Abstract
Phosphatidylinositol 3 kinase—protein kinase B (PI3K-AKT) pathway has been considered as major drug target site due to its frequent activation in cancer. AKT regulates the activity of various targets to promote tumorigenesis and metastasis. Accumulation of reactive oxygen species (ROS) has been linked to oxidative stress and regulation of signaling pathways for metabolic adaptation of tumor microenvironment. Hydrogen peroxide (H2O2) in this context is used as ROS source for oxidative stress preconditioning. Antioxidants are commonly considered to be beneficial to reduce detrimental effects of ROS and are recommended as dietary supplements. Quercetin, a ubiquitous bioactive flavonoid is a dietary component which has attracted much of interest due to its potential health-promoting effects. Present study is aimed to analyze PI3K-AKT signaling pathway in H2O2 exposed Dalton’s lymphoma ascite (DLA) cells. Further, regulation of PI3K-AKT pathway by quercetin as well as PI-103, an inhibitor of PI3K was analyzed. Exposure of H2O2 (1mM H2O2 for 30min) to DLA cells caused ROS accumulation and resulted in increased phosphorylation of PI3K and downstream proteins PDK1 and AKT (Ser-473 and Thr-308), cell survival factors BAD and ERK1/2, as well as TNFR1. However, level of tumor suppressor PTEN was declined. Both PI-103 & quercetin suppressed the enhanced level of ROS and significantly down-regulated phosphorylation of AKT, PDK1, BAD and level of TNFR1 as well as increased the level of PTEN in H2O2 induced lymphoma cells. The overall result suggests that quercetin and PI3K inhibitor PI-103 attenuate PI3K-AKT pathway in a similar mechanism.
Introduction
PI3K is crucial signal transducing enzyme regulating cell proliferation, cell survival, differentiation, apoptosis and angiogenesis [1, 2]. It is essential for activation of AKT which plays a central role in both physiological and pathological signaling mechanisms. PI3K-AKT pathway is major drug target due to its frequent activation in cancer [3–9]. PI3K is a lipid kinase, responsible for phosphorylation of PIP2 to PIP3 which is the activation site for AKT (or protein kinase B/PKB) and PDK. PI3K family is divided into three classes (class I, II & III) which differ in structure, substrate preference, tissue distribution, mechanism of activation and in function [10–12]. Class I PI3K has a long history of association with cancer. It is a heterodimer composed of a catalytic subunit P110α and regulatory subunit p85α [11–15]. PI3K dependant AKT activation leads to multistep process involving both membrane translocation and phosphorylation [16]. AKT is phosphorylated at Thr-308 in kinase activation loop and Ser-473 at carboxyl terminal. Thr-308 is phosphorylated by PDK1 whereas PDK2 is responsible for phosphorylation of Ser-473 [17, 18]. PDK1 is a crucial kinase required for normal mammalian development [19, 20]. AKT is comprised of 3 isoforms: AKT1, AKT2 and AKT3 according to different tissue distribution and biological activities. AKT1 plays a major role in regulation of cell survival and angiogenesis [3, 11, 21].
Cell survival is promoted by AKT mediated phosphorylation and inhibition of pro-apoptotic protein BAD [22]. BAD is a member of Bcl-2 family that promotes cell death by displacing Bax from binding to Bcl-2 and Bcl-xL [23]. Inactivation of BAD is also mediated through phosphorylation by ERK activated p90 ribosomal S6 kinase [24]. ERK is widely expressed signaling molecule that participates in regulation of a large variety of processes including cell adhesion, cell cycle progression, cell migration, cell survival, differentiation, metabolismand proliferation [24]. PI3K activation is responsible for ERK1/2 phosphorylation [25]. PKCα-mediated activation of ERK1/2 has also been reported through MEK [26].
Highly reactive oxygen species like hydrogen peroxide (H2O2), superoxide anion (O2•−), hydroxyl radicals (OH.) etc., produced in cells are grouped as reactive oxygen species (ROS) which confer reactivity to different biological targets. It has been suggested that ROS is selected by nature for adaptation to changes in environmental nutrients and oxidative environment during evolution [27]. Disturbance in balance between production of ROS and organism’s antioxidant defence system leads to accumulation of ROS causing oxidative stress. Oxidative stress is closely related to all aspects of cancer. It has been linked to hyper-activation of signaling pathways and metabolic adaptations of tumor microenvironment. Sustained oxidative stress in tumor microenvironment is due to production of ROS by tumor cells themselves and by activated neutrophils and macrophages. Recent reports indicate that apart from gene mutation by oxidative damage of cellular macromolecules including DNA, ROS has direct or indirect role in modulation of signal transduction and transcription factors to regulate cell survival, proliferation and migration [27–31]. Oxidative stress has been linked to hyper-activation of signaling pathways and metabolic adaptations of tumor microenvironment.
Induction of lymphoma cells by external exposure of H2O2 is hypothesized to be counterbalanced by an antioxidant supplement. Being flavonoid, quercetin (QUE) is an antioxidant rich in onion, grape, red wine etc. It has attracted much of interest due to its potential health-promoting effects against cardiovascular diseases, diabetes, thrombosis, longevity, including prevention against certain forms of cancer [32]. Being a dietary product, QUE is less likely to induce normal tissue toxicity and is reported to have no adverse health effects [11, 32, 33].
H2O2 is most commonly used as source of ROS for oxidative stress preconditioning [29, 34]. It is formed by dismutation of superoxide (O2•−) spontaneously or enzymatically, catalyzed by superoxide dismutase (SOD). H2O2 is more stable as compared to other ROS having half life of 1ms, and serves as inter as well as intracellular signaling molecule as it easily crosses membrane and diffuses from site of production [35, 36]. Aquaporin 8 (AQP8) in the inner mitochondrial membrane has a role in transport of H2O2 [33].
PI3K is activated by several factors including ROS [37]. ROS mediated signaling leading to activation of PI3K-AKT pathway plays an important role in the development of cancer. Estrogen induced ROS is reported to cause increased phosphorylation of AKT in breast cancer. Recruitment of ROS is required for PI3K-AKT activation in T-cell acute lymphoblastic leukemia cells [38]. Tumor microenvironment not only plays a pivotal role during cancer progression and metastasis but also has profound effects on therapeutic efficacy. AKT is an important driver of the tumor glycolytic phenotype and stimulates ATP generation. Increased intracellular glucose level is necessaryfor enhanced glycolytic metabolism. Lactate dehydrogenase (LDH)-A is a glycolytic enzyme that favours the conversion of pyruvate to lactate. It is important for maintenance and progression of tumor growth as oxygen availability is limiting in tumor microenvironment. Elevated LDH-A is important for tumor initiation to meet energy demand. High LDH-A in DL mice has been previously reported by our group which has been suppressed by various natural antioxidants [39]. We have earlier reported decreased activity of LDH-A by QUE which is correlated with markedly delayed tumor formation in DL mice [11]. AKT stimulates glycolysis by increasing the expression and membrane translocation of glucose transporters and by phosphorylating key glycolytic enzymes such as hexokinase and phosphofructokinase2 as well as phosphorylating mTOR [40]. Activated mTOR stimulates protein and lipid biosynthesis and cell growth in response to sufficient nutrient and energy conditions during tumorigenesis. PI-103 is a potent, cell permeable, inhibitor of catalytic subunit of PI3K (P110α) which competes with ATP binding site. It represents an exploratory compound for investigating the therapeutic relevance of PI3K inhibitors in cancer [41]. PI-103 inhibits proliferation and invasion of a wide variety of cancer cells and shows corresponding modulation of various cancer biomarkers [41, 42].
