Summary
Background
Previous case-control studies have suggested that the absence of a swallow-tail appearance in the substantia nigra on high-resolution SWI, representing nigrosome-1, has high accuracy to identify Parkinson’s disease (PD). The first goal of our study was to evaluate nigrosome-1 ex vivo using optimized high-resolution susceptibility sensitive MRI. Our second goal was to evaluate its diagnostic value in vivo using a clinical 3T SWI sequence to differentiate between PD and atypical parkinsonism (AP) in a cohort of patients with early-stage parkinsonism.
Material/Methods
Case-control pilot study to evaluate nigrosome-1 ex vivo (2 PD, 2 controls), using high-resolution susceptibility sensitive sequences at 11.7 T MRI. Next, evaluation of nigrosome-1 in vivo using a clinical 3 T SWI sequence in a prospective cohort of 60 patients with early-stage parkinsonism (39 PD, 21 AP). Moreover, 12 control subjects were scanned.
The bilateral substantia nigra was evaluated by two neuroradiologists for the presence, absence or indecisive presence of nigrosome-1. The discriminative power was evaluated by Receiver-Operating Characteristic.
Results
We identified nigrosome-1 in ex vivo control subjects. Nigrosome-1 was not identified in the ex vivo PD cases. In our prospective clinical cohort study, the AUC for the swallow-tail sign to discriminate between PD and AP was 0.56 (0.41–0.71) for reader 1 and 0.68 (0.55–0.82) for reader 2.
Conclusions
The diagnostic accuracy of the swallow-tail sign was marginal to discriminate between PD and AP using our clinical 3 T SWI sequence.
MeSH Keywords: Brain Diseases, Magnetic Resonance Imaging, Parkinson Disease, Parkinsonian Disorders
Background
Parkinson’s disease (PD) is a neurodegenerative disorder with incidence increasing with age. The diagnosis of PD is made on clinical grounds, based on classic motor symptoms including bradykinesia, rest tremor or rigidity [1,2]. Conventional brain MRI lacks a diagnostic marker specific for PD [3]. Previously, studies have been published with promising results regarding T 1 and T 2 signal intensity changes of the substantia nigra (SN) in PD [4,5], which could not be reproduced in follow-up studies. In the diagnostic work-up of parkinsonism, brain MRI is therefore mainly used to exclude other, more rare causes of parkinsonism such as vascular damage or hydrocephalus. Also, abnormalities on brain MRI could support the diagnosis of a neurodegenerative disorder other than PD, usually referred to as atypical parkinsonism (AP). These include multiple system atrophy (MSA), progressive supranuclear palsy (PSP), dementia with Lewy bodies (DLB) and corticobasal syndrome (CBS).
New MRI techniques such as diffusion tensor imaging (DTI) and magnetization transfer imaging (MTI) may have the potential to provide a new diagnostic marker specific for PD [6], but clinical application of these techniques is limited because study results are not consistent and validated diagnostic criteria are generally lacking [6,7]. This also accounts for quantitative measures of iron content of the SN in PD, demonstrated as increased susceptibility on T 2* or Susceptibility Weighted Imaging (SWI) sequences [8–10].
In recent in vivo and postmortem studies using high-resolution susceptibility sensitive MR imaging techniques (7 T but also 3 T MRI), a new diagnostic marker has been reported with high accuracy to identify PD [11–13]. A subregion in the ventro-lateral part of the substantia nigra lacking susceptibility and bordered by two lines of susceptibility, resembling a swallow-tail (Figure 1), represents nigrosome-1 and is identified in healthy controls. The absence of this swallow-tail configuration has been reported in PD, with observed good discrimination between PD and control subjects (reported accuracy 91–96%) [14]. It is not known whether the absence of the swallow-tail sign on SWI could discriminate PD from AP.
Figure 1.
