Abstract
Objectives
The objectives of this review are to discuss the scope of neurologic injuries in newborns with congenital heart disease, the mechanisms of injury, including pre-natal, pre- intra- and postoperative factors, neurodevelopmental outcomes and therapeutic strategies for the timely intervention and prevention of neurologic injury.
Data Source
MEDLINE, PubMed
Conclusion
At the current time, important research is underway to (1) better understand the developing brain in the fetus with complex congenital heart disease, (2) to identify modifiable risk factors in the operating room and intensive care unit in order to maximize long-term neurodevelopmental outcomes and (3) develop strategies to improve family psychosocial health, childhood development and health-related quality of life following hospital discharge. Crucial in this effort is the identification of an early post-operative surrogate variable with good predictive validity for long-term outcomes. If an appropriate surrogate variable for long term outcomes can be identified, and measured relatively early after surgical intervention for complex congenital heart disease, reliable clinical trials can be undertaken to improve upon current outcomes.
Keywords: neurologic injury, pediatrics, cardiac surgery, cardiopulmonary bypass, outcomes
Introduction
Prior to the early 1980s, it was uncommon for children with complex congenital heart disease (CHD) to survive into later childhood. The nearly simultaneous advances in congenital cardiac surgery, echocardiography, and intensive care medicine were coupled with the availability of prostaglandins and the developing discipline of interventional cardiology. Together, these factors resulted in a dramatic fall in surgical mortality, with complex repairs taking place at increasingly younger ages. At many large centers, palliative surgery followed by later repair was replaced by primary repair in infancy, while staged reconstructive surgery for various forms of functionally univentricular heart, including those with the hypoplastic left heart syndrome, was improving with steadily falling rates of surgical mortality. As a result, the early part of the 21st century has seen an increasing number of children entering primary and secondary schooling. Research into their academic and behavioral outcomes has revealed some sobering realizations about the outcomes in these children. For the purposes of this manuscript, complex CHD will refer to morphological abnormalities significant enough to require surgical or catheter intervention as neonates or young infants.
Scope of the Problem
An estimated 30,000 to 40,000 children are born in North America each year with CHD and approximately one third require surgical intervention during the first year of life. For the group of children with complex CHD, neurodevelopmental disabilities are common, affecting approximately half of the survivors as they mature (1–6). These disabilities are typically mild, but also commonly occur in combination, and occassionally are quite debilitating. Formal evaluations of preschool and school-aged children born with complex CHD demonstrate a pattern of neurodevelopmental sequelae which may appear alone or in combination. These include mild cognitive impairment; oral-motor dyscoordination, expressive speech and language abnormalities; impaired visual-spatial and visual-motor skills; attention-deficit/hyperactivity disorder (ADHD); motor delays; learning disabilities; and later problems with executive function and diminished health-related quality of life (Table 1). Indeed, neurodevelopmental challenges are more common in children and young adults with complex CHD than all cardiovascular problems combined. The need for early intervention, rehabilitative services, and special education reduces the quality of life for these children and their families, as well as resulting in significant costs to society (1). As children progress through school, low scores in terms of academic achievement, learning disabilities, behavioral problems, difficulties with social cognition and attention deficit/hyperactivity disorder may result in academic failure, development of poor skills in both the classroom and socially, low self-esteem, behavioral disinhibition, and ultimate delinqunecy. Given these findings, there is active interest in better understanding the mechanisms of brain injury in these children, in order to design treatment trials and improve long-term outcomes for future patients. In addition, there is active interest in adapting techniques used to treat these disabilities in children without complex CHD to this growing population (7–9).
Table 1.
Neurological, Developmental and Psychosocial Challenges Which Occur With Increased Frequency in Children, Adolescents and Young Adults Born With Critical Congenital Heart Disease
|
Legend: CNS= Central Nervous System; CT=Computerized Tomography; MRI=Magnetic Resonance Imaging
Mechanisms of Injury
Central nervous system (CNS) injury in children with CHD is a result of a complex interaction of patient-specific factors and environmental influences, including, but not limited to, the effects of various interventions such as cardiac surgery and perioperative care (figure 1). The risk of a poor developmental outcome varies according to the specific cardiac defect. In addition, there is significant interindividual variation in developmental outcome, even among children with the same cardiac defect. Although cerebral ischemia before, during, and after the surgical repair of complex CHD has been proposed to be the primary mechanism of CNS injury, additional in-hospital and later factors may contribute to neurologic dysfunction. These factors can broadly be divided into three main categories and time-frames: (1) prenatal, (2) perioperative, and (3) post-discharge. From a research perspective, it is difficult to separate out the relative contributions of these three mechanistic categories, as they co-exist in the majority of neonates.
Figure 1.

Current model relating measured neurodevelopmental outcomes at various timepointswith potential etiologic factors in children with complex congenital heart disease. ICU=Intensive Care Unit
Pre-natal Mechanisms of Brain Injury
There is growing recognition that the brain is abnormal at birth in many neonates with complex CHD. Particularly with the recent advances in fetal and post-natal Magnetic Resonance Imaging (MRI), imaging studies have identified a surpringly high incidence of white matter injury, stroke and hemmorhage, as well as brain immaturity at birth (10–13). MRI and previously reported echocardiography-Doppler studies have confirmed abnormalities of fetal blood flow and reduced substrate delivery that lead to immaturity of the developing brain (14–17). In addition, there is an increased incidence of congenital structural CNS abnormalities in association with complex CHD (10, 12, 18). In combination, these functional and anatomic abnormalities seen in the newborn with complex CHD might best be considered coexisting “Congenital Brain Disease” (CBD), and appear to be present in nearly half of these neonates.