The present study is aimed to analyze the molecular mechanism of PI3K dependent modulation in H2O2 induced DLA cells. Further, the impact of QUE is compared with PI-103 to regulate H2O2 induced PI3K-AKT pathway.
Materials and Methods
Chemicals
All chemicals used were of molecular biology and analytical grade. Quercetin, Dichlorofluorescein diacetate (H2DCFDA), horseradish peroxidise (HRP) conjugated β-actin, anti-rabbit phospho p85α were purchased from Sigma Aldrich (St. Louis, MO); PI-103 from Cayman (Ann Arbor, MI); anti-rabbit AKT1, anti-rabbit phospho AKT Ser-473, anti-rabbit phospho AKT Thr-308, anti-rabbit p85α, anti-rabbit PTEN, anti-rabbit phospho BAD, anti-rabbit phospho PDK1 from Cell Signaling Technology (Danvers, MA); anti-rabbit ERK1/2, anti-rabbit phospho ERK1/2, anti-rabbit TNFR1 from Biovision (Milpitas, CA); anti-rabbit PKCα from Santa Cruz Biotechnology (Dallas, Texas), HRP conjugated goat anti-rabbit secondary antibody from Bangalore Genei (Bangalore, India); enhanced chemiluminescence (ECL) Super Signal Kit from Pierce Biotechnology (Rockford, IL) and H2O2 from S D Fine Chem Limited (Mumbai, India).
Cell culture and treatment
Dalton’s lymphoma ascite (DLA) cells were grown in vitro from ascite cells collected from DL bearing mice. AKR strain mice were bred and maintained under standard laboratory conditions with proper human care, as per the guidelines of Institutional Animal Ethical Committee, Banaras Hindu University, Varanasi, at 25±2°C under a 12h light/12h dark schedule with standard mice feed and drinking water ad libitum. All the experiments were approved by Central Animal Ethical Committee, Banaras Hindu University (Letter no. Dean/10-11/92). Healthy adult male mice (16–20 weeks old and 30±2g) were used in experimental work. About 1x106 viable ascite cells in 1mL of PBS per mouse were transplanted to adult male mice intraperitoneally (i.p.) as described earlier [11, 43].
DL mice were sacrificed by humanely euthanization (cervical dislocation after anesthetized; mice were exposed to ether presented on a cotton gauze inside a small chamber and ether concentration was approximately 80μL/L of volume of the container). Under aseptic condition, DLA cells were grown and maintained in RPMI (Roswell Park Memorial Institute) medium supplemented with 10% FBS (fetal bovine serum), 2mM L-glutamine, 100IU/mL penicillin and 100μg/mL streptomycin. Cells were incubated in CO2 incubator (Sanyo) with a humidified atmosphere and 5% CO2 at 37°C.
Dose and time for H2O2 treatment—1mM for 30min was based on literature survey [34] as well as pilot study performed in our lab. Approximately, 5 X 105DLA cells/mL containing culture medium on 6 well plates was exposed to H2O2 and further proceeds for biochemical and molecular analysis. In another experiment, DLA cells were pre-treated with PI-103 (10μM) or QUE (300μM) for 6h and treated with H2O2.
Total ROS measurements
Total ROS level was determined by oxidative conversion of nonfluorescent 2’, 7’-dichlorofluorescein diacetate (H2DCFDA) to highly fluorescent 2’, 7’-dichlorofluorescein (DCF) as described previously [44]. Cell extracts of amount 100μl were incubated at 37°C for 60min with 2mM of H2DCFDA (sigma) in PBS. Fluorescence was recorded at 485nm (excitation) and 527nm (emission) with fluorescence spectrophotometer (HITACHI F-3000). The level of ROS in each sample was determined by observing fluorescence (absorbance)/mg protein.
Flow cytometric analysis of ROS
Cellular ROS contents were measured by flow cytometry. Briefly, 5 X 105cells were seeded on 6 well plates and exposed to H2O2 at 1mM for 30min. Further, cells were washed with PBS and stained with 10μM of H2DCFDA at 37°C for 30min. Cells were collected and fluorescence was analyzed using a FACS Calibur flow cytometer (BD Bioscience, CA). Data were analyzed by CellQuestTM pro-software (BD Bioscience, CA).
Cytotoxicity by MTT Assay
DLA cell cytotoxicity was analysed as described previously [45], approximately 30 x 103 cells were seeded in each well of a 96 well microtiter plates in 100 μL complete culture media containing PI-103 (10 μM) and QUE (300 μM) for 6 h. Thereafter, cells were treated with H2O2 for 30 min. Equivalent concentration of DMSO (vehicle) was added to control wells. After incubation, 100 μL of MTT solution (stock 5 mg/ml in PBS) was added into each well for 3 h. Pellet was dissolved in 100 μL of DMSO and the absorption of formazan solution was measured at 570 nm using a micro plate reader (ECIL).
Western blotting
Cells were lysed in buffer containing 20mM Tris-HCl (pH 7.4), 150mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100 and 1mM PMSF as described previously [43]. Cellular debris was spun down at 14,000g for 20min at 4°C and supernatant was used as whole protein extract. Isolated protein was quantified using Bradford reagent. Equal amount of protein from each sample was separated using 10% SDS-PAGE and transferred to a PVDF membrane overnight at 4°C. Membrane was blocked in 5% non-fat milk in PBS (pH 7.4) for 2h at RT. Further, Membrane was probed separately with primary antibodies anti-rabbit phospho p85α (1:500 dilution), anti-rabbit AKT1 (1:1000 dilution), anti-rabbit phospho AKT Ser-473 (1:1000 dilution), anti-rabbit phospho AKT Thr-308 (1:1000 dilution), anti-rabbit p85α (1:1000 dilution), anti-rabbit PTEN (1:1000 dilution), anti-rabbit PKCα (1:500 dilution), anti-rabbit phospho BAD (1:1000 dilution), anti-rabbit phospho PDK1 (1:1000 dilution), anti-rabbit ERK1/2 (1:500 dilution), anti-rabbit phospho ERK1/2 (1:500 dilution), anti-rabbit TNFR1 in 1% BSA and 0.05% Tween-20 in PBS (PBST; pH 7.4) overnight at 4°C. After thorough washing in 1X PBS for 3min, blot was incubated with HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:2500 dilution; Bangalore Genei, Bangalore, India) in PBST (pH 7.4) containing 5% non-fat milk and 0.05% Tween-20 for 2h at RT. Immunoreactive protein was detected using ECL Super Signal Kit (Pierce Biotechnology) on X-ray film. Intensity of bands was analyzed by densitometric scanning using Gel Doc System (Alpha InnotechEC). Relative densitometric values were calculated after normalization with β-actin.
Statistical analysis
All experiments were repeated three times independently and one representative image is presented in the figures. Student’s t-test was used for statistical analysis between control and H2O2-treated groups. One-way analysis of variance (ANOVA) followed by Tukey test were used for statistical analysis to compare the significant difference between control, H2O2-treated group, and H2O2-treated with PI-103 or QUE treated group. Data represent as mean ± S.E.M. * (#) denotes significant differences at level of p < 0.05. # indicates significant difference between control vs H2O2-treated group; and * H2O2 treated group vs H2O2 with PI-103 or QUE treated group.
