Abstract
We report results of a retrospective analysis of 44 patients with relapsed and high-risk multiple myeloma (MM) undergoing allogeneic CD34-selected hematopoietic stem-cell transplantation (CD34-selected HSCT) from human leukocyte antigen (HLA)-compatible donors. Patients had multiply relapsed disease including relapse at <15 months after autologous transplant and most patients (28/44; 65%) also had high-risk cytogenetics. Before transplant, patients received busulfan (0.8 mg/kg X 10 doses), melphalan (70 mg/m2 X 2 days), fludarabine (25 mg/m2 X 5 days), and rabbit anti-thymocyte globulin (2.5 mg/kg X 2 days). Patients with 10/10 HLA-matched donors were treated prophylactically with low doses of donor lymphocyte infusions (0.5 to 1 X 106 CD3+/kg) starting at 4–6 months post CD34-selected HSCT. Acute (grade II–IV) graph-versus-host disease (GVHD) and transplant-related mortality at 12 months were 2% and 18%, respectively. Chronic GVHD was not observed in any patient. Overall and progression-free survival at 2 years was 54% and 31%, respectively. By multivariate analyses, the outcomes of CD34-selected HSCT were influenced by presence of extramedullary disease, disease status prior to CD34-selected HSCT and age.
This study demonstrates notable safety and efficacy of CD34-selected HSCT in patients with multiply relapsed MM including those with high-risk cytogenetics.
Introduction
Multiple myeloma (MM) is a malignant disease of plasma cells, with an estimated 25,000 new MM diagnoses annually, and about 11,000 projected patients to die of the disease every year.1,2,3 Approximately 25% of MM patients are considered “high-risk” as defined by routine cytogenetics. Despite the introduction of immunomodulatory agents and proteasome inhibitors patients with high-risk myeloma continue to do poorly, even with tandem autologous stem cell transplantation with a median survival of approximately 3 years.3,4
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potential curative treatment available for patients with multiple myeloma. Despite the potential advantages of graft-versus-tumor immune responses and a tumor-free source of stem cells, the success rate of patients undergoing conventional high-dose conditioning with allogeneic bone marrow or peripheral blood stem-cell transplantation has been historically compromised by high incidences of acute and/or chronic graft-versus-host disease (GVHD) and transplant-related mortalities (TRM) exceeding 40% at day 100 post-transplant.5–7 The introduction of non-myeloablative conditioning regimens in the treatment of myeloma has reduced associated toxicities and TRM, but high rates of acute and chronic GVHD persist.8–10 In addition, results from transplants with non-myeloablative regimens have been poor in patients with multiply relapsed disease.11,12 CD34+ selection has been effectively used in other hematologic malignancies as a strategy that allows intensification of the conditioning regimen while at the same time reducing the risks of acute and chronic GVHD. We have extensively studied CD34 selection in a variety of hematologic malignancy and have shown in retrospective analysis that long-term results of disease free survival and overall survival are comparable to unmanipulated grafts with significantly lower rates of acute and chronic GVHD. 13,14 Since 2007, we began performing CD34 selected allogeneic HCT in patients with relapsed MM. To determine the long-term disease specific outcomes as well as determinants of prognosis we performed a retrospective analysis of transplant outcomes on the initial 44 patients treated that are summarized herein.
Patients and Methods
Patients
We assessed the safety, toxicity, and efficacy of allogeneic CD34-selected HSCT in patients with high-risk, multiply relapsed MM at Memorial Sloan Kettering Cancer Center (MSKCC). The study was approved by the Institutional Review/Privacy Board at MSKCC and by the Food and Drug Administration.
Patients included in this study had relapsed multiple myeloma following autologous stem-cell transplantation (auto-SCT). Relapse had to occur either with normal cytogenetics within 15 months following the autologous transplant or with high-risk cytogenetics. Patients had to have achieved at least a partial response (PR) following additional chemotherapy or second salvage auto-SCT. Patients with an HLA-matched related or unrelated donor (genotypically matched at all A, B, C, DRB1, and DQB1 loci, as tested by DNA analysis) and patients who had an unrelated donor with only one antigen or one allele mismatch at the HLA A, B, C, DRB1, or DQB1 loci were eligible for entry on this protocol. All patients on study with at least 1 year of follow up post-CD34-selected HSCT at the time of analysis are presented in this report; encompassing patients who underwent allogeneic HSCT between 11/28/2007 and 10/9/2013. T-cell depletion was performed by positive CD34 selection using the Isolex 300i (Nexell Therapeutics, Irvine, CA, USA) followed by rosetting with sheep erythrocytes for the initial 13 patients (2008–09) and by CD34+ enrichment by the CliniMACS CD34 Reagent System (Miltenyi Biotech, Bergisch Gladbach, Germany) in 31 patients thereafter. Patients did not receive immunosuppressive therapy after transplantation. All patients signed written informed consent for their treatment trials.
Conditioning regimen
The preparative regimen began with busulfan at 0.8 mg/kg/dose every 6 hours for 10 doses intravenously (IV) and was administered on days −9 to −7. Busulfan doses were adjusted based on the pharmacokinetics of the first dose. Melphalan 70 mg/m2/day IV was given on days −7 to −6, and fludarabine 25 mg/m2/day IV was administered on days −6 to −2. Busulfan and melphalan doses were adjusted if the patient was >125% of ideal body weight as calculated on an adjusted ideal body. Rabbit anti-thymocyte globulin (ATG) was administered at 2.5 mg/kg/day on days −3 and −2. Methylprednisolone was given at 2 mg/kg/day for 2 days with the ATG administration and was discontinued thereafter.
Donor lymphocyte infusions
Recipients of 10/10 HLA-matched allografts were treated prophylactically with 5 X 105 CD3+/kg from matched donors at 4–6 months post-transplant. A second infusion of 5 X 105 CD3+/kg was administered 3–4 months following the first infusion. A third dose of 1 X 106 CD3+/kg was administered 2–4 months following the second infusion. Recipients of HLA-mismatched allografts were only treated preemptively with 1 X 105 CD3+/kg at diagnosis of relapse or progression, but no sooner than 4–6 months post-transplant. A second infusion of 5 X 105 D3+/kg was administered 1–3 months following the first infusion. A third infusion of 1 X 106 CD3+/kg could be administered 3–4 months following the second infusion. All patients were eligible for second and third doses of DLI only in the absence of GVHD.
Response criteria
Responses to CD34-selected HSCT and DLI were assessed 3 monthly intervals according to the International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple Myeloma.15 Patients were deemed to have progressed if they had an increase from their lowest response value by >25% of any of the following 1) M-spike (absolute increase must be >0.5g/dL); 2) in patients who do not produce a measurable M-spike, the difference in the involved-uninvolved free light chains (absolute increase must be >10mg/dL); 3) BM involvement by MM cells; or 4) the development of new, or increase in size of old, bone lesions or soft tissue plasmacytomas.