Fetal Cerebrovascular Physiology and Oxygen Delivery
Ultrasound studies in the fetus have revealed that cerebral vascular resistance is altered in the presence of congenital cardiac disease. Fetuses with left-sided disease such as the hypoplastic left heart syndrome, have been found to have decreased cerebral vascular resistance compared to normal (14, 15). In patients with aortic atresia, the fetal cardiac output from the arterial duct must deliver flow cephalad to the brain as well as caudad to the low resistance placenta. It is speculated that cerebral vascular resistance must therefore be lower than normal to allow adequate blood flow to the developing brain. In contrast, fetuses with right-sided obstructive lesions such as tetralogy of Fallot have been shown to have increased fetal cerebral vascular resistance (15). In these children, it is speculated that the obstruction to flow into the pulmonary arteries changes the usual delivery from the patent arterial duct caudad to the placenta. In these cases, the left ventricle must contribute to placental blood flow antegrade from the ascending aorta, with a resultant increase in cerebral vascular resistance. The impact of these alterations in fetal cerebral vascular resistance is unclear, but almost certainly plays a role in subsequent neurological development and has significant implications for the transition from fetal to neonatal circulation.
In the normal fetus, the intracirculatory patterns created by the normal fetal connections result in preferential streaming of the most highly oxygenated fetal blood to the developing brain, and most desaturated blood to the placenta. When significant structural disease exists within the heart, these beneficial patterns are likely to be altered. Recently confirmed by fetal magnetic resonance T-2 relaxation measurements, fetuses with transposition of the great arteries have the blood with the lowest saturation of oxygen returning to the ascending aorta and brain, while blood with the highest saturation returns to the abdominal organs and placenta (17, 19). Speculation on the consequences of the transposed fetal circulation (as an explanation for the high incidence of macrosomia in these infants) dates back nearly 50 years and has also been offered as an explanation for the increased incidence of relative microcephaly and long-term developmental challenges seen so often in transposition of the great arteries (20). Complete mixing, as seen in those with functionally univentricular hearts, and limitations on compensatory lowering of cerebrovascular resistance, produce reduced fetal cerebral oxygen delivery. The contribution of the placenta adds complexity to the issue as it has been noted that placenta weights are much lower than normal and placental vascularity is abnormal (21). Furthermore, MRI measurements of umbilical vein oxygen saturations have been shown to be much lower than expected, suggesting placental dysfunction (17).
It has long been recognized that the neurologic status of newborns with complex CHD is frequently abnormal prior to open heart surgery, including tone abnormalities, abnormal posturing, weak cry, and poor coordination of suck, swallow, and breathing (22, 23). Following birth, cerebral blood flow has been shown to be significantly lower than normal in some patients, due to low cardiac output, competition with pulmonary vascular resistance, or a low diastolic blood pressure (“steal”) secondary to a patent ductus arteriosus (24). In some lesions such as total anomalous pumonary venous return with obstruction and transposition of the great arteries with an intact atrial and ventricular septum, profound hypoxemia and acidosis may result immediately after birth secondary to the uncorrected complex CHD. Certain procedures, such as balloon atrial septostomy, have been linked to an increased risk of stroke by some authors (25, 26) but not others (27–29). Genetic syndromes, present in a significant proportion of children with complex CHD, play a role in abnormalities of brain structure as well as developmental delays (30). Finally, all patients with a right to left shunt have the potential for air or particulate embolism to reach the brain from intravenous catheters. Hypoxemia, low cardiac output, and cardiac arrest in patients with uncorrected complex CHD may contribute to CNS ischemia, injury and developmental delay (31, 32), adding to the abnormalities that may be present at birth (10–13, 33).
Microcephaly
Head circumference at birth is a surrogate for growth of the brain, and in neonates without congenital cardiac disease, microcephaly is independently associated with later developmental delays and academic difficulties. The incidence of microcephaly at birth is increased in children with complex CHD, approaching 25% of children in some reports (10, 33–35), persists into later infancy, and is associated with later developmental abnormalities (36). While the causes are speculative, and most certainly multifactorial, Shillingford et al reported on a series of children with the hypoplastic left heart syndrome where the median head circumference was only at the 18th percentile. In this study, patients with microcephaly had significantly smaller ascending aortas than those without, suggesting that reduced blood flow to the brain from the left ventricle secondary to anatomical hypoplasia of the ascending aorta may result in diminished brain growth (34).
Decreased CNS Maturity
Microcephaly, structural and biochemical immaturity of the white matter (33) and delay in cortical folding and white matter myelination (11) have led researchers to delve into investigations of fetal brain development. In her landmark paper, Dr. Limperopoulos performed fetal brain MRIs on 50 fetuses with CHD and 55 fetuses without (16). Cross-sectional data from this study reveals striking differences in brain growth, with CHD fetus’ diverging from normal at the beginning or the 3rd trimester of development. Fetuses with aortic arch anomalies fared the worst, with the most stagnant brain growth. Further work from this dataset showed measures of fetal cortical complexity similarly diverged from normal starting at the same time (37).