Results
Cytotoxicity of 1 mM of H2O2 was checked on DLA cells and no significant difference in cell viability was found.
H2O2 induces ROS accumulation
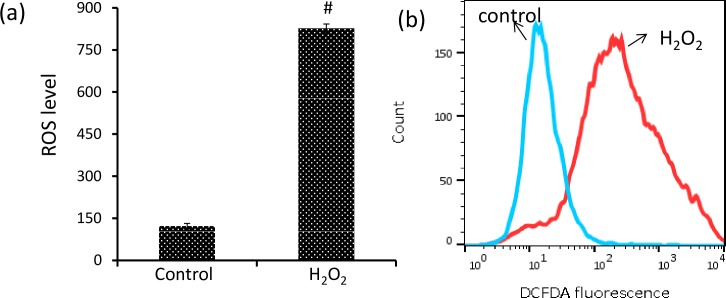
The effect of H2O2 was analyzed on ROS level of DLA cells in terms of absorbance (fluorescence/mg protein) using green fluorescent dye, H2DCFDA. ROS level was increased significantly by approximately 5.7 fold in H2O2 induced DLA cells [Fig 1A]. Increased absorbance of DCF with H2O2 was further confirmed through flow cytometry. Amount of ROS generation was indicated by shift in fluorescence [Fig 1B]. The result indicates that ROS accumulates in H2O2 induced DLA cells. Elevated level of ROS formation has been observed in animals suffering with different types of cancer including DL, which results in oxidative stress [43, 44].
Fig 1. H2O2 induces ROS accumulation.
(a) Homogenates of DLA cells from 3 set of each group were pooled separately and used for total ROS measurement (b) Cells were loaded with H2DCFDA (10μM) for 30min and amount of ROS generated was indicated by shift in fluorescence as detected by flow cytometry. Student’s t-test was used for statistical analysis. Data represent as mean ± S.E.M. of three independent experiments. * denotes significant differences at the level of p < 0.05 between H2O2-treated and control group.
H2O2 modulates PI3K signaling
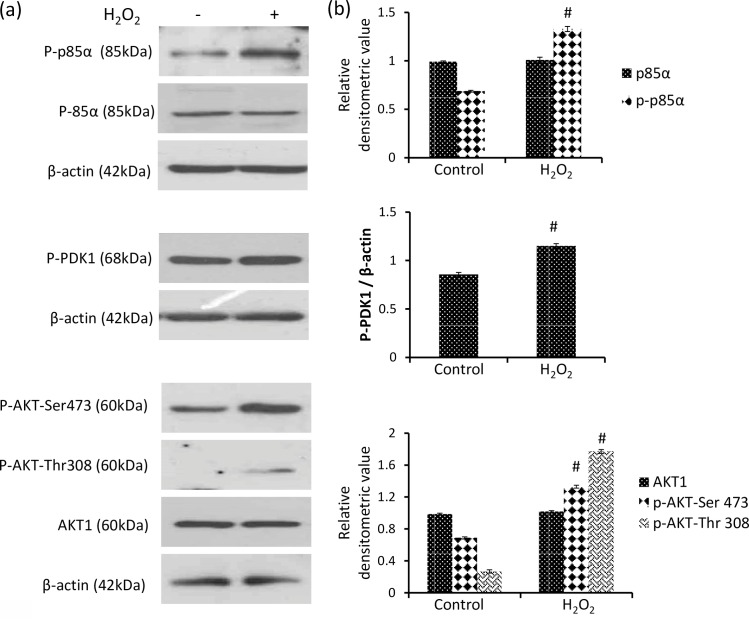
PI3K signaling cascade has been linked to cell proliferation, survival and angiogenesis. Effect of H2O2 was investigated on the regulatory subunit p85α and downstream enzymes PDK1 and AKT. Phosphorylation of p85α was significantly increased by approximately 1.93 fold with H2O2, however protein level of p85α was found to be unchanged [Fig 2]. The result shows activation of PI3K by covalent modification without affecting its translation. Further, H2O2 increased phosphorylation of trafficking enzyme PDK1 significantly by approximately 34.4% in DLA cells [Fig 2].
Fig 2. H2O2 activates PI3K signalling.
(a) Western analysis of p85α, phospho p85α, phospho PDK1, phospho AKT Thr-308, phospho AKT Ser-473 and AKT1 (b) respective densitometric scanning of band after normalization with β-actin. Student’s t-test was used for statistical analysis. Data represent as mean ± S.E.M. of three independent experiments. * denotes significant differences at the level of p < 0.05 between H2O2-treated and control group.
AKT is an essential downstream target of PI3K signaling pathway that provides a survival signal. AKT activation is a multistep process involving both membrane translocation and phosphorylation. H2O2 increased phosphorylation of AKT at Ser-473 and Thr-308 significantly by approximately 93.4% and 6.63 fold respectively as compared to control DLA cells [Fig 2]. However, level of AKT1 was found unaffected showing that H2O2 modulates PI3K pathway by activation of AKT without affecting the protein level.
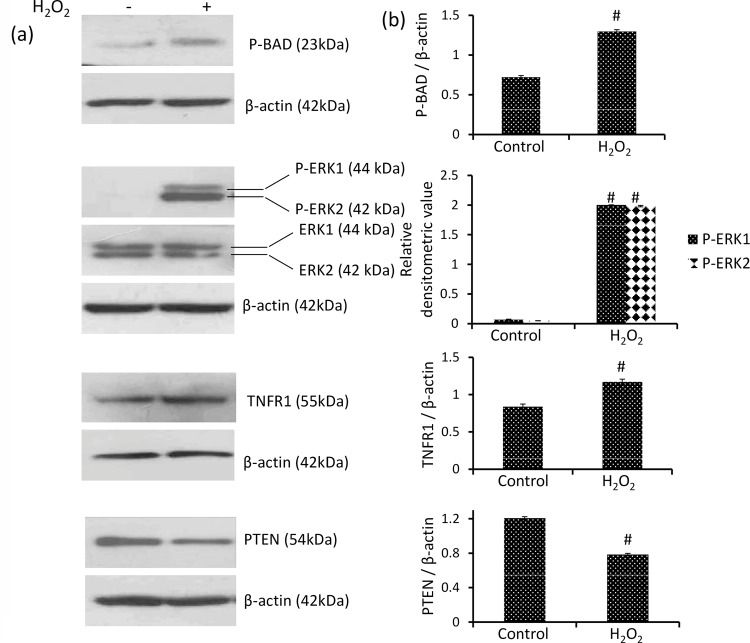
H2O2 induces phosphorylation of BAD and ERK1/2 and the expression of TNFR1
BAD provides a link between cell death machinery and survival signaling. De-phosphorylated BAD forms heterodimers with Bcl-2 and Bcl-xL inactivating them and allowing Bax/Bak triggered apoptosis. BAD phosphorylation forms BAD-(14-3-3) protein heterodimers which leaves Bcl-2 free to inhibit Bax-triggered apoptosis. Therefore BAD phosphorylation is considered asanti-apoptotic, whereas dephosphorylation of BAD as pro-apoptotic signal. H2O2 increased phosphorylation of BAD significantly by approximately 80% in DLA cells as compared to control showing its inactivation [Fig 3].
Fig 3. H2O2 induces the phosphorylation of BAD, ERK1/2 and TNFR1 level and down-regulates PTEN.