Cytogenetics and FISH analyses
Bone-marrow samples were collected before HSCT and at 30 days, 100 days, 6 months, 12 months, and 24 months post-HSCT. Analysis by MSKCC clinical laboratories was performed for immunohistochemistry of CD138 and light chains. Cytogenetics and fluorescence in situ hybridization (FISH) were performed on magnetic-bead–selected CD138 positive cells isolated from bone-marrow aspirates. For the purpose of this study, patients were considered to have high-risk cytogenetics if they had at least one of the following: gain 1q, deletion 17p, complex cytogenetics, t(4;14), or t(14;16) by FISH analyses or deletion 13 by karyotyping.
Biostatistics
Overall survival (OS) and progression-free survival (PFS) from the time of HSCT were evaluated using Kaplan-Meier methodology. The logrank test and Cox proportional hazard regression were used to compare the effect of disease and transplant characteristics on the time-to-event endpoints. Cumulative incidence functions were used to estimate the incidences of grade II–IV acute GVHD and non-relapse mortality (NRM). Competing risks for NRM were relapse, and for acute GVHD were relapse and death in the absence of GVHD. All analyses were conducted using the R statistical package. 16
Results
Patient characteristics
The pre-transplant characteristics of these patients, cytogenetics, and lines of treatment are detailed in Table I. Median follow-up among survivors was 24.8 months (range, 11.2–81.2 months). The median age at the time of the study transplant was 55.5 years (range, 32–68 years). All patients had prior auto-SCT followed by a relapse within 15 months. Eighteen of the 44 patients (40%) had two prior auto-SCTs. Additionally, 29/44 patients (65%) had high-risk cytogenetics and 13/44 patients (29%) were diagnosed with extramedullary disease manifestations prior to CD34-selected HSCT. All patients had 3–10 prior lines of treatment; 16 patients (36%) had >6 prior lines of treatment, 16 (36%) had 5–6 prior lines, and 12 (27%) had 3–4 lines. Median time from diagnosis to CD34-selected HSCT was 41 months. For 32 patients (72%), 10/10 HLA-matched donors were available (14 sibling donors; 18 matched unrelated donors), while the remaining 12 patients (28%) had 9/10 HLA-mismatched unrelated donors.
Table I.
| UPN# | MM | Cytogenetics | Prior Lines of TX (Detail) | Pri or Lines of TX | Age at BMT | BMT | Match | Donor |
|---|---|---|---|---|---|---|---|---|
| 1 | IgG Kappa | Normal | TD, Mel + tandem auto SCT, RVD | 3 | 42 | 11/28/2007 | 9/10 | Unrelated |
| 2 | IgA Lambda | t(4;14), del 13q | BD x 6; Mel + auto SCT; RD; VD | 4 | 38 | 6/18/2008 | 10/10 | Related |
| 3 | IgG Kappa | del 13q | TD; Mel + auto SCT; VTD | 3 | 32 | 8/20/2008 | 10/10 | Related |
| 4 | IgG Lambda | del 17p, del 13q | TD; Bortez; auto SCT; VAD; VP-16/Cytoxan | 5 | 57 | 3/4/2009 | 10/10 | Unrelated |
| 5 | IgG Kappa | t(4;14) | BIRD; Cytoxan, Mel + auto SCT; RVD | 3 | 69 | 4/30/2009 | 10/10 | Related |
| 6 | IgG Kappa | t(4;14) | TD→RVD; Cytoxan→Mel + tandem auto SCT; BDD | 3 | 54 | 9/3/2009 | 10/10 | Unrelated |
| 7 | IgG Lambda | 11q23, t(4;14), del 17p | VAD x 4; Mel + tandem auto SCT; RVD x 9 | 3 | 54 | 10/23/2009 | 10/10 | Related |
| 8 | IgG Lambda | t(4;14), del 13q | RD; Mel + tandem auto SCT; BD, XRT; DCEP; RVD | 5 | 49 | 11/20/2009 | 10/10 | Unrelated |
| 9 | IgA Lambda | del 17p, t(4;14) | TD; BT x 3; AMD310–3102→Mel + auto SCT #1; RD→RVD; monoclonal antibody BT-062; DCEP + Thal; Mel + Bortez + auto SCT #2 | 7 | 48 | 12/17/2009 | 10/10 | Related |
| 10 | IgG Kappa | MLL, del 13q | BDD, TD; Mel + auto SCT; CyBorD; RVD | 4 | 57 | 12/24/2009 | 8/10 | Unrelated |
| 11 | IgA Kappa | del 13q, del 14q32 | TD, XRT; auto SCT #1; VTD; Mel + auto SCT #2 | 4 | 46 | 1/15/2010 | 10/10 | Related |
| 12 | IgA Kappa | del 13q, 1q23 | TD; Cytoxan→Mel + auto SCT #1; RD; BD; DT-PACE; Mel + auto SCT #2 | 6 | 68 | 1/21/2010 | 10/10 | Related |
| 13 | IgG Kappa | t(4;14), del 13q | BDD; Mel + auto SCT; RVD | 3 | 56 | 3/5/2010 | 10/10 | Related |
| 14 | IgG Kappa | Normal | XRT, RD x 5; Auto SCT; RVD x 3; VDT-PACE x 2 | 4 | 65 | 8/13/2010 | 10/10 | Related |
| 15 | IgG Kappa | Normal | BDD x 3; TD x 2; Mel + auto SCT; XRT; RD x 5; CyBorD; Mel + auto SCT #2, XRT | 7 | 63 | 8/19/2010 | 9/10 | Unrelated |