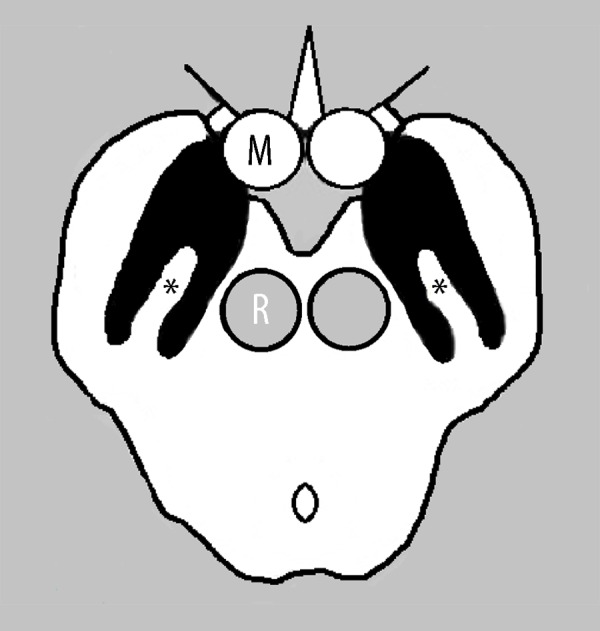
Schematic representation of nigrosome-1 on SWI (asterisks), a subregion in the ventrolateral substantia nigra pars compacta lacking susceptibility bordered by two lines of susceptibility resembling a swallow tail. R – red nucleus, M – mammillary body.
The first goal of our pilot study was to evaluate the swallow-tail appearance in the substantia nigra ex vivo, using optimized high-resolution susceptibility sensitive MRI sequences. Our second goal was to evaluate the diagnostic value of the swallow-tail sign to differentiate between PD and AP in a cohort of patients with early-stage parkinsonism using a clinical 3 T SWI sequence.
Material and Methods
Ex vivo case-control study (11.7 T MRI)
Formalin-fixed postmortem midbrain samples of 2 patients with histopathologically confirmed PD (76 y/o male and 81 y/o female) and 2 controls (76 and 84 y/o females without a neurodegenerative disorder) were collected. Slices of the midbrain including the lower part of the substantia nigra were rehydrated with phosphate buffered saline for one week to reduce fixation-induced T2 shortening. A unilateral part of the midbrain samples (measuring approximately 10×10×5 mm), was placed in a syringe filled with Galden D40 perfluoropolyether (Solvay Solexis, New York). The samples were stored at room temperature for 72 hours prior to scanning and air bubbles were removed. This method is in accordance with a previous ex vivo MRI study of our group [15].
Imaging was performed on an 11.7-T BioSpec Avance III small animal MRI system (Bruker BioSpin, Ettlingen, Germany) equipped with an actively shielded gradient set of 600 mT/m and operated by the ParaVision 5.1 software platform. A circular polarized volume resonator was used for signal transmission, and an actively decoupled mouse brain quadrature surface coil with integrated combiner and preamplifier (Bruker BioSpin) for signal reception. After standard adjustments, 2D and 3D gradient echo sequences were acquired. Details of the scanning protocol are provided in Table 1.
Table 1.
Details of the MRI scanning protocols used.
| Study/MR field strength | Sequence | TR/TE | Flip angle ° | Matrix size | Voxel (mm) | No. of averages | Acquisition time |
|---|---|---|---|---|---|---|---|
| Ex vivo/11.7 T | 2D MGE* | 1248/5 | 12 | 512×512 | 0.079×0.079×0.64 | 2 | 16 min |
| 3D MGE* with 11 echos | 57/3.4–53.4 | 30 | 256×256 | 0.112×0.112×0.112 | – | 47 min | |
| In vivo SWI 3 mm sliced/3 T | 3D gradient echo SWI | 29/20 | 15 | 384×384 | 0.63×0.63×x3 | 1 | 4 min, 42 sec |
MGE – multi gradient echo.
The images were visually inspected by two neuroradiologists (FJAM and BG) for the presence of a swallow-tail appearance in the substantia nigra pars compacta, in order to identify nigrosome-1.
Clinical cohort and case-control study (clinical 3T SWI)
A prospective observational study of 60 patients presenting with early stage parkinsonism, part of a larger clinical cohort study, was performed. Those patients were prospectively recruited as part of a larger clinical cohort study. The work described was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The medical ethics committee of our hospital (CMO Arnhem-Nijmegen) approved the study and all participants gave written informed consent prior to inclusion. Patients were recruited at our outpatient movement disorder clinic in the period 2010–2012.
Study inclusion criteria were clinical signs and symptoms of parkinsonism (hypokinetic-rigid syndrome) with uncertain clinical diagnosis and disease duration of less than 3 years. Exclusion criteria were age under 18 years, prior brain surgery, presence of another neurodegenerative disorder and unstable co-morbidity.