(a) Western analysis of phospho BAD, ERK1/2,phospho ERK1/2, TNFR1 and PTEN (b) respective densitometric scanning of band after normalization with β-actin. Student’s t-test was used for statistical analysis. Data represent as mean ± S.E.M. of three independent experiments. * denotes significant differences at the level of p < 0.05 between H2O2-treated and control group.
The other kinase ERK1/2 is also known to inactivate BAD by phosphorylation. ERK1/2 is found to be activated in H2O2 induced DLA cells as indicated by its significant increase in phosphorylation as compared to negligible phosphorylation of ERK1/2 in control group [Fig 3]. Although, total level of ERK1/2 was unaffected by H2O2 exposure to DLA cells [Fig 3].
Further, the receptor TNFR1 is known to promote cancer growth via PI3K and NF-κB-dependent pathways. Level of TNFR1 was increased significantly by approximately 40% in H2O2 induced DLA cells as compared to control [Fig 3].
H2O2 declines level of tumor suppressor PTEN
PTEN suppresses PI3K signaling by dephosphorylation of PIP3 to PIP2. The level of PTEN was observed to be decreased by approximately 35% in H2O2 induced DLA cells as compared to control [Fig 3].
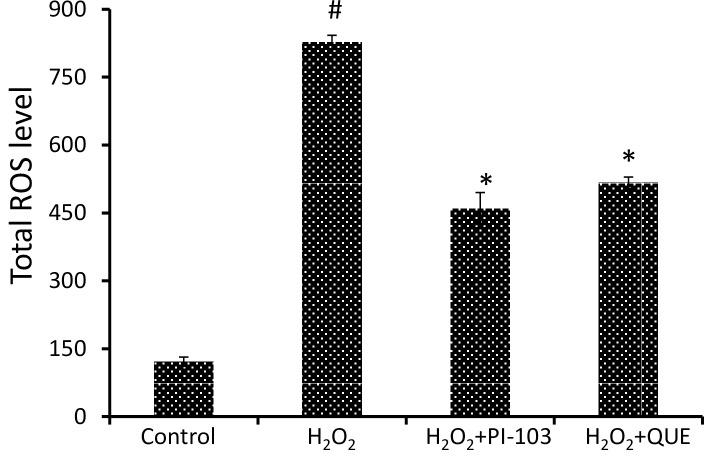
PI-103 and QUE decrease H2O2 induced ROS level
The effect of antioxidant QUE was compared with PI-103, a known inhibitor of PI3K on ROS level in terms of absorbance (fluorescence/mg protein) using green fluorescent dye, H2DCFDA. Both PI-103 and QUE decrease H2O2 induced ROS level approximately by 44% and 38% respectively in DLA cells [Fig 4].
Fig 4. PI-103 and QUE decrease H2O2 induced ROS level.
Homogenates of DLA cells from 3 set of each group were pooled separately and used for total ROS measurement. DLA cells were pre-treated with PI-103 and QUE; then post-treated with H2O2. Data represent as mean ± S.E.M. * (#) denotes significant differences at level of p < 0.05. # indicates significant difference between control vs H2O2-treated group; and * H2O2 treated groups vs PI-103/QUE treated group.
PI-103 and QUE suppresses cell proliferation
Effect of PI-103 and QUE on cell viability was analyzed in H2O2 induced DLA cells by MTT assay which measures mitochondrial dehydrogenase as cell viability. PI-103 and QUE decrease cell viability approximately by 47% and 41% respectively in H2O2 exposed DLA cells [S1 Fig]. However, H2O2 doesn’t significantly affect the DLA cell viability.
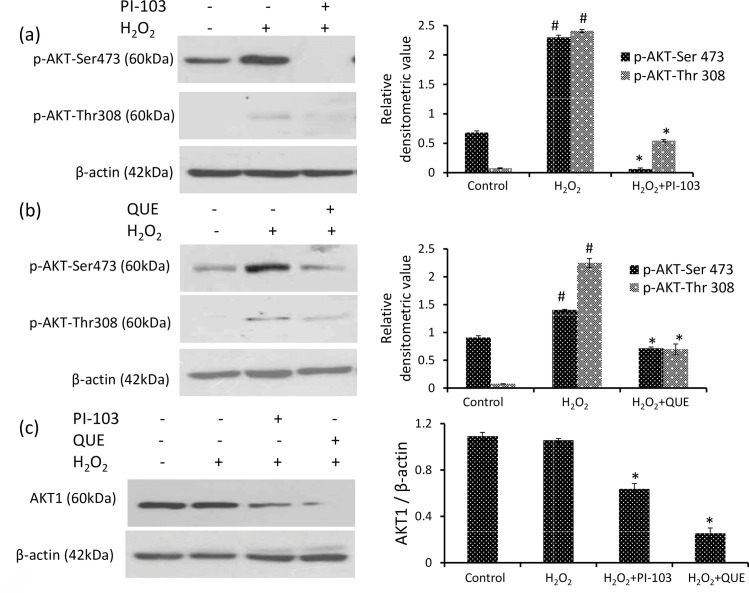
PI-103 and QUE attenuate H2O2 induced phosphorylation of AKT
Regulation of PI3K activity by QUE was compared with PI-103, an inhibitor of PI3K. PI-103 significantly decreased phosphorylation of AKT at Ser-473 and Thr-308 approximately by 97% and 77% respectively as compared to that of H2O2 induced DLA cells [Fig 5A]. QUE showed a similar effect as PI-103 showing significant decrease in phosphorylation of AKT at Ser-473 and Thr-308 approximately by 50% and 70% respectively as compared to H2O2 induced DLA cells [Fig 5B].
Fig 5. PI-103 and QUE attenuate phosphorylation of AKT Thr-308 & AKT Ser-473.
(a) and (b) Western analysis of phospho AKT Thr-308, AKT Ser-473 and respective densitometric scanning of band after normalization with β-actin. (c) Western analysis of AKT1 and respective densitometric scanning of band after normalization with β-actin. DLA cells were pre-treated with PI-103 and QUE; then post-treated with H2O2. Data represent as mean ± S.E.M. * (#) denotes significant differences at level of p < 0.05. # indicates significant difference between control vs H2O2-treated group; and * H2O2 treated groups vs PI-103/QUE treated group.
PI-103 & QUE decline AKT1 level
Both PI-103 and QUE declined the protein level of AKT1 approximately by 40% and 75% respectively as compared to H2O2 induced DLA cells [Fig 5C]. QUE showed better effect than PI-103.