| 16 | IgG Kappa | Normal | BD x 2; BDD x 2; BDD/Rev x 1; Mel + tandem auto SCT, Thal maintenance; RVD x 6; DT-PACE x 2 | 6 | 58 | 8/25/2010 | 9/10 | Unrelated |
| 17 | IgA Lambda | extra1q, del(13q), t(4;14) | COP + MP; Thal; Thal; BDD x 3; Mel + auto SCT #1, Thal maintenance; RD x 4; RVD; BDD x 2; VDT-PACE x 4; Mel + auto SCT #2 | 10 | 59 | 9/8/2010 | 9/10 | Unrelated |
| 18 | IgG Kappa | del(13q), der(1) | TD→Dex x 5; Mel + tandem auto SCT, TD; XRT; Bortez + Doxil; Revlimid; BD; RD; DCEP x 7 | 8 | 61 | 11/10/2010 | 9/10 | Unrelated |
| 19 | IgG Lambda | Normal | CyD x 2; BD x 2; Mel + auto SCT #1; VD; RD/Mel; Mel + auto SCT #2 | 6 | 57 | 12/2/2010 | 10/10 | Related |
| 20 | IgG Kappa | Normal | TD x 4; RVD→RD x 5; Mel + auto SCT #1; RVD | 4 | 54 | 12/10/2010 | 10/10 | Unrelated |
| 21 | IgG Kappa | p53, tri 17, 5p, 11, 15 | BiRD x 5; Mel + auto SCT #1; RVD; Rev maintenance; CyBorD x 5; VDT-PACE x 2; Mel + auto SCT #2 | 7 | 37 | 3/2/2011 | 10/10 | Unrelated |
| 22 | IgG Kappa | Normal | BDD x 3; TD x 2; Rev; Mel + auto SCT #1, Rev maintenance; Mel + auto SCT #2, Rev maintenance; VDT-PACE x 2; R-VDC x 3 | 7 | 49 | 4/14/2011 | 10/10 | Unrelated |
| 23 | Nonsecretory | del(20q), del(13q), del(17p), p53 | pulse dose dex; VAD x 4; BD x 4; Mel + auto SCT #1, BD maintenance; RD; Mel + auto SCT #2; BiRD maintenance | 7 | 63 | 4/20/2011 | 10/10 | Related |
| 24 | IgG Kappa | MLL, del(13q), IgH, p53 | pulse dose dex x 2; BBD x 2; TE x 2; Mel + tandem auto SCT, XRT, maintenance Thal; RVD x 10 | 5 | 45 | 5/26/2011 | 10/10 | Unrelated |
| 25 | IgG Lambda | extra 1q23 and 19p13, IgH, MLL, del p53, extra of 1q, 1p, del(13) and del(17p), extra 4, 11, and 14 | TD x 3; BD x 3; RD, VD-PACE x 1; VD-PACE x 3; Mel + auto SCT; Bortez mainenance | 7 | 60 | 6/3/2011 | 10/10 | Unrelated |
| 26 | IgG Lambda | extra 1q25, mono 13, Der3, 15p, 15q, trans IgH locus, del(17p) | TD x 1; RD x 4; Cytoxan→Mel + auto SCT #1; XRT/-Dex→RD x 6; Mel + auto SCT #2, Rev maintenance; CyBorD x 3; CyBorD x 2 | 7 | 62 | 8/31/2011 | 10/10 | Unrelated |
| 27 | IgG Lambda | Dup(1q), del(4p), 1q25, tri(9), mono(13), tri 15, mono 16, loss p53 gene, MLL | RVD→RD x 4; Mel + auto SCT #1; RVD→RD→R; DCEP + RVD→RD; BD + Benda; VDT-PACE x 1; Mel + auto SCT #2 | 7 | 56 | 9/21/2011 | 9/10 | Unrelated |
| 28 | IgG Kappa | del(1)(p13p22), +3, +5,+9, +11, del(13), (q12q14), del(14)(q24), der(16), t(11;16), p13.1;q24 | RVD x 9; XRT; Mel + auto SCT #1; VD x 3; RVD; VDT-PACE x 3; Mel + auto SCT #2 | 7 | 61 | 10/21/2011 | 9/10 | Unrelated |
| 29 | Lambda LC | Normal | T-BiRD; Mel + auto SCT; maintenance Rev; RD; RVD x 1→VD x 6 | 5 | 56 | 12/29/2011 | 10/10 | Unrelated |
| 30 | IgG Lambda | Normal | RVD x 6; Cytoxan→Mel + auto SCT #1, maintenance Rev; ClaPD x 5; Car x 3; VDT-PACE; Mel + auto SCT #2 | 6 | 50 | 2/1/2012 | 10/10 | Related |
| 31 | IgA Kappa | extra 1q25, trisomy 5, 9, 15; del12p1q | XRT, BD x 2, BDD x 2; Cytoxan→Mel + auto SCT, Rev maintenance; CyBorD x 4; VDT-PACE x 3 | 5 | 59 | 4/20/2012 | 9/10 | Unrelated |
| 32 | Nonsecretory | mono 13, t(11;14) | TD x 3; Bortez + TD x 2; Mel + tandem auto SCT; Rev/Dex/Lorvotuzumab/Mertansine x 9; BD x 4 | 5 | 52 | 8/1/2012 | 10/10 | Unrelated |
| 33 | Kappa LC | Normal | RVD x 4; Mel + auto SCT, boost for graft failure; Rev/Bortez maintenance; Vel-CT→Vel-C x 2 | 4 | 48 | 9/5/2012 | 10/10 | Unrelated |
| 34 | IgG Lambda | del 13q, del20q, extra 1q25, del4, 12, 16 | RVD x 4; VDT-PACE; Mel + auto SCT #1; CyBorD x 4; Bortez/Mel + auto SCT #2 | 5 | 44 | 12/28/2012 | 10/10 | Unrelated |
| 35 | IgG Lambda | at relapse: t(11;14), gain of chromosomes 11 and 14, del 13q | VAD + Cytoxan; BiRD; BT-D; Mel/Benda + auto SCT; DT-PACE; CRd | 6 | 62 | 1/2/2013 | 10/10 | Unrelated |
| 36 | IgA Kappa | extra copy of 1q, t(7;15), mono 7, 13, 14, 22 | first pulse dose Dex; BDD; TD x 2; Mel + auto SCT #1; RVD x 4→RD→RD→TD; Mel + auto SCT #2; CyBorD x 4; VDT-PACE x 3 | 8 | 64 | 1/11/2013 | 9/10 | Unrelated |
| 37 | IgG Lambda | t(4;14), tri 5, 9, 15 t(4;14), extra | BD x 4; Mel + auto SCT #1; Mel + auto SCT #2; BD x 4, VAD x 3;RD x 6, RVD, VTD; ClaPD; Cytoxan/Car | 8 | 57 | 3/15/2013 | 1010 | Related |