After a mean clinical follow-up of 24.6 (±12.4) months, ‘silver standard’ diagnoses could be made by two experienced clinicians (AR, RE): 39 patients were diagnosed with PD and 21 patients with AP (13 MSA-P, 3 PSP, 3 DLB, 1 vascular parkinsonism, 1 CBS). Those diagnoses were made according to international diagnostic criteria [16–21], based on neurological signs that developed during the course of the disease (as identified during repeat neurological exams), rate of disease progression and treatment response. In addition, 12 controls were included. Demographic criteria of the study groups are summarized in Table 2. In comparison to PD, disease severity and severity of motor symptoms were higher for AP (p<0.05).
Table 2.
Demographic data of the prospective study group.
| PD (n=39) | AP (n=21) | Controls (n=12) | |
|---|---|---|---|
| Mean age (yrs) | 61.5 (9.1) | 64.8 (7.7) | 71.4 (7.4) |
| Gender (M: F) | 24: 15 | 9: 12 | 8: 4 |
| Disease duration (months) | 21.6 (11.9) | 28.4 (11.1) | – |
| MMSE | 28.5 (1.6) | 28.2 (1.5) | – |
| H&Y | 1.7 (0.65)* | 2.4 (0.61)* | – |
| UPDRS-III | 32.4 (12)* | 45.7 (12.8)* | – |
PD – Parkinson’s disease; AP – atypical parkinsonism; MMSE – mini-mental state examination; H&Y – Hoehn and Yahr staging scale; UPDRS-III – Unified Parkinson Disease Rating Scale – III.
Student’s t-test p-value <0.05.
At baseline of this study, all patients had a 3-T brain MRI scan (Magnetom Trio, Siemens, Erlangen, Germany), which included a clinical SWI sequence with a high in-plane resolution but 3-mm slice thickness (0.63×0.63×3 mm voxels). Details of the scanning protocol are provided in Table 1.
These SWI sequences were visually evaluated by two neuroradiologists (FJAM and SS), blinded to clinical information and diagnoses. The bilateral substantia nigra was scored for the unambiguous presence, unambiguous absence, or indecisive presence of the swallow-tail sign. Unilateral absence of the swallow-tail sign was considered indicative of PD irrespective of the other side, as the onset of PD can be asymmetrical.
Cohen’s kappa co-efficient was used to evaluate intra- and inter-rater variability. Agreement was graded as: kappa <0.20, poor agreement; 0.21–0.4, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; >0.80, excellent agreement. One reader (FJAM) scored all MRI studies twice with a two-week interval in order to evaluate the intra-rater variability.
The discriminative power of the swallow-tail appearance was evaluated for both readers with the Receiver-Operating-Characteristic (ROC).
Results
Ex vivo case-control study (11.7 T MRI)
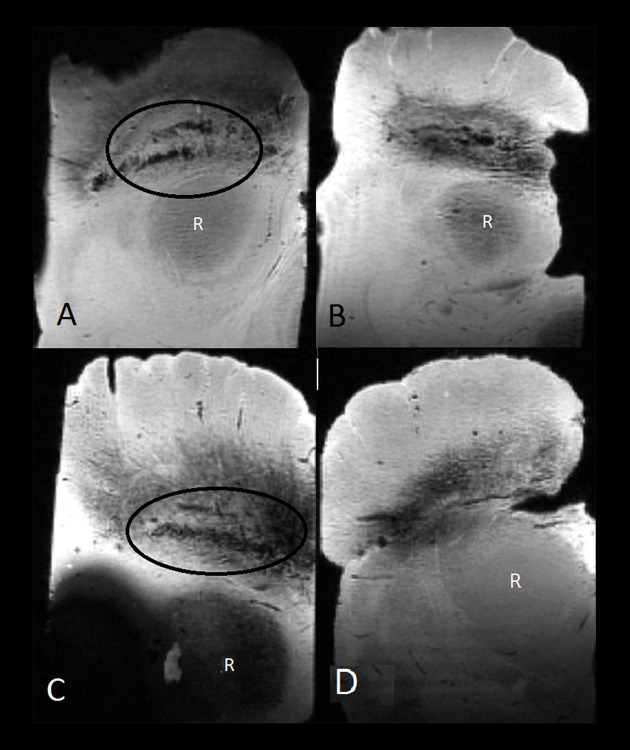
Nigrosome-1 was identified by a swallow-tail appearance in the ventrolateral substantia nigra pars compacta on the MR images of the two control samples, while nigrosome-1 was not identified in the two PD samples (Figure 2).
Figure 2.
Ex vivo midbrain samples scanned with a multi-gradient echo sequence on a 11.7-T MRI scanner. A swallow tail appearance of nigrosome-1 is identified in the lower part of the substantia nigra pars compacta (encircled) in two control subjects (A, C). Nigrosome-1 could not be identified in the two samples diagnosed with Parkinson’s disease (B, D). R – red nucleus.