Periventricular Leukomalacia/White Matter Injury (PVL/WMI)
Injury to the white matter, a common finding in premature infants, has been increasingly recognised in full-term neonates with complex CHD. In premature infants, severe degrees of periventricular leukomalacia have been associated with cerebral palsy, while mild degrees of injury have been associated with developmental delay, motor difficulties, and behavioral disorders, a developmental ‘phenotype’ remarkably similar to school-age children with complex CHD. Investigations into the causes of PVL/WMI in neonates with complex CHD have led to the recognition that preoperative factors and patient-specific factors (heart diagnosis, age at surgery, prenatal diagnosis, genetics) rather than surgical or postoperative factors are the major risks. The etiology regarding the alterations in fetal circulation effecting brain growth and maturation was first suggested by Miller and McQuillen in 2007 (33). In that report, the authors used diffusion tensor imaging and MR spectroscopy to demonstrate significant differences in white matter microstructure and biochemistry between newborn infants with complex CHD and infants without CHD. Soon afterward, we reported an MRI-based observational metric called the Total Maturation Scale that demonstrated brain maturation in full-term presurgical infants with CHD was equivalent to the expected brain maturation of a 35-week premature infant (11). Others have since shown that the Total Maturation Scale predicted not only the risk for pre- and postoperative WMI but also abnormalities on neurodevelopmental testing in childhood and adolescents (38–39). While delayed brain maturation results in populations of vulnerable premyelinating oligodendrocytes (40) to PVL/WMI, the actual injury likely results from deficient cerebral blood flow, low oxygen saturations or a combination of the two. Lynch et al showed that daily falls in cerebral oxygen saturations between birth and surgery increased the risk for postoperative PVL/WMI in babies with the hypoplastic left heart syndrome (41). Here, cerebral blood flow, measured at the same time, failed to compensate for the falling saturations. Thus PVL/WMI results from a combination of cellular vulnerability and limitations in oxygen delivery. Petit et al found similar findings in neonates with transposition of the great arteries (27) that a longer wait to surgery with unrepaired complex CHD resulted in increased injury to the CNS.
Genetic Susceptibility to Neurologic Injury and Developmental Dysfunction
All of the above risk factors do not fully explain either the high frequency or the pattern of neurodevelopmental dysfunction described in children with complex CHD, suggesting that other patient-specific factors may be important determinants of neurologic injury. Intellectual development and cognitive function are highly heritable and probably are dependent on multiple genes, as well as on environmental factors. Numerous inherited defects or syndromes that are associated with compromised mental development and intellectual capacity (e.g., Down syndrome, Williams syndrome, DiGeorge syndrome) may have complex CHD as one of the phenotypic outcomes. Although the genetic basis for most cardiac defects has not been delineated, specific genetic anomalies have been implicated in the pathogenesis of some defects. For example, microdeletions of chromosome 22 are associated with DiGeorge syndrome and a variety of heart defects, including tetralogy of Fallot, truncus arteriosus, and interruption of the aortic arch. Developmental abnormalities are present in all children with 22q11 microdeletions, even those with no cardiac abnormalities (42). Thus, children with cardiac defects and 22q11 microdeletions may be developmentally impaired independent of the cardiac defect and cardiac surgery, however, recent studies suggest that the effects may be additive (43–45).
Risk of disease or injury in response to an environmental stimulus is a complex interaction between genetic susceptibility and environmental exposures. Interindividual variation in “disease risk” and in the response to environmental factors is significant. The “risk” may be modified by age, gender, ethnicity, and the extent of exposure to environmental factors. Multiple genes are involved in determining an individual’s response to a specific environmental factor. Interindividual variation in response to environmental exposures, such as cardiac surgery, probably is due in part to genetic polymorphisms. Common genetic variants, often due to single nucleotide substitutions, occur with a frequency of greater than 1%. For a child with complex CHD, environmental factors include cardiac surgery, use and/or duration of deep hypothermic circulatory arrest (DHCA), the inflammatory response to the synthetic surface of the cardiopulmonary bypass circuit, need for repeated operations, response to pressor or sedating medications, and socioeconomic status. The role of genetic polymorphisms in determining susceptibility to CNS injury in children with CHD is not known. Recent studies suggest that polymorphisms of apolipoprotein E (ɛ2 polymorphism) may be predictors of adverse neurodevelopmental sequelae following infant cardiac surgery (46–49), and similar finding have been reported in adults with the ɛ4 polymorphism (49–51). Antagonistic pleotropy is the term to describe how a polymorphism may be beneficial early, but harmful later in life (52). It is likely that multiple genes modulate the CNS response to cardiopulmonary bypass, DHCA, and other environmental factors modifying the risk and pattern of injury (53).
The underlying cardiac diagnosis may have a significant and independent impact on neurodevelopmental outcome and may modulate the effects of neuroprotective strategies. Presence of a ventricular septal defect in patients with transposition of the great arteries is a significant risk factor for poor developmental outcome (54–56). In a study of the effect of intraoperative pH management, developmental and neurologic outcomes were evaluated in infants undergoing repair of a variety of cardiac defects at less than 9 months of age who were randomized to either alpha-stat or pH-stat blood gas management strategy during deep hypothermic cardiopulmonary bypass (57). Children with transposition of the great arteris with or without ventricular septal defect, tetralogy of Fallot, isolated ventricular septal defect,, atrioventricular canal defect, truncus arteriosus, and total anomalous pulmonary venous return were enrolled. There was no effect of treatment on the Psychomotor Developmental Index (PDI) score of the Bayley Scores of Infant Development. The Mental Developmental Index (MDI) score, however, varied significantly depending on treatment group and diagnosis. For patients with transposition of the great arteries and tetralogy of Fallot, use of pH-stat resulted in a slightly higher MDI, although the difference was not statistically significant. Of interest, in the ventricular septal defect subgroup, the treatment effect was opposite with use of alpha-stat management, resulting in significantly improved scores. Cardiac diagnosis had a significant effect on outcomes: PDI and MDI scores were significantly higher in the transposition of the great arteries group compared with those noted for the other cardiac defects.