| 38 | IgG Kappa | of 1, extra of MLL Normal | RVD x 3.5; XRT; VDT-PACE x 2; Mel + auto SCT; BiRD maintenance; VDT-PACE x 2 | 6 | 60 | 4/12/2013 | 9/10 | Unrelated |
| 39 | IgG Lambda | Normal | RD x 4; Cytoxan→Mel + auto SCT; RD; Bortez→BD; CyBorD; VDT-PACE x 2 | 6 | 55 | 4/18/2013 | 10/10 | Unrelated |
| 40 | IgG Lambda | mono 13, IgH rearrangement, t(11;14), extra 1q25, del 13q14.3, 4 copies ETV6, 4 copies CBFB, 4 copies of 17 | RVD; RVD + Cytoxan; Mel + VDT-PACE; Mel/VRD-PACE + Auto SCT; Mel/VDT-PACE x 2; Mel/VRD-PACE + 2nd Auto SCT; maintenance alternating VRD and VMD; VDT-PACE; VDT-PACE/Mel + auto SCT #2; Car/Dex | 9 | 39 | 6/14/2013 | 10/10 | Unrelated |
| 41 | IgA Kappa | loss of p53, extra MLL | RVD x 3; Mel + auto SCT #1; Bortez maintenance; Mel + auto SCT #2, Rev maintenance; RVD; VDT-PACE x 1 | 6 | 48 | 8/8/2013 | 10/10 | Related |
| 42 | IgG Kappa | multiple copies MLL, extra copies 19p13 | TD; Cytoxan; mel + auto SCT #1, Thal maintenance; RD; RVD x 4; Car/Mel + auto SCT #2 | 6 | 56 | 8/21/2013 | 10/10 | Unrelated |
| 43 | IgG Kappa | RD x 2; RVD x 3; VDT-PACE;; Mel + tandem auto SCT; anti-PD1 antibody; Carfilzomib/Cytoxan/Dex x 6 | 7 | 53 | 10/3/2013 | 9/10 | Unrelated | |
| 44 | Kappa LC | del(13q), 1 copy IgH | RVD x2; VD x 1; Cytoxan; RD, RVD, RD, Rev maintenance, RD; Mel + auto SCT; Dex; CyBorD x 2; Pom/Dex x 3; Car-Pom-d; VDT-PACE | 10 | 46 | 10/9/2013 | 9/10 | Unrelated |
Abbreviations
BDD = Bortezomib, Doxil, Dexamethasone
BD = Bortezomib, Dexamethasone
BiRD = Biaxin, Clarithromycin, Revlimid, Dexamethasone
BT-D = Biaxin, Thalidomide, Dexamethasone Carfilzomib,
Car-Pom-d = Pomalidomide, Dexamethasone Clarithromycin,
ClaPD = Pomalidomide, Dexamethasone
COP = Cyclophosphamide, Vincristine, Prednisone
CRd = Carfilzomib, Revlimid, Dexamethasone
CyBorD = Cyclophophamide, Bortezomib, Dexamethasone
CyD = Cyclophosphamide, Dexamethasone Dexamethasone,
DCEP = Cyclophosphamide, Etoposide, Cisplatin Revlimid,
LD = Dexamethasone
MP = Melphalan, Prednisone
RVD = Revlimid, Velcade, Dexamethasone
R-VDC = Revlimid, Velcade, Dexamethasone, Cyclophosphamide
RD = Revlimid, Dexamethasone
TD = Thaldomide, Dexamethasone
TAD = Thalidomide, Adriamycin (doxorubicin), Dexamethasone
T-BiRD = Thalidomide, Biaxin, Clarithromycin, Revlimid, Dexamethasone
VAD = Vincristine, Adriamycin, Dexamethasone
VEL-CT = Velcade, Cyclophosphamide, Thalidomide
VB = Velcade, Bendamustine
VDT-PACE = Velcade, Dexamethasone, Thalidomide, Cisplatin, Adriamycin, Cyclophosphamide, Etoposide
VTD = Velcade, Thalidomide, Dexamethasone
Graft composition and engraftment
T-cell depletion performed by both methods achieved a median of 2.4 X 103 CD3+/kg (range, 4.72 X 102 to 1.29 X 104 CD3+/kg) for all patients. See Table II for complete graft composition of all 44 patients. No significant differences in the graft composition were observed when T-cell depletion was performed for the initial 13 patients by positive CD34 selection followed by rosetting with sheep erythrocytes compared to the subsequent CD34+ enrichment in the other patients (data not shown). All patients engrafted promptly at a median of 10 days post CD34-selected HSCT (range, 9–12 days). None of the patients developed graft failure or graft rejection.
Table II.
| Graft composition (N = 44pts) | |||||
|---|---|---|---|---|---|
| CD34+/kg | CD3+/kg | CD4+/kg | CD8+/kg | CD3−CD56+/kg | |
| Median | 8.06 x 106 | 2.39 x 103 | 1.10 x 103 | 1.05 X 103 | 9.56 x 102 |
| Range | 2.0 x 106 – 1.72 x 107 | 4.73 x 102 – 1.29 x 104 | 0 – 5.01 x 103 | 0 – 3.44 X 103 | 0 – 1.39 x 104 |
Overall survival and progression-free survival
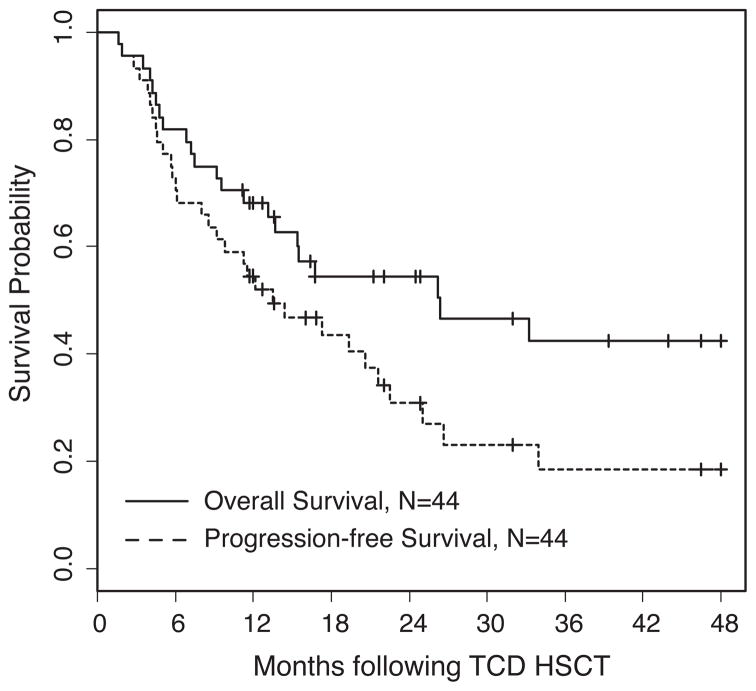
The clinical outcomes of all 44 patients are shown in Figure 1. The median PFS of 13.5 months translates into a PFS for all patients of 31% (95% CI: 0.19, 0.5) at 2 years and 18% (95% CI: 0.09, 0.40) at 4 years with an OS of 54% (95% Confidence Interval [CI] 0.41, 0.72) at 2 years and 42% (95% CI: 0.28, 0.63) at 4 years. There was no difference in outcome based on transplants from related (n = 14) vs unrelated (n = 30) donors or unrelated 10/10 matched (n = 18) vs 9/10 matched (n = 12) donors (Table III).
Figure 1.
Overall and progression-free survival of 44 patients with multiply relapsed multiple myeloma undergoing CD34-selected HSCT.