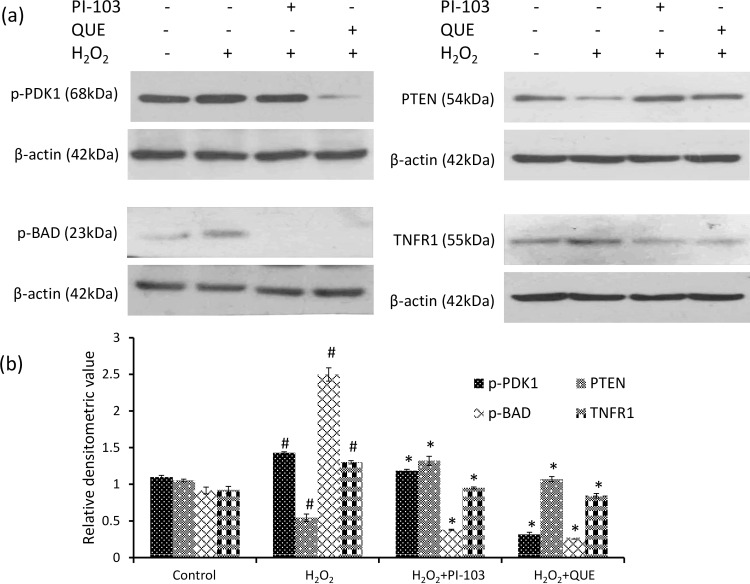
PI-103 & QUE decrease H2O2 induced phosphorylation of PDK1
Both PI-103 and QUE decreased phosphorylation of PDK1 significantly by approximately 17% and 77% respectively as compared to H2O2 induced DLA cells [Fig 6]. QUE was found to be more efficient in regulation of PDK1 activity than PI-103.
Fig 6. PI-103 and QUE attenuate H2O2 induced phosphorylation of PDK1, BAD, expression of TNFR1 and induce PTEN level.
(a) Western analysis of phospho PDK1, PTEN, phospho BAD and TNFR1 (b) respective densitometric scanning of band after normalization with β-actin. DLA cells were pre-treated with PI-103 and QUE; then post-treated with H2O2. Data represent as mean ± S.E.M. * (#) denotes significant differences at level of p < 0.05. # indicates significant difference between control vs H2O2-treated groups; and * H2O2 treated groups vs PI-103/QUE treated group.
PI-103 & QUE attenuate H2O2 induced phosphorylation of BAD
BAD is downstream signaling protein of AKT. Phosphorylation of BAD is considered as anti-apoptotic, whereas its dephosphorylation favours pro-apoptotic activity. Both PI-103 and QUE significantly down-regulated phosphorylation of BAD approximately by 85% and 90% respectively as compared to H2O2 induced DLA cells reflecting dephosphorylation of BAD [Fig 6].
PI-103 & QUE reduce H2O2 induced TNFR1 level
The result showed significant down-regulation in protein level of TNFR1 approximately by 27% and 35% by PI-103 and QUE respectively as compared with H2O2 induced DLA cells [Fig 6] indicating reduced cell survival.
PI-103 & QUE induce PTEN level
The level of tumor suppressor PTEN, a negative regulator of PI3K is increased significantly by both PI-103 and QUE approximately by 2.43 fold and 2 fold respectively as compared to H2O2 induced DLA cells [Fig 6]. Induced level of PTEN reflects down-regulation of PI3K activity by PI-103 as well as QUE.
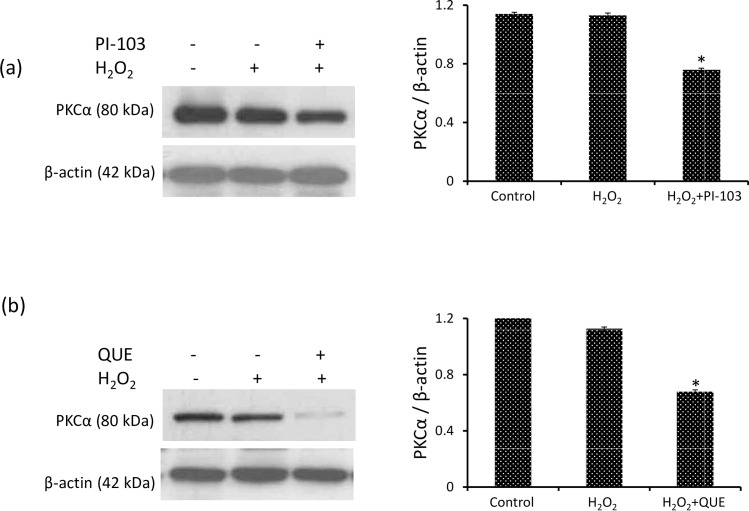
PI-103 & QUE down-regulate PKCα level
PDK1 regulates the activity of downstream protein PKCα, a key regulator of cell growth and differentiation in mammalian cells and activation of PKCα is believed to promote tumor progression. Level of PKCα was unaffected by H2O2 exposure to DLA cells. However, both PI-103 and QUE attenuate the level of PKCα approximately by 32% and 42% respectively as compared to H2O2 induced DLA cells [Fig 7A and 7B]. The effect of QUE was better than that of PI-103.
Fig 7. PI-103 & QUE downregulate PKCα level.
(a) and (b) Western analysis of PKCα and respective densitometric scanning of band after normalization with β-actin. DLA cells were pre-treated with PI-103 and QUE; then post-treated with H2O2. Data represent as mean ± S.E.M. * (#) denotes significant differences at level of p < 0.05. # indicates significant difference between control vs H2O2-treated groups; and * H2O2 treated groups vs PI-103/QUE treated group.
The overall result shows similar effect of QUE and PI-103, which supports that antioxidant QUE is a potential down-regulator of PI3K-AKT signaling pathway.
Discussion
Previously we have reported hyper-activation of PI3K-AKT pathway involved in tumor cell survival in Dalton’s lymphoma bearing mice and QUE attenuates the hyper-activation bringing the pathway towards normal [11]. The molecular mechanism of PI3K-AKT pathway has been established by using PI-103, a potent and selective inhibitor of PI3K [41]. PI-103 is reported to show therapeutic activity against a range of cancer by inhibition of angiogenesis, invasion and metastasis [42, 46]. It has a number of metabolic hotspots, especially the phenol ring, which are shown to be extensively glucuronidated, resulting in rapid plasma and tissue clearance [41]. In the present study the regulation of PI3K-AKT pathway by PI-103 is compared with QUE in lymphoma cells in vitro. QUE is a dietary antioxidant with almost no side effect as reported by Harwood et al., 2007. The lymphoma cells were exposed to oxidative insult in vitro by 1mM H2O2 for 30 min.
Oxidative stress is an evident feature of almost all cancerous cells as compared to normal, which activates several signaling cascade to achieve adaptation for survival and immortalization [47–49]. Numerous signaling pathways are involved in tumor cell survival including PI3K-AKT pathway [1, 4]. Hyper-activation of PI3K pathway is frequent in human cancer and has been considered as a major drug target in cancer treatment [5–9]. PI3K is activated by several factors including oxidative stress. Previously we have reported that QUE attenuates ROS accumulation in DL mice as well as in HepG2 cells [11, 12].
In present study, short time exposure of H2O2 to ascite cells from DL mice in vitro is found to induce accumulation of ROS causing oxidative stress. The ROS level was decreased after treatment of H2O2 induced DLA cells with antioxidant QUE as well as PI3K inhibitor PI-103. ROS is involved in various cellular processes that positively and negatively regulate cell fate. It is reported to promote cell proliferation and survival in several cell types [29]. On the other hand, it has been shown to induce apoptosis in certain cell types and organs [37, 50]. Oxidative stress is suggested to be a key pathogenic factor in cisplatin-induced ototoxicity in vivo [51].