Clinical cohort and case-control study (clinical 3 T SWI)
Table 3 demonstrates the distribution of nigrosome-1 as identified by both readers in PD, AP and controls. Inter-rater variability proved to be fair (kappa 0.35). Intra-rater variability proved to be moderate (kappa 0.44). For reader 2, nigrosome-1 was identified in the majority of PD patients (56%), while this was the case in a minority of PD patients for reader 1 (23%).
Table 3.
The absence, presence and indecisive presence of nigrosome-1 in Parkinson’s disease, atypical parkinsonism and controls.
| Nigrosome-1 absent | Nigrosome-1 present | Nigrosome-1 indecisive | |
|---|---|---|---|
| Reader 1 | |||
| PD (n=39) | 23 (59%) | 9 (23%) | 7 (18%) |
| AP (n=21) | 14 (67%) | 6 (29%) | 1 (5%) |
| Controls (n=12) | 1 (8%) | 8 (67%) | 3 (25%) |
| Reader 2 | |||
| PD (n=39) | 13 (33%) | 22 (56%) | 4 (10%) |
| AP (n=21) | 14 (67%) | 7 (33%) | 0 (0%) |
| Controls (n=12) | 6 (50%) | 5 (42%) | 1 (8%) |
PD – Parkinson’s disease; AP – atypical parkinsonism.
The ability of the swallow-tail sign to discriminate between PD and controls resulted in an AUC of 0.72 (0.58–0.87) for reader 1 and 0.58 (0.39–0.77) for reader 2. For the discrimination between PD and AP, the AUC was 0.56 (0.41–0.71) for reader 1 and 0.68 (0.55–0.82) for reader 2.
Discussion
A compartmental organization of the substantia nigra, consisting of the nigral matrix and nigrosomes, was first described based on the immunohistochemical staining of calbindin in striatonigral afferent fibers [22]. It was reported that the largest of the nigrosomes called nigrosome-1, located in the ventro-lateral substantia nigra, was most affected in PD exhibiting the maximum depletion of dopaminergic cells [23]. Based on the results of previous case-control studies [11–14], the swallow-tail sign seems to be a promising new diagnostic MRI marker in the diagnostic work-up of parkinsonism. In contrast to quantitative MRI techniques, this possible new diagnostic marker can be easily implemented in everyday clinical practice because there is no requirement for complicated post-processing prior to visual assessment. Using an 11.7-T MRI scanner, we were able to identify the swallow-tail appearance of nigrosome-1 in the substantia nigra pars compacta in ex vivo midbrain samples of controls. In two midbrain samples with histopathologically confirmed PD, this structure was absent. Yet, no studies have been published evaluating nigrosome-1 in AP, and evaluating its diagnostic value in discriminating PD from AP.
In our prospective observational cohort study, the diagnostic accuracy of the swallow-tail sign to differentiate between PD and AP was marginal, with limited intra- and inter-rater agreement. This also accounts for the differentiation between PD and controls, with a diagnostic performance clearly lower than the case-control studies published previously. A likely explanation for this discrepancy is that the 3-mm slices of our clinical SWI protocol were too thick to reliably visualize nigrosome-1, despite its high in-plane resolution. This explanation is supported by the fact that a swallow-tail configuration in the substantia nigra was missing in a significant part of the control subjects on this sequence. The minimal required spatial resolution of SWI to reliably identify nigrosome-1 has not been reported, though an optimized 3D high-resolution SWI is probably crucial with increased confidence at higher magnetic field strengths. In a very recent case-control study comparing 7 T and 3 T for the evaluation of nigrosome-1 [23], the confidence in revealing the inner structure of the substantia nigra on 3 T was inferior to that of 7 T as demonstrated by lower intra- and inter-observer agreement at 3 T. Also, the diagnostic accuracy to identify PD proved to be slightly lower for 3 T (86%) in comparison to 7 T MRI (96%). Increased T 2* contrast and magnetic susceptibility effects of paramagnetic substances at higher magnetic fields is the explanation the authors consider the most likely for the superior imaging performance of 7T, rather than a higher spatial resolution which only differed slightly between 3 T and 7 T [24].
Furthermore, it can be debated whether a swallow-tail configuration identified on our relatively thick-sliced SWI sequence actually represents nigrosome-1 in the inner structure of the substantia nigra, or would be a reflection of the closely related substantia nigra and subthalamic nucleus. This bears the risk of a false-positively identified nigrosome-1.