Table III.
| N | Overall Survival | Progression-free Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2-yr (95% CI) | 4-yr (95% CI) | HR (95% CI) | P-value | 2-yr (95% CI) | 4-yr (95% CI) | HR (95% CI) | P-value | ||
| Overall | 44 | 0.54 (0.41–0.72) | 0.42 (0.28–0.63) | - | - | 0.31 (0.19–0.5) | 0.18 (0.09–0.4) | - | - |
| Gender | 0.91 | 0.46 | |||||||
| Female | 13 | 0.57 (0.34–0.95) | 0.43 (0.2–0.92) | (reference) | 0.51 (0.29–0.9) | - | (reference) | ||
| Male | 31 | 0.53 (0.38–0.75) | 0.42 (0.26–0.68) | 1.05 (0.43–2.57) | 0.24 (0.12–0.47) | 0.19 (0.08–0.43) | 1.35 (0.6–3.03) | ||
| Age | 0.007 | 0.02 | |||||||
| Less than 55 | 20 | 0.79 (0.62–1) | 0.7 (0.5–0.98) | (reference) | 0.46 (0.26–0.79) | 0.27 (0.11–0.68) | (reference) | ||
| 55 or older | 24 | 0.35 (0.19–0.62) | 0.21 (0.08–0.52) | 3.72 (1.43–9.65) | 0.2 (0.09–0.45) | 0.13 (0.04–0.42) | 2.46 (1.17–5.14) | ||
| Prior Lines of Therapy | 0.34 | 0.44 | |||||||
| 3–4 lines | 12 | 0.67 (0.45–0.99) | 0.57 (0.35–0.94) | (reference) | 0.42 (0.21–0.81) | 0.25 (0.09–0.67) | (reference) | ||
| 5–6 lines | 16 | 0.60 (0.39–0.91) | 0.48 (0.26–0.88) | 1.17 (0.38–3.57) | 0.41 (0.22–0.76) | 0.27 (0.1–0.75) | 0.99 (0.40–2.41) | ||
| > 6 lines | 16 | 0.33 (0.14–0.76) | - | 2.04 (0.70–5.95) | 0 | 0 | 1.64 (0.65–4.12) | ||
| Donor | 0.68 | 0.55 | |||||||
| Related | 14 | 0.56 (0.35–0.9) | 0.38 (0.18–0.78) | (reference) | 0.30 (0.12–0.73) | 0.1 (0.02–0.62) | (reference) | ||
| Unrelated | 30 | 0.53 (0.37–0.76) | 0.47 (0.3–0.73) | 0.84 (0.36–1.95) | 0.32 (0.18–0.56) | 0.25 (0.12–0.52) | 0.80 (0.39–1.65) | ||
| Match | 0.65 | 0.33 | |||||||
| 10/10 Unrelated | 18 | 0.57 (0.36–0.9) | 0.57 (0.36–0.9) | (reference) | 0.36 (0.18–0.72) | 0.36 (0.18–0.72) | (reference) | ||
| 9/10 Unrelated | 12 | 0.44 (0.22–0.89) | 0.29 (0.1–0.85) | 1.29 (0.43–3.89) | 0.25 (0.08–0.73) | 0.12 (0.02–0.72) | 1.60 (0.63–4.08) | ||
| Extramedullary Manifestation | 0.01 | 0.003 | |||||||
| Absent | 31 | 0.66 (0.51–0.86) | 0.53 (0.36–0.79) | (reference) | 0.41 (0.26–0.67) | 0.30 (0.15–0.58) | (reference) | ||
| Present | 13 | 0.31 (0.14–0.7) | 0.21 (0.07–0.64) | 2.90 (1.27–6.60) | 0.08 (0.01–0.51) | 0 | 2.99 (1.45–6.18) | ||
| Pre-Transplant Status | 0.03 | 0.12 | |||||||
| VGPR/CR | 23 | 0.62 (0.44–0.87) | 0.62 (0.44–0.87) | (reference) | 0.4 (0.24–0.68) | 0.22 (0.09–0.53) | (reference) | ||
| PR | 21 | 0.47 (0.29–0.74) | 0.19 (0.06–0.6) | 2.56 (1.1–5.97) | 0.14 (0.03–0.7) | 0.14 (0.03–0.7) | 1.66 (0.82–3.39) | ||
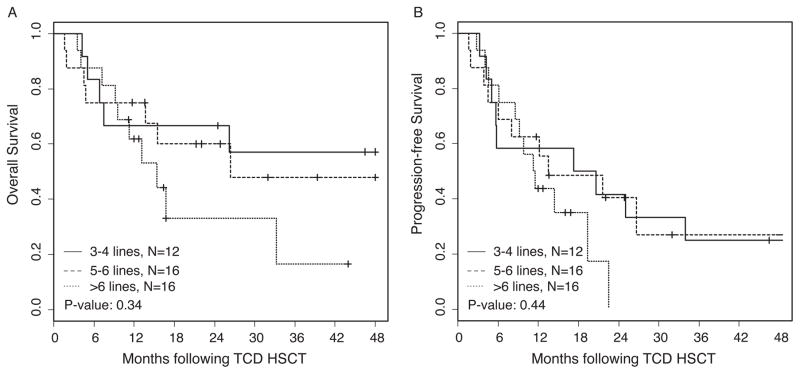
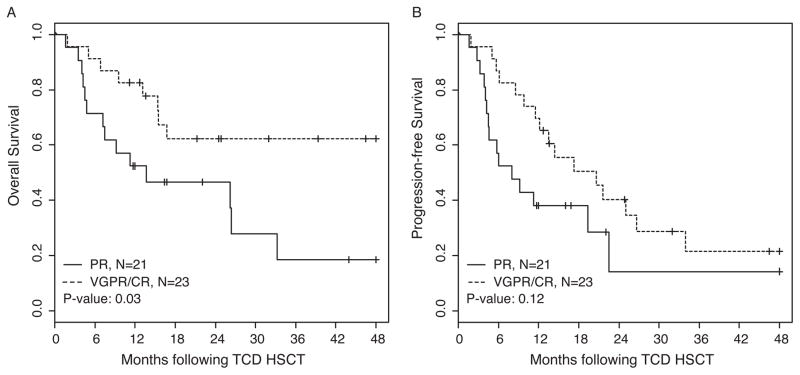
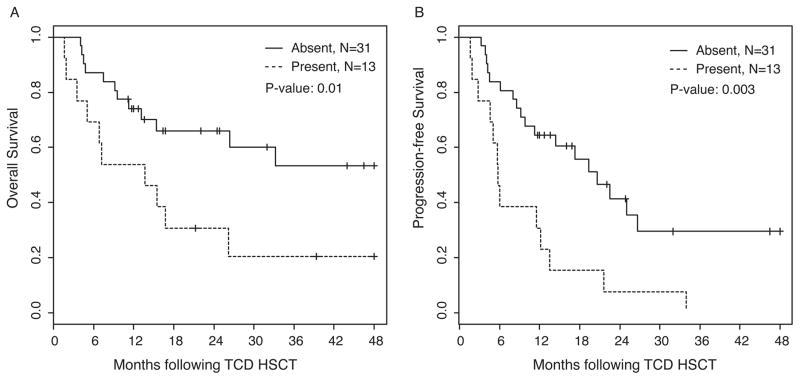
When we analyzed the OS and PFS based on the number of lines of therapy administered prior to CD34-selected HSCT, we found a trend towards better OS and PFS in patients with ≤6 lines of treatment compared to those with >6 lines of treatment. For these analyses, auto-SCT followed by maintenance therapy and tandem auto-SCT plus maintenance therapy were considered a single line of treatment (Figure 2). OS at 2 years for patients with 3–4 lines, 5–6 lines, and >6 lines of treatment were 67% (95% CI: 0.45, 0.99), 60% (95% CI: 0.39, 0.91), and 33% (95% CI: 0.14, 0.76), respectively. PFS at 2 years for patients with 3–4, 5–6, and >6 lines of treatment were 42% (95% CI: 0.21, 0.81), 41% (95% CI: 0.22, 0.76), and 0%, respectively. As demonstrated in Figure 3A, there is a significant difference (P = 0.03) in OS based on disease status prior to CD34-selected HSCT. Patients achieving a very good partial response or a complete response (VGPR/CR; n = 23) demonstrated 2-year OS estimates of 62% (95% CI: 0.44, 0.87) and while those with only a PR (n = 21) had 2-year OS estimates of 47% (95% CI: 0.29, 0.74). There was a trend but no significant difference in PFS (P = 0.12) in these two subgroups (Figure 3B), with 40% (95% CI: 0.24, 0.68) and 14% (95% CI: 0.03, 0.70), respectively. We also performed CD34-selected HSCT for 13 patients with relapsed MM and high-risk cytogenetics who were diagnosed with extramedullary manifestation of disease prior to allotransplant. Patients with extramedullary disease had significantly poorer OS and PFS when compared to those without extramedullary disease. As shown in Figure 4, the OS and PFS at 2 years for patients with extramedullary disease was only 31% (95% CI: 0.14, 0.7) and 8% (95% CI: 0.01, 0.51), compared to 66% (95% CI: 0.51, 0.86) and 41% (95% CI: 0.26, 0.67), respectively, for patients without extramedullary manifestation. The OS and PFS results including 95% Confidence Intervals for all patient cohort analyses at 2 and 4 years post CD34-selected HSCT are summarized in Table III. Overall, the two-year cumulative incidence of relapse was 51% (95% CI: 0.34–0.66) in our patient population.