The effect of oxidative stress is analyzed on signaling pathway of proto-oncogene PI3K. The best elucidated mechanism of PI3K activation involves association of p85α subunit with specific phospho-tyrosine on membrane. Under basal condition, regulatory subunit p85α stabilizes catalytic subunit p110α and inhibits its catalytic activity. Phosphorylation of p85α alleviates the inhibition, causing activation of PI3K [15]. Increased phosphorylation of p85α has been linked with various cancers [10, 13, 15]. H2O2 is found to increase phosphorylation of p85α in DLA cells in vitro without affecting its level. The result reflects that H2O2 activates PI3K by increasing tyrosine kinase activity [52]. Earlier we have reported down-regulation of PI3K by antioxidant QUE in ascite cells of DL mice [11]. PI3K catalyzes phosphorylation of lipid associated PIP2 in to PIP3, which recruits AKT as well as PDK to plasma membrane. PDK dependant phosphorylation of AKT leads to its activation [16].
PDK1 is a crucial kinase required for normal mammalian development [19, 20]. However, it is frequently elevated in cancer with parallel increased phosphorylation of downstream kinase AKT at Thr-308 [20]. Exposure of lymphoma cells to H2O2 to DLA cells increased phosphorylation of PDK1. Both QUE and PI-103 show down-regulation of phosphorylation of PDK1 in H2O2 induced DLA cells.
Full activity of AKT requires phosphorylation at Ser-473 and Thr-308. Since hyperactivation of AKT supports tumor cell survival, it becomes strategic target in cancer therapeutics [16, 53]. PDK1 is responsible for phosphorylation of Thr-308 [17, 18]. Our results demonstrate that H2O2 increased phosphorylation of both Ser-473 and Thr-308 region of AKT without affecting its level in DLA cells. The result suggests that H2O2 activates PI3K signaling pathway via PDK1 and AKT. An inhibitory effect on H2O2 mediated AKT activation is shown by PI-103 as well as QUE. In addition, the level of AKT1 is also decreased by both PI-103 and QUE where QUE shows better effect than PI-103. A similar decrease in AKT1 level after QUE treatment to DL mice has been reported earlier [11]. QUE has classically been defined as a broad spectrum inhibitor against PI3K [54]. QUE inhibits AKT1/2 on platelet spreading [54]. Inhibition of AKT1/2 by QUE suggested that the flavonol either by a direct effect on this Ser/Thr kinase or most significantly as a regulation of the enzyme downstream of PI3K. PI-103 has been reported to inhibit PI3K/AKT activity and blocks the proliferation in human leukemic cell lines [55].
This is consistent with our finding of declined level of tumor suppressor PTEN in DLA cells exposed to H2O2. Tumor suppressor PTEN exerts feedback regulation of PI3K signaling by dephosphorylation of PIP3 and thus imposes negative regulation on AKT activity. PTEN is frequently lost or mutated in cancer [56, 57]. Our finding is supported by earlier report of PTEN inactivation by H2O2 [58]. Modulation in activity of both AKT and PTEN play a central role in tumorigenesis. Upregulation of tumor suppressor PTEN level by QUE is similar to the effect of PI-103 in DLA cells. A similar increase in tumor suppressor p53 level by QUE has been reported earlier in DL mice [11]. Upregulation of PTEN by QUE and PI-103 indicates down-regulation of AKT signaling.
AKT promotes cell survival by phosphorylating and inhibiting pro-apoptotic protein BAD [22]. BAD is a member of Bcl-2 family which promotes cell death by displacing Bax from binding to Bcl-2 and Bcl-xL [23, 59–61]. BAD function is regulated by phosphorylation. Dephosphorylated BAD forms a heterodimer with Bcl-2 and Bcl-xL, inactivating them and allowing Bax/Bak triggered apoptosis. Phosphorylation of BAD leads to its binding to 14-3-3 protein, which leaves Bcl-2 free to inhibit Bax-triggered apoptosis. Thus BAD phosphorylation promotes cell survival. H2O2 increases phosphorylation of BAD in DLA cells. The result is supported by earlier report [34]. Both QUE and PI-103 decreased phosphorylation of BAD in H2O2 induced cells suggesting reduced survival of DLA cells. Our results are in accordance with the report that AKT phosphorylates BAD to promote cell survival in vivo and in vitro [22]. Inactivation of BAD is also mediated through phosphorylation by ERK. Activation of ERK1/2 inhibits apoptosis by inactivation of caspase 8. PI3K activation is responsible for ERK1/2 phosphorylation [25]. Our result shows increased phosphorylation of ERK1/2 without affecting its level in H2O2 induced DLA cells.
PKCα is another trafficking molecule of PDK1, which is a key regulator of cell growth and differentiation and its activation is believed to promote tumor progression [62]. Level of PKCα is down-regulated by both QUE and PI-103 in H2O2 induced DLA cells. The result is in consistence with our previous reports in DL mice and HepG2 cells [11, 12].
Abundance of TNFR1 is known to promote cell survival via PI3K and NF-κB dependant pathway [58, 63–68]. Increased level of TNFR1 by H2O2 exposure supports induced survival of DLA cell. The result correlates with increased TNFR1 expression after exposure of ROS in neuronal cells [69]. Further, both QUE and PI-103 down-regulate level of TNFR1. Reduced level of TNFR1 by QUE is in accordance with our previous report in DL mice [35]. Together, down-regulated BAD and TNFR1 by QUE suggests reduced survival of H2O2 induced DLA cells.
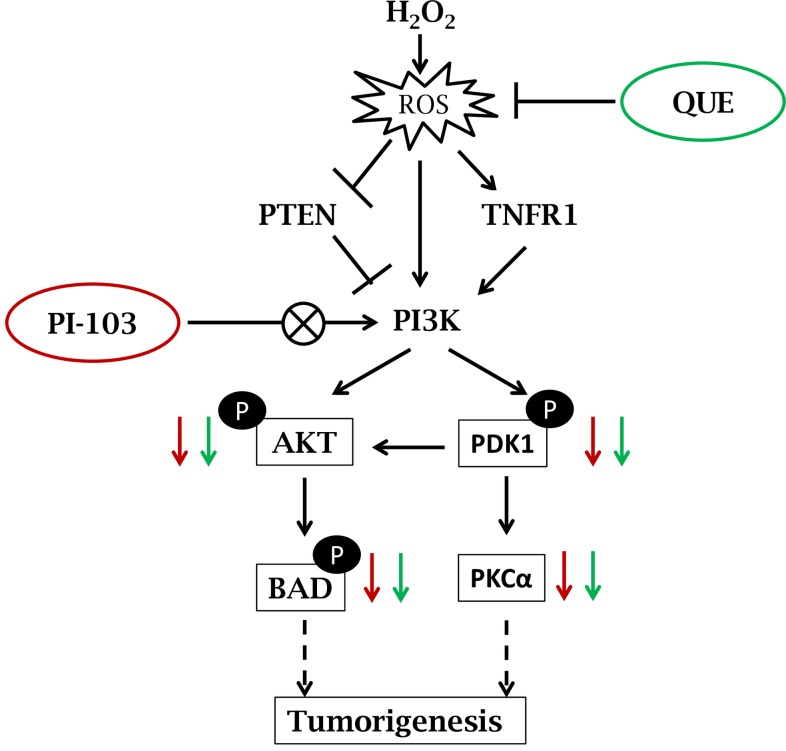
The results of current study suggest that H2O2 activates PI3K-AKT signaling via increasing ROS level. Overall effect of QUE is found similar to that of PI-103. Both PI-103 & QUE reduce phosphorylation of AKT and PDK1 whereas up-regulate level of tumor suppressor PTEN. This is consistent with decreased level of cell survival factors; p-BAD and TNFR1 in DLA cells in vitro. PI-103 and QUE mediated modulation of PI3K-AKT signaling pathway in DLA cells exposed to H2O2 is represented by schematic diagram [Fig 8]. The findings suggest that QUE modulates PI3K-AKT signaling pathway suppressing survival signals in prevention of lymphoma growth. This study may provide the base for implication of PI3K-AKT signaling in cancer targeting.