The Effect of Cardiac Surgery on the Brain
Even though there is increasing evidence that congenital and acquired CNS injury occurs in a significant fraction of children with CHD before surgery, many still focus on intraoperative management as the primary mechanism of CNS injury. This is due to the fact that, as opposed to all of the risk factors for abnormal neurological development discussed thus far, variation in intra-operative support, such as the conduct of bypass, is one of the few more easily modifiable risk factors which may be altered to improve long-term neurological outcomes. A partial list of factors which may contribute to CNS injury during surgical repair include hypoxemia, cerebral hypoperfusion, and cerebral embolism (particulate and/or air), as well as many of the details of mechanical support during surgery (DHCA or continuous cadioupulmonary bypass), use of hemodilution, the degree and rate of cooling, use of steroids, glucose management and type of blood gas management. Use of bypass exposes the blood to the foreign surfaces of the bypass circuit, initiating a systemic inflammatory response characterized by neutrophil activation, complement activation, and increased circulating levels of inflammatory cytokines. This inflammatory response may result in increased capillary permeability, tissue edema, and organ dysfunction. When continuous cardiopulmonary bypass is utilized, perfusion to the body and brain is maintained. When DHCA is utilized, there is a period of obligate global cerebral ischemia followed by reperfusion. Use of DHCA provides a bloodless surgical field, facilitating meticulous completion of the repair, and decreases the duration of blood exposure to the bypass circuit, but at the cost of a period of global cerebral ischemia. Continuous cardiopulmonary bypass - either in a typical manner or via regional techniques maintains perfusion to the brain and body but increases the duration of blood exposure to the bypass circuit, which may increase the severity of the inflammatory response. Use of continuous bypass avoids the period of cerebral ischemia but results in a greater increase in total body water and potentially more severe dysfunction of other organs, such as the heart and lungs (58, 59). These multiple facets of bypass have received considerable attention, and have been the subject of active research. Of the many potential modifiable technical features of intraoperative support mentioned above, there are three that been most extensively studied, particularly with randomized clinical trials.
pH Management
At Children’s Hospital, Boston, developmental and neurological outcomes were evaluated in infants undergoing biventricualr repair of a variety of cardiac defects at less than nine months of age who were randomized to either alpha-stat or pH-stat management during deep hypothermic cardiopulmonary bypass (60). Although there were some benefits reported with the use of pH-stat management for outcomes in the immediate peri-operative period, the use of either strategy was not consistently related to either improved or impaired neurodevelopmental outcomes in childhood (61). On the Bayley Scales of Infant Development, there was no effect of treatment on the PDI. The MDI, in contrast, varied significantly depending on the underlying anatomical diagnosis. For patients with transposition of the great arteries and tetralogy of Fallot, use of pH-stat resulted in a slightly higher mental developmental index, although the difference was not statistically significant. In patients with a ventricular septal defect, the effect was opposite, with use of alpha-stat management resulting in significantly improved scores. There was a significant effect of cardiac diagnosis on outcomes. Both scores of the Bayley examinations were significantly higher in those with transposition of the great arteries compared to the other cardiac defects. Despite the equivocal data in this early report, with no longer-term follow-up yet available nor confirmatory data from other randomised trials, many centers are currently utilizing pH-stat management – particularly during cooling on bypass - in all operations on neonates and infants. Further research in this area, based upon additional potential modifiers, for example, cardiac diagnosis, age, genetics and severity of pre-operative hypoxemia should continue.
Hematocrit During Bypass
During cardiopulmonary bypass, hemodilution has been widely applied based upon the notion that increased viscosity is detrimental during periods of profound or even moderate hypothermia. Work in animals suggesting that higher hematocrit levels conferred better cerebral protection has also been more extensively investigated in two human randomized clinical trials (62, 63). The results of these trials indicated that hematocrit levels during bypass below 24% were associated with lower scores in the psychomotor development index of the Bayley Scales of Infant Development, although no further improvement was seen comparing hematocrit levels of 25% to 35%. In addition, lower hematocrit levels were associated with a more positive fluid balance after surgery and higher serum lactate levels. Pooled data from these two studies was analyzed and an inflection point was determined to be at around 28% (64). These findings have been confirmed by multiple authors, and higher hematocrits during bypass are being utlized by most centers (65).