Figure 2. Overall and progression-free survival based on lines of treatment prior to CD34-selected HSCT.
For these analyses, auto-SCT followed by maintenance therapy and tandem auto-SCT plus maintenance therapy were calculated as a single line of treatment as detailed in Table I.
Figure 3. Overall and progression-free survival based on disease status prior to CD34-selected HSCT.
Partial remission (PR) vs very good partial remission (VGPR) / complete remission (CR)
Figure 4.
Overall and progression-free survival based on presence (n = 13) or absence (n = 31) of extramedullary manifestation prior to CD34-selected HSCT.
Based on the univariate results above and listed in Table III, a multivariable model for OS was constructed. The three factors remained significant in the multivariable model; the model included extramedullary manifestation (HR: 3.18 (95% CI: 1.34–7.58); p-value 0.009), pre-transplant disease status (less than VGPR, HR: 3.80 (95% CI: 1.56–9.18); p-value: 0.003) and age (HR: 3.96 (95% CI: 1.44–10.83); p-value: 0.007). Among the 13 patients with extramedullary disease only 4 patients had > 6 lines of treatment prior to CD34-selected HSCT. We were also interested in analyzing the effect of the conditioning regimen of busulfan/melphalan/fludarabine (Bu/Mel/Flu) on patients who did not achieve a CR prior to CD34-selected HSCT. After salvage treatment, at the time of conditioning chemotherapy 21/44 (48%) patients were in CR or VGPR. The remaining 52% of patients had overt residual disease at the time of transplant. Using the post-salvage treatment outcome as a new baseline to assess their response to Bu/Mel/Flu conditioning chemotherapy, we demonstrated that this regimen had potent additional anti-myeloma activity. At 100 days post CD34-selected HSCT evaluation, there was an overall response rate (CR+PR) of 70%, including the induction of CRs in 39%, in this relatively refractory, multiply relapsed patient population that had suboptimal responses to salvage therapy. Overall, the Bu/Mel/Flu conditioning was very well tolerated as evidenced by only 1/44 (2%) patient death by 100 days post-transplant, low overall TRM, and the rapid engraftment in these patients after CD34-selected HSCT.
Graft-versus-host disease and non-relapse mortality
Standard Blood and Marrow Transplant Clinical Trials Network and International Bone Marrow Transplant Registry systems clinical criteria as defined by Rowlings et al17 were used to establish and grade acute GVHD.
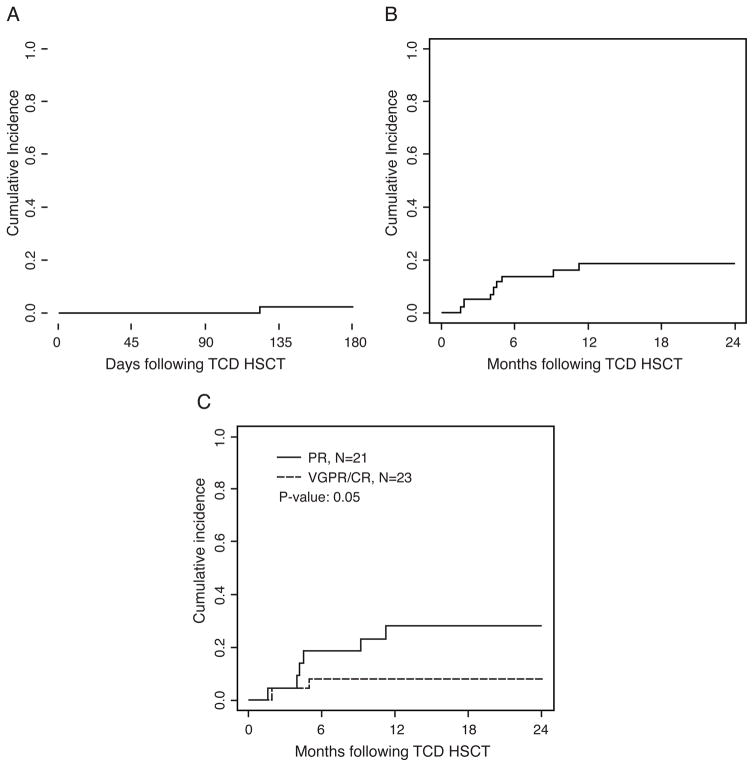
The event of grade II–IV acute GVHD was seen at a low rate (2%; 95% CI: 0.002, 0.11) (Figure 5A). Only one patient developed acute GVHD of the lower GI tract and died of complications thereof. There was no observed GVHD after DLI infusions. No patients were diagnosed with chronic GVHD.
Figure 5. Acute GVHD and non-relapse mortality (NRM).
(A) Acute GVHD (grade II–IV) to days +180 post CD34-selected HSCT, (B) NRM post CD34-selected HSCT and (C), NRM based on disease status (PR; n = 21) or (VGPR/CR; n = 23) prior to CD34-selected HSCT.