Fig 8. Schematic representation showing PI-103 and QUE mediated modulation of PI3K-AKT signaling pathway.
Exposure of H2O2 induces ROS accumulation, which in turn activates TNFR1 and PI3K level. Activated PI3K phosphorylates AKT via activated PDK1 and thereby induces downstream survival factor BAD. This is consistent with reduced level of tumor suppressor PTEN—negative regulator of PI3K pathway. Enhanced level of ROS by H2O2 is suppressed by both PI-103 & QUE which in turn down-regulates phosphorylation of AKT, PDK1 and BAD. Additionally, QUE and PI-103 increase the level of PTEN and decrease TNFR1 as well as PKCα.
Supporting Information
DLA cells were pre-treated with PI-103/QUE and post-treated with H2O2, then subjected to MTT dye reduction assay. The percentage viable cells (relative to control) were plotted against concentration. The data at each point represent mean ± S.E.M. * denotes significant differences at level of p < 0.05 between H2O2 treated group vs PI-103/QUE-treated groups.
(TIF)
Acknowledgments
Research was supported by University Grants Commission (UGC), India. MV is thankful to UGC-CAS programme to Department of Zoology for infrastructural facilities; and Interdisciplinary School of Life Sciences (ISLS), BHU, Varanasi, for providing Flow Cytometry (FACS) facility.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by grants received by University Grant Commission, India (Grant No. p-01/653). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vogt PK, Hart JR, Gymnopoulos M, Jiang H, Kang S, Bader AG, et al. Phosphatidylinositol 3-kinase: the oncoprotein. Curr Top MicrobiolImmunol. 2010; 347:79–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci. 2009; 34(3):115–27. 10.1016/j.tibs.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 3.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004; 9(6):667–76. [DOI] [PubMed] [Google Scholar]
- 4.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008; 27(41):5497–510. 10.1038/onc.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr Genomics. 2007; 8(5):271–306. 10.2174/138920207782446160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown KK, Toker A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000Prime Rep. 2015; 7:13 10.12703/P7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurya AK and Vinayak M. Abstract A07: Decline in the growth of murine T-cell lymphoma via modulation of PI3K signaling pathway: key role of quercetin and PI-103. Mol Cancer Ther. 2015; 14:A07 10.1158/1538-8514.PI3K14-A07 [DOI] [Google Scholar]
- 8.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. BiochimBiophysActa. 2008; 1784(1):159–85. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009; 8(8):627–44. 10.1038/nrd2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–41 10.1038/nrm2882 [DOI] [PubMed] [Google Scholar]
- 11.Maurya AK, Vinayak M. Quercetin regresses Dalton's lymphoma growth via suppression of PI3K/AKT signaling leading to upregulation of p53 and decrease in energy metabolism. Nutr Cancer. 2015; 67(2):354–63. 10.1080/01635581.2015.990574 [DOI] [PubMed] [Google Scholar]
- 12.Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol Biol Rep. 2015; 42(9):1419–29. 10.1007/s11033-015-3921-7 [DOI] [PubMed] [Google Scholar]
- 13.Berenjeno IM, Vanhaesebroeck B. PI3K regulatory subunits lose control in cancer. Cancer Cell. 2009; 16(6):449–50. 10.1016/j.ccr.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008; 27(41):5486–96. 10.1038/onc.2008.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geering B, Cutillas PR, Vanhaesebroeck B. Regulation of class IA PI3Ks: is there a role for monomeric PI3K subunits? BiochemSoc Trans. 2007; 35:199–203. [DOI] [PubMed] [Google Scholar]
- 16.Dibble CC, Manning BD. A molecular link between AKT regulation and chemotherapeutic response. Cancer Cell. 2009; 16(3):178–80. 10.1016/j.ccr.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 17.Chan CH, Jo U, Kohrman A, Rezaeian AH, Chou PC, Logothetis C, Lin HK. Posttranslational regulation of Akt in human cancer. Cell Biosci. 2014; 4(1):59 10.1186/2045-3701-4-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010; 2(1):19–42. [PMC free article] [PubMed] [Google Scholar]
- 19.Gagliardi PA, di Blasio L, Primo L. PDK1: A signaling hub for cell migration and tumor invasion. BiochimBiophysActa. 2015; 1856(2):178–188. [DOI] [PubMed] [Google Scholar]
- 20.Fyffe C, Falasca M. 3-Phosphoinositide-dependent protein kinase-1 as an emerging target in the management of breast cancer. Cancer Manag Res. 2013; 5:271–80. 10.2147/CMAR.S35026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl AcadSci U S A. 2001; 98(20):10983–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997; 91(2):231–41. [DOI] [PubMed] [Google Scholar]
- 23.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995; 80(2):285–91. [DOI] [PubMed] [Google Scholar]
- 24.Jr RR. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacological Research. 2012;66:105–143 10.1016/j.phrs.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 25.Bell KA, O'Riordan KJ, Sweatt JD, Dineley KT. MAPK recruitment by beta-amyloid in organotypic hippocampal slice cultures depends on physical state and exposure time. J Neurochem. 2004; 91(2):349–61. [DOI] [PubMed] [Google Scholar]
- 26.Mauro A, Ciccarelli C, De Cesaris P, Scoglio A, Bouché M, Molinaro M, Aquino A, Zani BM. PKCalpha-mediated ERK, JNK and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J Cell Sci. 2002; 115:3587–99. [DOI] [PubMed] [Google Scholar]
- 27.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014; 24(10):453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011; 194(1):7–15. 10.1083/jcb.201102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal. 2009; 11(11):2655–71. 10.1089/ARS.2009.2728 [DOI] [PubMed] [Google Scholar]
- 30.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell.2007; 26(1):1–14. [DOI] [PubMed] [Google Scholar]
- 31.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006; 312(5782):1882–3. [DOI] [PubMed] [Google Scholar]
- 32.D'Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia. 2015. October; 106:256–71. 10.1016/j.fitote.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 33.Harwood M, Nikiel BD, Borzelleca JF, Flamm GW, Williams GM (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food ChemToxicol 45: 2179–2205 [DOI] [PubMed] [Google Scholar]
- 34.Pronsato L, Boland R, Milanesi L. Testosterone exerts antiapoptotic effects against H2O2 in C2C12 skeletal muscle cells through the apoptotic intrinsic pathway. J Endocrinol. 2012; 212(3):371–81. 10.1530/JOE-11-0234 [DOI] [PubMed] [Google Scholar]
- 35.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. BiochimBiophysActa. 2006; 1758(8):994–1003. [DOI] [PubMed] [Google Scholar]
- 36.Sousa-Lopes A, Antunes F, Cyrne L, Marinho HS. Decreased cellular permeability to H2O2 protects Saccharomyces cerevisiae cells in stationary phase against oxidative stress. FEBS Lett. 2004; 578(1–2):152–6. [DOI] [PubMed] [Google Scholar]