Deep Hypothermic Circulatory Arrest
Much has been written on the potentially deleterious effects of prolonged circulatory arrest with profound hypothermia in cardiac surgery for neonates and infants. It is generally agreed that more prolonged periods of uninterrupted circulatory arrest will result in an increased risk of adverse neurological outcomes (66, 67). Close inspection of the data, however, shows that the effects of short durations of circulatory arrest are inconsistently related to adverse outcomes, and that the effect of circulatory arrest is not a linear phenomenon. As mentioned previously, the effects are most likely modified by other pre- and postoperative factors related to the patient. Some reports, most in an earlier era of cardiac surgery demonstrate a detrimental effect of circulatory arrest on a variety of outcomes relating to the CNS, while some demonstrate either an inconsistent effect or no effect. Some have taken the stance that, since the majority of studies suggest a negative effect of circulatory arrest, it should be avoided at all costs. Innovative and challenging strategies have been designed to provide continuous cerebral perfusion during reconstruction of the aortic arch or intracardiac repair. The avoidance of circulatory arrest, however, by necessity requires an increased duration of cardiopulmonary bypass (68). This has consistently been shown to have an adverse effect on outcomes in both the short and longer term. A randomized trial comparing circulatory arrest to continuous cerebral perfusion completed at the University of Michigan demonstrated no improvement in developmental scores at one year of age (69). Similar findings were reported in a contemporaneous but non-randomized study at Children’s Hospital of Boston (70). It seems imprudent to change practice based upon studies with only short-term developmental assessment. Developmental studies in infants have very limited predictive validity for long-term outcomes, either for patients with or without CHD.
Perhaps the best conducted study in this regard, which emphasizes this point, is the Boston Circulatory Arrest Study (58, 71–82). In this study, a cohort of children with transposition of the great arteris undergoing the arterial switch operation were randomly assigned to intra-operative support with predominantly DHCA or to predominantly cardiopulmonary bypass at low flow. Earlier reports suggested that the group as a whole was performing below expectations in many aspects of evaluation, with worse outcomes for those undergoing circulatory arrest in the areas of post-operative seizures (71), motor skills at 1 year of age (72), as well as behavior, speech, and language by the age of 4 years (73–75). Mean intelligence quotient at the age of 4 was lower than expected at 93, with no difference according to treatment assignment (75). Many centers began avoiding even short periods of circulatory arrest based upon these and other reports. Neurodevelopmental analyses when the patients were aged 8 years revealed that the intelligence quotients for the cohort as a whole are now closer to normal, at 98 versus the population mean of 100 (76). The patients did demonstrate significant deficits in visual-spatial and visual-memory skills, as well as in components of executive functioning such as working memory, hypothesis generation, sustained attention, and higher-order language skills. In other words, the children had difficulty coordinating skills in order to perform complex operations. Those repaired using circulatory arrest scored worse on motor and speech functioning, while those undergoing bypass at low flow demonstrated worse scores for impulsivity and behavior. When compared to a normative sample, parents of the entire cohort reported significantly higher frequencies of attention problems, developmental delay, and problems with learning and speech. More than one-third of the population required remedial services at school, and one in ten had repeated a grade. At age 16, no significant impact was seen based upon intraoperative management; the early negative effects of hypothermic arrest were no longer seen, and in fact, some outcomes were worse in the arm randomized to low-flow cardiopulmonary bypass (80). However, additional concerns became apparent: executive dysfunction and “theory of mind” abnormalities were prevalent (79), patients were 4 times more likely to be taking psychotropic medications compared to cardiovascular medications, and the number who received behavioral therapies and/or additional help at school increased to 65% (79). One-third had brain abnormalities detected on MRI (80, 81). Additional recent investigations confirm these abnormalities in multiple centers throughout the world (29, 39, 83, 84).
Whether current modifications of bypass techniques will improve the outcomes in the long term remains the subject of ongoing study. This well-designed trial, with superb follow-up, enrolled neonates who were planned to undergo an arterial switch operation between 1988 and 1992. Hence, the results reflect the peri-operative and surgical care delivered in that era, and thus may not be generalizable to the current era, or to other congenital cardiac lesions. For example, some features of routine post-operative care in that era, including extension of the anaesthetic period for at least 48 hours, active rewarming in the intensive care unit after surgery, and hyperventilation to reduce the risk of pulmonary hypertension, may each independently adversely affect neurodevelopmental outcomes. In addition, those patients randomized to predominantly continuous bypass also underwent a relatively brief period of circulatory arrest. Thus, the study does not compare use of circulatory arrest to no circulatory arrest. The results, nonetheless, serve to show the multiple factors which influence developmental outcome at school age, and show that factors related to poorer outcome, such as DHCA, which seem apparent and significant on early testing, may be attenuated or even abolished during longer-term follow-up, as other factors assume a more important role. More recently pooled 2-year neurodevelopmental testing data from over 1700 patients from 22 international centers collected from 1996 to 2009 were analyzed. PDI and MDI scores (77.6 ± 18.8 and 88.2 ± 16.7, respectively) were lower than normative means, and after controlling for a variety of risks, MDI improved only 0.38 points/year, hardly a drastic effect from over a decade of modifying surgical and medical care strategies (2).
