properties. Toxicology 2007, 230, 90-104. [DOI] [PubMed] [Google Scholar]
- 44.Sayes C. M., Wahi R., Kurian P. A., Liu Y., West J. L, Ausman K. D., Warheit D. B., Colvin V. L. Correlating Nanoscale Titania Structure With Toxicity: a Cytotoxicity and Inflammatory Response Study With Human Dermal Fibroblasts and Human Lung Epithelial Cells. Toxicol. Sci. 2006, 92, 174-185. [DOI] [PubMed] [Google Scholar]
- 45.Oberdorster G., Elder A., Rinderknecht A. Nanoparticles and the Brain: Cause for Concern? J. Nanosci. Nanotechnol. 2009, 9, 4996-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller R. H., Shegokar R., Keck C. M. 20 Years of Lipid Nanoparticles (SLN and NLC): Present State of Development and Industrial Applications. Curr. Drug Discov. Technol. 2011. [DOI] [PubMed] [Google Scholar]
- 47.Pumera M. Nanotoxicology: the Molecular Science Point of View. Chem. Asian J. 2011, 6, 340-348. [DOI] [PubMed] [Google Scholar]
- 48.Wittmaack K. Excessive Delivery of Nanostructured Matter to Submersed Cells Caused by Rapid Gravitational Settling. ACS Nano. 2011. [DOI] [PubMed] [Google Scholar]
- 49.Weng J., Ren J. Luminescent Quantum Dots: a Very Attractive and Promising Tool in Biomedicine. Curr. Med. Chem. 2006, 13, 897-909. [DOI] [PubMed] [Google Scholar]
- 50.Agnihotri S. A., Mallikarjuna N. N., Aminabhavi T. M. Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery. J. Control Release 2004, 100, 5-28. [DOI] [PubMed] [Google Scholar]
- 51.Lee L. J. Polymer Nano-Engineering for Biomedical Applications. Ann. Biomed. Eng 2006, 34, 75-88. [DOI] [PubMed] [Google Scholar]
- 52.Hofheinz R. D., Gnad-Vogt S. U., Beyer U., Hochhaus A. Liposomal Encapsulated Anti-Cancer Drugs. Anticancer Drugs 2005, 16, 691-707. [DOI] [PubMed] [Google Scholar]
- 53.Moghimi S. M., Szebeni J. Stealth Liposomes and Long Circulating Nanoparticles: Critical Issues in Pharmacokinetics, Opsonization and Protein-Binding Properties. Prog. Lipid Res. 2003, 42, 463-478. [DOI] [PubMed] [Google Scholar]
- 54.Geiser M., Rothen-Rutishauser B., Kapp N., Schurch S., Kreyling W., Schulz H., Semmler M., Im H. V, Heyder J., Gehr P. Ultrafine Particles Cross Cellular Membranes by Nonphagocytic Mechanisms in Lungs and in Cultured Cells. Environ. Health Perspect. 2005, 113, 1555-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porter A. E., Muller K., Skepper J., Midgley P., Welland M. Uptake of C60 by Human Monocyte Macrophages, Its Localization and Implications for Toxicity: Studied by High Resolution Electron Microscopy and Electron Tomography. Acta Biomater. 2006, 2, 409-419. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Garcia E., Andrieux K., Gil S., Kim H. R., Le D. T., Desmaele D., d’Angelo J., Taran F., Georgin D., Couvreur P. A Methodology to Study Intracellular Distribution of Nanoparticles in Brain Endothelial Cells. Int. J. Pharm. 2005, 298, 310-314. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee O., Meera P., Ghosh S., Kubendran S., Kiran K., Manjunath K. R., Subhash M. N., Benegal V., Brahmachari S. K., Majumder P. P., Jain S. Evidence of Linkage and Association on 18p11.2 for Psychosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 868-873. [DOI] [PubMed] [Google Scholar]
- 58.Sood N., Bennett W. D., Zeman K., Brown J., Foy C., Boucher R. C., Knowles M. R. Increasing Concentration of Inhaled Saline With or Without Amiloride: Effect on Mucociliary Clearance in Normal Subjects. Am. J. Respir. Crit Care Med. 2003, 167, 158-163. [DOI] [PubMed] [Google Scholar]
- 59.Vijaya L. K., Shetty P., Vottam K., Govindhan S., Ahmad S. N., Hasan Q. Tumor Necrosis Factor Alpha - C850T Polymorphism is Significantly Associated With Endometriosis in Asian Indian Women. Fertil. Steril. 2009. [DOI] [PubMed] [Google Scholar]
- 60.Norling L. V., Spite M., Yang R., Flower R. J., Perretti M., Serhan C. N. Cutting Edge: Humanized Nano-Proresolving Medicines Mimic Inflammation-Resolution and Enhance Wound Healing. J. Immunol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