- 37.Niwa K, Inanami O, Yamamori T, Ohta T, Hamasu T, Kuwabara M. Redox regulation of PI3K/Akt and p53 in bovine aortic endothelial cells exposed to hydrogen peroxide. Antioxid Redox Signal. 2003; 6:713–22. [DOI] [PubMed] [Google Scholar]
- 38.Silva A, Gírio A, Cebola I, Santos CI, Antunes F, Barata JT, Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells, Leukemia, 2011, 25(6):960–7. 10.1038/leu.2011.56 [DOI] [PubMed] [Google Scholar]
- 39.Mishra S, Vinayak M. Ellagic acid induces novel and atypical PKC isoforms and promotes caspase-3 dependent apoptosis by blocking energy metabolism. Nutr Cancer. 2014;66(4):675–81. 10.1080/01635581.2013.878735 [DOI] [PubMed] [Google Scholar]
- 40.Cairns RA, Harris IS, Mak TW, Regulation of cancer cell metabolism, Nat Rev Cancer, 2011, 11(2):85–95. 10.1038/nrc2981 [DOI] [PubMed] [Google Scholar]
- 41.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S,et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67(12):5840–50. [DOI] [PubMed] [Google Scholar]
- 42.Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A. et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30(12):4951–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Maurya AK, Vinayak M. Modulation of PKC signaling and induction of apoptosis through suppression of reactive oxygen species and tumor necrosis factor receptor 1 (TNFR1): key role of quercetin in cancer prevention. Tumour Biol. 2015. 36, 8913–24. 10.1007/s13277-015-3634-5 [DOI] [PubMed] [Google Scholar]
- 44.Das L, Vinayak M. Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS One. 2014; 9(6):e99583 10.1371/journal.pone.0099583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandey SK, Patel DK, Maurya AK, Thakur R, Mishra DP, Vinayak M, Haldar C, Maiti P, Controlled release of drug and better bioavailability using poly(lactic acid-co-glycolic acid) nanoparticles, Int J BiolMacromol, 2016, 89:99–110. [DOI] [PubMed] [Google Scholar]
- 46.Chaisuparat R, Hu J, Jham BC. Dual Inhibition of PI3Ka and mTOR as an Alternative Treatment for Kaposi's Sarcoma. Cancer Res 2008;68:8361–8368 10.1158/0008-5472.CAN-08-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 2013; 19(16):4309–14. 10.1158/1078-0432.CCR-12-1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66(23):3663–73 10.1007/s00018-009-0099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong L, Chuang CC, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367(1):18–25. 10.1016/j.canlet.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 50.Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007; 292(1):F440–7. [DOI] [PubMed] [Google Scholar]
- 51.Fang B, Xiao H. Rapamycin alleviates cisplatin-induced ototoxicity in vivo, BiochemBiophys Res Commun, 2014, 448(4):443–7. [DOI] [PubMed] [Google Scholar]
- 52.Cohen B, Yoakim M, Piwnica-Worms H, Roberts TM, Schaffhausen BS. Tyrosine phosphorylation is a signal for the trafficking of pp85, an 85-kDa phosphorylated polypeptide associated with phosphatidylinositol kinase activity. Proc Natl AcadSci U S A. 1990(12):4458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faes S and Dormond O, PI3K and AKT: Unfaithful Partners in Cancer, Int J MolSci, 2015, 16(9), 21138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro-Núñez L, Lozano ML, Martínez C, Vicente V, Rivera J, Effect of quercetin on platelet spreading on collagen and fibrinogen and on multiple platelet kinases, Fitoterapia, 2010, 81(2):75–80. 10.1016/j.fitote.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 55.Park S, Chapuis N, Bardet V, Tamburini J, Gallay N, Willems L, Knight ZA, Shokat KM, Azar N, Viguié F, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D, PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML, Leukemia, 2008, 22(9):1698–706. 10.1038/leu.2008.144 [DOI] [PubMed] [Google Scholar]
- 56.Papa A, Wan L, Bonora M, Salmena L, Song MS, Hobbs RM, Lunardi A, Webster K, Ng C, Newton RH, Knoblauch N, Guarnerio J, Ito K, Turka LA, Beck AH, Pinton P, Bronson RT, Wei W, Pandolfi PP. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell. 2014; 157(3):595–610. 10.1016/j.cell.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009; 4:127–50. 10.1146/annurev.pathol.4.110807.092311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI3-kinase signalling via inactivation of PTEN. EMBO J. 2003; 22(20):5501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balmanno K and Cook SJ, Tumour cell survival signalling by the ERK1/2 pathway, Cell Death Differ,2009, 3, 368–77. [DOI] [PubMed] [Google Scholar]
- 60.Weyhenmeyer B, Murphy AC, Prehn JH and Murphy BM, Targeting the anti-apoptotic Bcl-2 family members for the treatment of cancer, ExpOncol, 2012, 34(3), 192–9. [PubMed] [Google Scholar]
- 61.Howells CC, Baumann WT, Samuels DC and Finkielstein CV, The Bcl-2-associated death promoter (BAD) lowers the threshold at which the Bcl-2-interacting domain death agonist (BID) triggers mitochondria disintegration, J TheorBiol, 2011, 271(1), 114–23. [DOI] [PubMed] [Google Scholar]
- 62.Xie Z, Zeng X, Waldman T, Glazer RI. Transformation of mammary epithelial cells by 3-phosphoinositide- dependent protein kinase-1 activates beta-catenin and c-Myc, and down-regulates caveolin-1. Cancer Res. 2003, 63(17):5370–5. [PubMed] [Google Scholar]
- 63.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999; 401(6748):82–5. [DOI] [PubMed] [Google Scholar]
- 64.Gu L, Findley HW, Zhu N, Zhou M. Endogenous TNFalpha mediates cell survival and chemotherapy resistance by activating the PI3K/Akt pathway in acute lymphoblastic leukemia cells. Leukemia. 2006; 20(5):900–4. [DOI] [PubMed] [Google Scholar]
- 65.Oshima H, Ishikawa T, Yoshida GJ, Naoi K, Maeda Y, Naka K, Ju X, Yamada Y, Minamoto T, Mukaida N, Saya H and Oshima M, TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells, Oncogene, 2014, 33(29), 3820–9. 10.1038/onc.2013.356 [DOI] [PubMed] [Google Scholar]
- 66.Wajant H and Scheurich P, TNFR1-induced activation of the classical NF-κB Pathway, FEBS J, 2011, 278(6), 862–76. 10.1111/j.1742-4658.2011.08015.x [DOI] [PubMed] [Google Scholar]
- 67.Rivas MA, Carnevale RP, Proietti CJ, Rosemblit C, Beguelin W, Salatino M, Charreau EH, Frahm I, Sapia S, Brouckaert P, Elizalde PV and Schillaci R, TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways, Exp Cell Res, 2008, 314(3), 509–29. [DOI] [PubMed] [Google Scholar]
- 68.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, Balkwill FR and Hagemann T, The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans, J Clin Invest, 2009, 119(10), 3011–23. 10.1172/JCI39065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma F, Zhang L, Westlund KN. Reactive oxygen species mediate TNFR1 increase after TRPV1 activation in mouse DRG neurons. Mol Pain. 2009; 5:31 10.1186/1744-8069-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DLA cells were pre-treated with PI-103/QUE and post-treated with H2O2, then subjected to MTT dye reduction assay. The percentage viable cells (relative to control) were plotted against concentration. The data at each point represent mean ± S.E.M. * denotes significant differences at level of p < 0.05 between H2O2 treated group vs PI-103/QUE-treated groups.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.