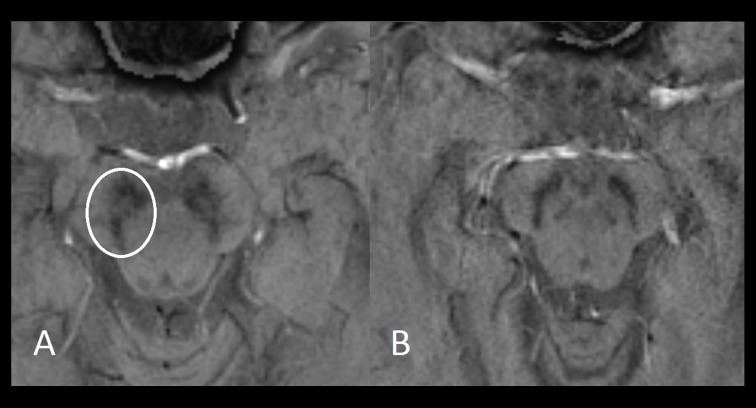
Another discrepancy with previous studies is that a swallow-tail configuration in the substantia nigra was frequently seen in our prospective cohort of patients diagnosed with PD. Our PD patients were probably scanned earlier in the course of the disease (mean disease duration of 21.6 months) than the case-control studies published previously (which included patients with disease duration of up to 10 years) [12,14,24]. The absence of nigrosome-1 in the substantia nigra in PD on SWI is probably not explained by predominant degeneration or volume loss, but is most likely explained by pathologically increased iron accumulation with increased susceptibility on SWI as a result [14]. However, in previous studies increase of the iron concentrations of the substantia nigra did not seem to be related to the disease duration in PD, while it was correlated with the severity of motor symptoms [25,26]. Another possible explanation for the absence of nigrosome-1 in PD is decreased neuromelanin content of the substantia nigra with decreased iron storage capacity leading to more free iron with paramagnetic properties [27]. It needs to be determined whether the absence of nigrosome-1 on SWI in PD is related to disease duration and whether it correlates with loss of presynaptic dopamine transporters, as demonstrated by nuclear scan techniques. In case the absence of nigrosome-1 on SWI was not related to disease duration, it could prove to be a useful diagnostic MRI marker in the work-up of early-stage parkinsonism. It needs to be determined whether it is PD-specific and not valid for atypical parkinsonism, as absence of the swallow-tail sign was also noted in AP in our clinical cohort (Figure 3).
Figure 3.
Axial 3 T SWI images of the midbrain (0.63×0.63×3-mm voxel) demonstrating a swallow tail configuration in the substantia nigra in a patient diagnosed with dementia with Lewy bodies (A), and its absence in a patient diagnosed with vascular parkinsonism (B).
There are some limitations to our study. Our in vivo and ex vivo case-control studies included only a few subjects. Because case-control studies with a larger amount of subjects have been published recently, we only aimed to reproduce the identification of the healthy nigrosome-1, and its absence in PD. A limitation of our clinical prospective observational cohort is that we do not have any post-mortem confirmation of the diagnoses, and therefore we cannot fully rule out misdiagnosis. Well-designed prospective clinical cohort studies are warranted for assessing the diagnostic accuracy of the swallow-tail sign in early-stage parkinsonism. Conducting such a study is challenging because obtaining histopathological confirmation for larger study populations is practically impossible. Although high rates of misdiagnosis have been reported on for the clinical diagnosis, pathological studies show high accuracy levels (>90%) for the clinical diagnosis when the diagnosis was made by a movement disorder specialist after a minimal follow-up of 2 years [28].
Conclusions
The diagnostic accuracy of the swallow-tail sign was marginal to discriminate between PD and AP using our clinical 3 T SWI sequence with high in-plane resolution but 3-mm slice thickness. The diagnostic value of the swallow-tail sign in the work-up of early-stage parkinsonism using an optimized high-resolution SWI sequence needs to be determined in future well-designed prospective clinical cohort studies.
Acknowledgements
We thank Andor Veltien for acquisition of the ex vivo MRI data. We thank Michiel Kleinnijenhuis for providing advice.