As shown in Figure 5B, the non-relapse mortality at one year was overall 18% (8/44 patients; 95% CI: 0.08, 0.31). Of these patients, 2 experienced nosocomial infections of oseltamivir-resistant influenza A during the neutropenic period and subsequently succumbed. One patient developed de novo acute toxoplasmosis following a business trip (against medical advice) only 3 months after transplant. Both patient and donor were seronegative for toxoplasmosis prior to transplant. Two patients developed antiviral drug-resistant cytomegalovirus disease and 2 patients died of gram-negative sepsis. The NRM was higher (P = 0.05) in patients who only achieved a PR (29%; 95% CI: 0.11, 0.49) compared to the patients in VGPR/CR (9%; 95% CI: 0.01, 0.25) prior to CD34-selected HSCT (Figure 5C).
Donor lymphocyte infusions
In order to boost the graft-versus-malignancy effect, for patients with 10/10 HLA-matched donors administration of 2–3 doses of DLI were planned prophylactically beginning at 4–6 months post-transplant. 19/31 patients with 10/10 matched donors received DLI, reasons to have not received DLI include illness or death (n=11) at the time of eligibility or GvHD (n=1). 5/13 patients with mismatched donors went on to receive DLI at the time of progression. Mismatched patients did not receive DLI because they are either still in remission (n=3), or illness or death (n=5). Six patients were in CR at the time of initial DLI. These patients outperformed the group as a whole with all 6 in CR at 1-year post-transplant and survival data as follows: 15.4 months, 60.2 months, and 4 patients still alive with OS ranging between 22–57 months (data not shown). A first dose of DLI was given to 18 patients with residual disease either because they did not reach CR from salvage and transplant conditioning or because they were given this dose at the time of progression. Of the 18 patients who received their initial dose of DLI with residual disease, 4 (22%) were restored to CR and were in CR at 1 year post-completion of an initial doses of DLI. Importantly, none of our patients developed GVHD as result of DLI at the doses administered.
Discussion
We demonstrate that CD34-selected HSCT has remarkable safety and improved efficacy when compared to historic controls of allogeneic transplants for MM and provides a platform to integrate post-transplant immunotherapeutic approaches to improve outcome.
Multiple previous studies have demonstrated a median PFS of only 7–8 months in patients with high-risk cytogenetics after high-dose therapy and autologous stem-cell transplantation.3,4 Outcome is even worse if patients relapse post-auto-SCT with high-risk cytogenetics or within a short time period.10,11 Risk-adapted strategies for these high-risk patients are warranted, such as consideration of allogeneic SCT, which provides a potential for prolonged remission or even cure. However, myeloablative allogeneic transplants for MM historically have been plagued with unacceptably high rates of GVHD and TRM.19 As a consequence, many institutions have shifted to providing non-myeloablative transplants for MM, which has reduced the TRM 8% to 16% in reported large institutional studies, but rates of GVHD remain unacceptably high. These studies report grade II–IV acute GVHD of 17% to 43% and chronic GVHD of 54% to 63%, with up to 32% of patients with extensive GVHD despite the long-term immunosuppressive therapy that is required following non-myeloablative transplants.8,9,20–22
In response to these restrictions and limited clinical options for patients with high-risk MM who may otherwise benefit from allogeneic transplant, we investigated myeloablative CD34-selected HSCT as a potentially safer alternative. Our results demonstrate only 2% acute GVHD (Figure 5A) and no chronic GVHD in our patient cohort, despite having 61% (27/44) of transplants coming from unrelated donors, nearly half of which (13/27; 48%) had a mismatched antigen/allele. The administration of DLI post-transplantation at calculated doses given on our study was not associated with GVHD.
Overall, our myeloablative conditioning regimen with Bu/Mel/Flu was very well tolerated with median engraftment on day 10 post-transplant. Although still favorable with 18% at one year (Figure 5B) and comparable to the NRM obtained after non-myeloablative transplants,9, 10, 18–20 the NRM in this study is higher compared to NRM obtained in other clinical trials with this chemotherapy regimen obtained at our center.23 This may be explained by the nosocomial infections of 2 patients with oseltamivir-resistant influenza on this study and one patient who developed de novo toxoplasmosis infection. Overall causes of NRM were infection (n=7) and GvHD (n=1). In addition, the NRM was particularly poor in patients who only achieved a PR compared to patients who were in VGPR or CR (29% vs 9%; P =0.05) (Figure 5C).
Overall, our median PFS of 13.5 months, which translates into PFS of 55% at one year and 31% at two years, compares very favorably to other reported studies, especially since all of our patients were heavily pretreated with multiple lines of chemotherapy and all relapsed after autologous transplant. In fact, 18/44 patients (40%) underwent two autologous transplants, of which 13/18 patients (72%) required a salvage auto-SCT to obtain at least a partial remission in order to proceed to CD34-selected HSCT (Table I). Our study also included a cohort of 13 patients who presented with extramedullary disease prior to CD34-selected HSCT. As shown in Figure 4, these patients had a particularly poor OS and PFS at 2 years of 31% and 8%, respectively. If patients with extramedullary disease were excluded from these analyses (n = 31), we achieved an OS and PFS of 66% and 41%, respectively, at 2 years and OS 53% and PFS 30% at 4 years (Table III).
The large institutional studies for patients with MM undergoing non-myeloablative transplants reported PFS ranging from 36% to 58% at 3 years.9, 10, 18–20 In contrast to our patient cohort, those patients underwent transplant exclusively from their sibling donors following an auto-SCT, and the majority of these patients had normal cytogenetics. In fact, transplants with non-myeloablative regimens showed only a 2-year PFS and OS of 19% and 32%, respectively, if patients had failed prior autologous bone-marrow transplantations.12 Outcome with non-myeloablative transplants was limited in all studies if patients presented with high-risk cytogenetics and/or chemo-insensitive disease.11,13 Strikingly, the limited outcomes of these studies were accompanied by high rates of acute and chronic GVHD as detailed above. This is in contrast to the low rate of acute GVHD and absence of chronic GVHD in our study, raising the overall question: Is chronic GVHD beneficial at all in patients undergoing allotransplant for MM? In fact, a recent publication from the European Society for Blood and Marrow Transplantation registry describes the lack of benefit of GVHD in a variety of diseases and particularly in patients with plasma-cell disorders,24 supporting the promising outcome in particular subsets of patients in the absence of GVHD in our study.
While overall our OS and PFS compare favorably to historical results, there is still room for improvement of the relapse rate, particularly for patients who have failed >6 lines of prior therapy or have extramedullary involvement. Given the safety of CD34-selected allogeneic transplant presented here, we may consider an earlier allogeneic transplant performed for patients with high-risk MM before multiple lines of chemotherapy have been administered and clinical responses are limited to partial remissions with remaining treatment options.
Recently, improvement in inducing complete remissions and PFS in patients with relapsed myeloma following 1–3 prior treatments with carfilzomib, lenalidomide, and dexamethasone has been reported.25 This combination provides a potentially new induction regimen that could be considered in multiply relapsed patients prior to CD34-selected HSCT. In young patients with relapsed high-risk disease, this regimen may also provide the clinical responses needed to improve the outcome following consolidative CD34-selected HSCT and provide long-term PFS.