Postoperative Factors
CNS injury may occur or be exacerbated in the postoperative period. As described, many studies have focused on the operating room as the site of CNS injury; however, events in the cardiac intensive care unit may be equally important. Cerebral ischemia can result from low oxygen delivery from decreased cardiac output, severe hypoxemia and/or severe anemia. Postoperative agitation, pain and/or hyperthermia may increase the metabolic needs of the brain, resulting in worsening CNS injury (85). In addition, postoperative cardiac arrest – with or without the need for mechanical circulatory support – may occur is as many as 20% of certain subgroups of newborns with complex CHD (86), and may result in significant CNS injury (86–90). Following cardiac surgery with bypass with or without DHCA, cerebral autoregulation may be impaired (91). Following surgery, especially in newborns and infants, there is a predictable and reproducible fall in cardiac output (58, 92–94). This period of decreased oxygen delivery, usually within the first 24 hours after surgery, represents a particularly vulnerable time for the CNS, especially if associated with increased oxygen consumption (95–97). At present, studies linking postoperative hemodynamic lability to long-term CNS outcomes are lacking. However, postoperative hypotension has been shown to be related to new or worsened white matter injury (98), especially if combined with hyperventilation, which may further reduce cerebral blood flow (99, 100). Despite theoretical concerns of adverse neurodevelopmental effects, postoperative hyperglycemia has not been shown to correlate with adverse longer-term neurodevelopmental outcomes (101, 102). Finally, work is currently underway to investigate the correlation of noise, aminoglyoside usage and subsequent hearing loss in school age children with complex CHD (Burnham N, personal communication).
Length of Stay
Compared to cardiac surgery at older ages, neonates with complex CHD may have protracted stays in the intensive care unit - averaging nearly a month in most reports - with a significant number of outliers with even considerably longer lengths of stay (103). Increased length of stay has been associated with increased risks of medical error, costs, parental stress, reoperation and other cardiac and non-cardiac morbidity (103–107). In the Boston Circulatory Arrest Study, length of stay was independently associated with worse cognitive function at 8 years of age, even after adjustment for factors related to the length of stay (e.g., sepsis, low cardiac output) or cognitive outcomes (e.g., maternal education, socioeconomic status) (76). Virtually all studies reporting short and longer term neurodevelopmental outcomes have two consistent factors independently related to worse outcomes: increased length of stay and lower socioeconomic status (77, 80, 108–110). While some aspects of length of stay may not be modifiable, many units are now actively investigating strategies to reduce length of stay (e.g., timing of surgery, early extubation, minimizing delayed sternal closure, etc.) in hopes of improving longer term outcomes. (While socioeconomic status is not modifiable per se, children from disadvantaged families may be at highest risk, and particular attention must be given to neurodevelopmental care during the hospitalization and after discharge.) One aspect of increased length of stay in particular is the use of prolonged sedation, including narcotics and benzodiazepines, along with the use of volatile anesthetic agents during cardiac surgery, which may adversely affect neurodevelopment. Increasingly it is being recognized that the cummulative exposure to these agents in infancy is related to worse outcomes (111–114).
Longer-term Effects of the ICU Stay
Finally, it is clear that multiple factors for adverse outcomes co-exist in neonates who experience a long initial hospital length of stay; all of these have been shown to increase parental stress, anxiety, and feelings of helplessness and inadequacy (figure 2a). This is superimposed on the early traumatic events of receiving the diagnosis of complex CHD, the uncertainty of survival, separation from the infant, possible setbacks, postpartum depression, witnessing medical procedures and paraphernalia, and vicarious trauma (witnessing events in other patients). Following discharge, home care of the neonate following surgery for complex CHD is exceptionally complex, with feeding issues common, multiple medications, feelings of inadequacy, disruption of the family routine, and many other issues (115–119). These factors incurred early on lead to an abnormal maternal-child dyad and ultimately to behavioral challenges (figure 2b), which almost certainly have long term effects on parenting styles, psychosocial health and the development of the “fragile child” (figure 3). Indeed, maternal worry and mental health (along with a small component of the child’s visual-perceptual skills) accounted for 27.9% of the variability in child behavior adjustment at the end of the first year of school, 5–10 times more explanatory than any surgical or intraoperative factor described to date (120). In his seminal work, McCusker and colleagues have shown in a randomized trial that perioperative efforts to reduce maternal worry utilizing advanced practice nursing have significant benefits to both the mother and child (121). Acute stress disorder in parents during the neonatal hospitalization is common (122), and has been shown to be related to symptoms of post-traumatic stress disorder later in life (122, 123), which may independently effect family functioning, child self-image and child-rearing schema. Attention to this important, modifiable risk factor for later neurodevelopment both in the inpatient and outpatient settings holds promise for improvement in our patients with complex CHD.
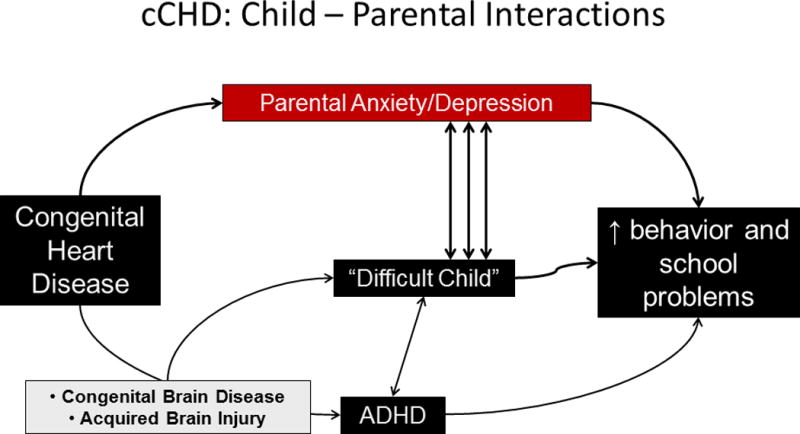
Figure 2.