Footnotes
Source of support: Parkinson Patiënten Vereniging and Van Alkemade Keuls Foundation
References
- 1.Factor SA, Weiner WJ. In: Parkinson’s disease, diagnosis and clinical management. Factor S, Weiner W, editors. New York: Demos Medical Publishing; 2007. [Google Scholar]
- 2.Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol. 2013;20(1):16–34. doi: 10.1111/ene.12022. [DOI] [PubMed] [Google Scholar]
- 3.Brooks DJ. Morphological and functional imaging studies on the diagnosis and progression of Parkinson’s disease. J Neurol. 2000;247:11–18. doi: 10.1007/pl00007755. [DOI] [PubMed] [Google Scholar]
- 4.Duguid JR, De La Paz R, DeGroot J. Magnetic resonance imaging of the midbrain in Parkinson’s disease. Ann Neurol. 1986;20(6):744–47. doi: 10.1002/ana.410200618. [DOI] [PubMed] [Google Scholar]
- 5.Braffman BH, Grossman RI, Goldberg HI, et al. MR imaging of Parkinson disease with spin-echo and gradient-echo sequences. Am J Roentgenol. 1989;152:159–65. doi: 10.2214/ajr.152.1.159. [DOI] [PubMed] [Google Scholar]
- 6.Lehéricy S, Sharman MA, Dos Santos CL, et al. Magnetic resonance imaging of the substantia nigra in Parkinson’s disease. Mov Disord. 2012;27(7):822–30. doi: 10.1002/mds.25015. [DOI] [PubMed] [Google Scholar]
- 7.Meijer FJA, Bloem BR, Mahlknecht P, et al. Update on diffusion MRI in Parkinson’s disease and atypical parkinsonism. J Neurol Sci. 2013;332:21–29. doi: 10.1016/j.jns.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: A potential biomarker of disease status. Neurology. 2008;70:1411–17. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- 9.Rossi ME, Ruottinen H, Saunamäki T, et al. Imaging brain iron and diffusion patterns: A follow-up study of Parkinson’s disease in the initial stages. Acad Radiol. 2014;21(1):64–71. doi: 10.1016/j.acra.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Haller S, Badoud S, Nguyen D, et al. Differentiation between Parkinson disease and other forms of Parkinsonism using support vector machine analysis of susceptibility-weighted imaging (SWI): Initial results. Eur Radiol. 2013;23(1):12–19. doi: 10.1007/s00330-012-2579-y. [DOI] [PubMed] [Google Scholar]
- 11.Kwon DH, Kim JM, Oh SH, et al. Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol. 2012;71:267–77. doi: 10.1002/ana.22592. [DOI] [PubMed] [Google Scholar]
- 12.Blazejewska AI, Schwarz ST, Pitiot A, et al. Visualization of nigrosome-1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology. 2013;81:534–40. doi: 10.1212/WNL.0b013e31829e6fd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noh Y, Sung YH, Lee J, Kim EY. Nigrosome 1 detection at 3T MRI for the diagnosis of early-stage idiopathic Parkinson disease: Assessment of diagnostic accuracy and agreement on imaging asymmetry and clinical laterality. Am J Neuroradiol. 2015;36(11):2010–16. doi: 10.3174/ajnr.A4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz ST, Afzal M, Morgan PS, et al. The ‘Swallow Tail’ appearance of the healthy nigrosome – a new accurate test of Parkinson’s disease: A case-control and retrospective cross-sectional MRI Study at 3T. PLoS One. 2014;9(4):e93814. doi: 10.1371/journal.pone.0093814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinnijenhuis M, Zerbi V, Küsters B, et al. Layer-specific diffusion weighted imaging in human primary visual cortex in vitro. Cortex. 2013;49(9):2569–82. doi: 10.1016/j.cortex.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–76. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65(12):1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 20.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):15–19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- 21.Zijlmans JC, Daniel SE, Hughes AJ, et al. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19(6):630–40. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 22.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D28k immunohistochemistry. Brain. 1999;122:1421–36. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- 23.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–48. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 24.Cosottini M, Frosini DF, Pesaresi I, et al. Comparison of 3T and 7T susceptibility-weighted angiography of the substantia nigra in diagnosing Parkinson disease. Am J Neuroradiol. 2015;36(3):461–66. doi: 10.3174/ajnr.A4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallis LI, Paley MN, Graham JM, et al. MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J Magn Reson Imaging. 2008;28(5):1061–67. doi: 10.1002/jmri.21563. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang Y, Wang J, et al. Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: An in vivo MR study. Brain Res. 2010;1330:124–30. doi: 10.1016/j.brainres.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Zecca L, Casella L, Albertini A, et al. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem. 2008;106:1866–75. doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- 28.Hughes AJ, Daniel SE, Ben Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]