The existence of a GVM effect has been directly confirmed by the results of DLI in patients who relapsed after failure of conventional allografts.26–29 Salama et al. reported results on 25 patients who received DLI (median dose 1 X 108 mononuclear cells/kg) for MM after relapsing after an allograft. Overall, 7 of 15 pts achieved a CR.28 Lokhorst reported on 27 patients receiving DLI following partially T-cell–depleted allotransplants.26 Overall, 14 of 27 patients responded, 5 with CRs. Responding patients received at least 1 X 108 mononuclear cells/kg. However, all responding patients developed GVHD following administration of the relatively high doses of donor T lymphocytes.
We administered doses of donor-derived CD3+ T cells in the range of 5 X 105/kg to 1 X 106/kg CD3+ from matched donors with a first dose administered 4–6 months post CD34-selected HSCT. We did not observe development of GVHD post-DLI, but found the development of donor-derived, antigen-specific T-cell responses, that correlated with clinical responses as previously described. 18 Our patients were not receiving post-transplantation immunosuppressive therapy, which likely significantly contributed to the observed outgrowth of donor-derived, antigen-specific, T-cell responses.18
In summary, we demonstrate that CD34-selected HSCT significantly reduces acute and chronic GVHD and associated transplant-related mortality. Given the high-risk, multiply relapsed nature of this patient population it is important to note that the reduction in toxicity does not compromise overall clinical responses when compared to historical and contemporary studies of allogeneic transplants for MM as evidenced by a “tail on the curve” that indicates durable responses in a cohort of these patients. This approach provides the safety and efficacy to consider risk stratification for younger patients with high-risk disease to undergo transplant at an earlier time before most chemo combinations have been exhausted. The lack of immunosuppressive therapy post CD34-selected HSCT provides further additional options to include post-transplant immunotherapeutic approaches to improve on disease recurrence. The presence of extramedullary disease is associated with a particularly poor outcome and the effect of immunotherapy on extramedullary sites remains to be determined.
Highlights.
CD34-selected HSCT demonstrates notable safety in patients with multiply relapsed MM
CD34-selected HSCT permits lasting remissions in the absence of graft-versus-host disease
CD34-selected HSCT provides a platform for adoptive immunotherapeutic approaches
Acknowledgments
GK is the recipient of a research grant from Otsuka Pharmaceuticals Inc in support of this trial.
Footnotes
Authorship Statement
ES provided clinical care of patients, analyzed data and contributed to the manuscript. SD performed biostatistical analyses and contributed to the manuscript. EO collected and analyzed data. HL, AL, DC, HH, NL, OL, SG, AC and SJ provided clinical care of patients and reviewed the manuscript. GK performed the study, provided clinical care, supervised data collection and analyses, and wrote the manuscript.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar S, Fonseca R, Lacy M, et al. Abnormal cytogenetics predict poor survival after high-dose therapy and autologous blood cell transplantation in multiple myeloma. Bone Marrow Transplant. 1999;24(5):497–503. doi: 10.1038/sj.bmt.1701943. [DOI] [PubMed] [Google Scholar]
- 4.Gertz MA, Lacy MQ, Dispenzieri A, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106(8):2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gahrton G, Tura S, Ljungman P, et al. Allogeneic bone marrow transplantation in multiple myeloma. European Group for Bone Marrow Transplantation. N Engl J Med. 1991;325(18):1267–1273. doi: 10.1056/NEJM199110313251802. [DOI] [PubMed] [Google Scholar]
- 6.Bensinger WI, Buckner CD, Anasetti C, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88(7):2787–2793. [PubMed] [Google Scholar]
- 7.Gahrton G, Tura S, Ljungman P, et al. Prognostic Factors in Allogeneic Bone-Marrow Transplantation for Multiple-Myeloma. Journal of Clinical Oncology. 1995;13(6):1312–1322. doi: 10.1200/JCO.1995.13.6.1312. [DOI] [PubMed] [Google Scholar]
- 8.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356(11):1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patriarca F, Einsele H, Spina F, et al. Allogeneic stem cell transplantation in multiple myeloma relapsed after autograft: a multicenter retrospective study based on donor availability. Biol Blood Marrow Transplant. 2012;18(4):617–626. doi: 10.1016/j.bbmt.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Efebera YA, Qureshi SR, Cole SM, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed multiple myeloma. Biol Blood Marrow Transplant. 2010;16(8):1122–1129. doi: 10.1016/j.bbmt.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashir Q, Khan H, Orlowski RZ, et al. Predictors of prolonged survival after allogeneic hematopoietic stem cell transplantation for multiple myeloma. Am J Hematol. 2012;87(3):272–276. doi: 10.1002/ajh.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50(4):493–498. doi: 10.1038/bmt.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 16.A language and environment for statistical computing. R Core Team; 2014. [Google Scholar]
- 17.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 18.Tyler EM, Jungbluth AA, O’Reilly RJ, Koehne G. WT1-specific T-cell responses in high-risk multiple myeloma patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation and donor lymphocyte infusions. Blood. 2013;121(2):308–317. doi: 10.1182/blood-2012-06-435040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24(6):929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 20.Rosinol L, Perez-Simon JA, Sureda A, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning Allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112(9):3591–3593. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 21.Vesole DH, Zhang L, Flomenberg N, et al. A Phase II trial of autologous stem cell transplantation followed by mini-allogeneic stem cell transplantation for the treatment of multiple myeloma: an analysis of Eastern Cooperative Oncology Group ECOG E4A98 and E1A97. Biol Blood Marrow Transplant. 2009;15(1):83–91. doi: 10.1016/j.bbmt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjorkstrand B, Iacobelli S, Hegenbart U, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011;29(22):3016–3022. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 23.Koehne G, Boulad F, Barker JN, Castro-Malaspina H. Two chemotherapy-based conditioning regimens compared to TBI-based conditioning secure consistent engraftment of T-cell depleted allogeneic HSCT, similarly low incidences of GVHD and favorable rates of disease-free survival (DFS) [Google Scholar]
- 24.Stern M, de Wreede LC, Brand R, et al. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28(11):2235–2240. doi: 10.1038/leu.2014.145. [DOI] [PubMed] [Google Scholar]
- 25.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 26.Lokhorst HM, Schattenberg A, Cornelissen JJ, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000;18(16):3031–3037. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 27.Mehta J, Singhal S. Graft-versus-myeloma. Bone Marrow Transplant. 1998;22(9):835–843. doi: 10.1038/sj.bmt.1701459. [DOI] [PubMed] [Google Scholar]
- 28.Salama M, Nevill T, Marcellus D, et al. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant. 2000;26(11):1179–1184. doi: 10.1038/sj.bmt.1702685. [DOI] [PubMed] [Google Scholar]
- 29.Alyea E, Weller E, Schlossman R, et al. T-cell--depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001;98(4):934–939. doi: 10.1182/blood.v98.4.934. [DOI] [PubMed] [Google Scholar]