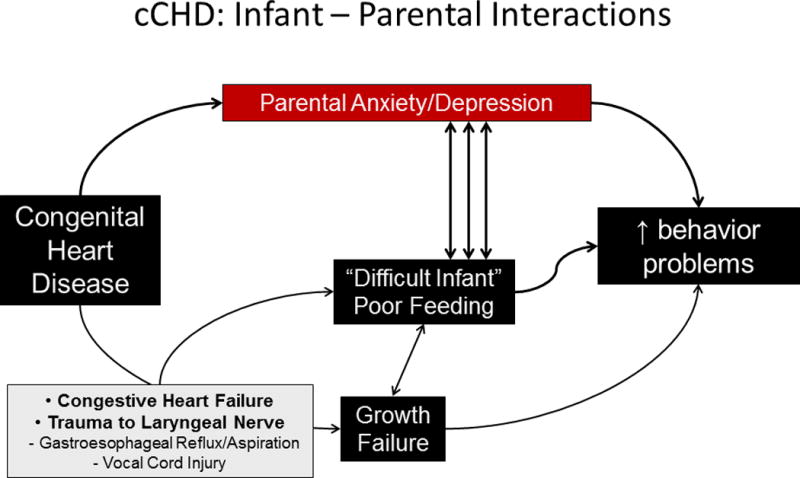
a. The potential interactions between complex congenital heart and brain disease, its treatment, and parental and patient outcomes in (a) the infant. ADHD=attention deficit hyperactivity disorder
b. The potential interactions between complex congenital heart and brain disease, its treatment, and parental and patient outcomes in (b) the child. ADHD=attention deficit hyperactivity disorder
Figure 3.
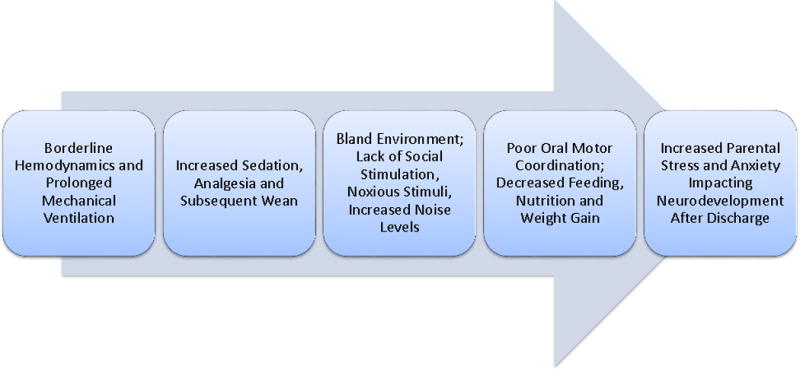
The progression of multiple factors related to adverse neurodevelopment and ‘the fragile child’ following a prolonged intensive care unit stay
Summary and Furture Directions
Although children with mild types of CHD appear to have normal CNS and neurodevelopmental outcomes (124–125), children with complex CHD constitute an at-risk population with a significant incidence of adverse developmental outcomes. Current techniques for developmental evaluation in neonates and infants are imprecise predictors of late outcomes. Evaluation of preschool- and school-aged children reveals a pattern of neurodevelopmental dysfunction characterized by mild cognitive impairment, motor dysfunction, impaired visual-spatial and visual-motor skills, and attention and academic difficulties. There are significant problems with expressive speech and language and a high incidence of learning disabilities. The factors resulting in CNS injury and developmental dysfunction in these children are multiple, and incompletely understood. Developmental dysfunction results from a complex interaction between patient-specific factors (genetic susceptibility, cardiac diagnosis, fetal development) and environmental factors (preoperative events, techniques of support during surgical repair, postoperative events, socioeconomic status). Currently, reported risk factors do not adequately explain the pattern or incidence of CNS injury following cardiac surgery in infants, suggesting that other patient-specific factors may modulate the response to CHD and cardiac surgery, increasing the risk of adverse neurodevelopmental sequelae. Children with complex CHD are at risk for cerebral ischemia before, during, and after cardiac surgery; therefore, factors, that impair CNS recovery following ischemia may be important determinants of long-term neurologic outcome.
At the current time, important investigations are underway to (1) understand the developing brain in the fetus with complex CHD, (2) to identify modifiable risk factors in the operating room and intensive care unit in order to maximize long-term neurodevelopmental outcomes and (3) develop strategies to improve family psychosocial health, childhood development and health-related quality of life following hospital discharge. Crucial in this effort is the identification of an early post-operative surrogate variable with good predictive validity for long-term outcomes. MRI is showing great promise in this area, with correlations now being seen with early structural changes, particularly in the white matter, with intermediate neurodevelopmental outcomes (81–83). If an appropriate surrogate variable for long term outcomes can be identified, and measured relatively early after surgical intervention for complex CHD, reliable clinical trials can be undertaken to improve upon current outcome.
Acknowledgments
Copyright form disclosures: Dr. Licht received support for article research from the National Institutes of Health and philanthropy (The S. and J. Wolfson Family).
Dr. Licht is supported by grants from the NINDS (R01NS72338, R01NS060653) and support from the June and Steve Wolfson Family Foundation.
Footnotes
Conflict of interest: Dr. Wernovsky disclosed that he does not have any potential conflicts of interest.
References
- 1.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management. A Scientific Statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 2.Gaynor JW, Stopp C, Wypij D, et al. Neurodevelopmental Outcomes after Cardiac Surgery in Infancy: A multi-center retrospective analysis of patient factors associated with early outcomes in 1,770 subjects. Pediatrics. 2015;135:816–25. doi: 10.1542/peds.2014-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16(Suppl 1):92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-Transposition of the Great Arteries Corrected with the Arterial Switch Procedure: Neuropsychological Assessment and Structural Brain Imaging. Circulation. 2011;124:1361–9. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackie AS, Vatanpour S, Alton GY, et al. Clinical Outcome Score Predicts Adverse Neurodevelopmental Outcome After Infant Heart Surgery. Ann Thorac Surg. 2015;99:2124–2132. doi: 10.1016/j.athoracsur.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Mussatto KA, Hoffmann RG, Hoffman GM, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014:3570–e577. doi: 10.1542/peds.2013-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long S, Eldridge B, Harris SR, Cheung M. Challenges in trying to implement an early intervention program for infants with congenital heart disease. Pediatric Physical Therapy. 2015;27:38–43. doi: 10.1097/PEP.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 8.Malkar MB, Jadcheria S. Neuro-motor mechanisms of Pharyngo-esophageal Motility in Dysphagic Infants with Congenital Heart Disease. Pediatr Res. 2014;76:190–196. doi: 10.1038/pr.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stieber NA, Gilmour S, Morra A, et al. Feasibility of improving the motor development of toddlers with congenital heart defects using a home-based intervention. Pediatr Cardiol. 2012;33:521–532. doi: 10.1007/s00246-011-0144-0. [DOI] [PubMed] [Google Scholar]
- 10.Mahle WT, Tavani F, Zimmerman RA, et al. The assessment of neurologic injury before and after congenital heart surgery using magnetic resonance imaging. Circulation. 2002;106(Suppl 1):I109–I114. [PubMed] [Google Scholar]
- 11.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller SP, McQuillen PS. Neurology of congenital heart disease: insight from brain imaging. Arch Dis Child Fetal neonatal Ed. 2007;92:F435–437. doi: 10.1136/adc.2006.108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block AJ, McQuillen PS, Chau V, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donofrio MT, Bremer YA, Schieken RM, et al. Autoregulation of cerebral blood flow in fetus with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436–443. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 15.Kaltman JR, Di H, Tian Z, Rychik J. Ultrasound. Obstet Gynecol. 2005;25:32–36. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 16.Limperopoulos C, Tworetzky W. evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glauser TA, Rorke LB, Weinberg PM, et al. Congenital brain anomalies associated wit the hypoplastic left heart syndrome. Pediatrics. 1990;85:984–990. [PubMed] [Google Scholar]
- 19.Prsa M, Sun L, van Amerom J, et al. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ Cardiovasc Imaging. 2014;7:663–670. doi: 10.1161/CIRCIMAGING.113.001859. [DOI] [PubMed] [Google Scholar]
- 20.Naeye RL. Transposition of the great arteries and prenatal growth. Arch Pathol. 1966;82:412–418. [PubMed] [Google Scholar]
- 21.Jones HN, Olbrych SK, Smith KL, et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta. 2015 doi: 10.1016/j.placenta.2015.08.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillon JE. Behavior of Newborns with Cardiac Distress. Am J Nurs. 1973;73:254–257. [PubMed] [Google Scholar]
- 23.Limperopoulos C, Majnemer A, Shevell MI, et al. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr. 2000;137:638–645. doi: 10.1067/mpd.2000.109152. [DOI] [PubMed] [Google Scholar]
- 24.Licht DJ, Wang J, Silvestre DW, et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841–849. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 25.McQuillen PS, Hamrick SE, Perez MJ, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–285. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee D, Lindsay M, Zhang Y, et al. Analysis of 8681 neonates with transposition of the great arteries: outcomes with and without Rashkind balloon atrial septostomy. Cardiol Young. 2010;20:373–380. doi: 10.1017/S1047951110000296. [DOI] [PubMed] [Google Scholar]
- 27.Petit CJ, Rome JJ, Wernovsky G, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–16. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Applegate SE, Lim DS. Incidence of stroke in patients with d-transposition of the great arteries that undergo balloon atrial septostomy in the University Healthsystem Consortium Clinical Data Base/Resource Manager. Catheter Cardiovasc Interv. 2020;76:129–131. doi: 10.1002/ccd.22463. [DOI] [PubMed] [Google Scholar]
- 29.Beca J, Gunn J, Coleman L, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53:1807–1811. doi: 10.1016/j.jacc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 30.Andelfinger G. Genetic factors in congenital heart malformation. Clin Genet. 2008;73:516–527. doi: 10.1111/j.1399-0004.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- 31.Aisenberg RB, Rosenthal A, Nadas AS, et al. Developmental delay in infants with congenital heart disease. Correlation with hypoxemia and congestive heart failure. Pediatr Cardiol. 1982;3:133–137. doi: 10.1007/BF02312960. [DOI] [PubMed] [Google Scholar]
- 32.Kurth CD, Steven JL, Montenegro LM, et al. Cerebral oxygen saturation before congenital heart surgery. Ann Thorac Surg. 2001;72:187–192. doi: 10.1016/s0003-4975(01)02632-7. [DOI] [PubMed] [Google Scholar]
- 33.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 34.Shillingford AJ, Ittenbach RF, Marino BS, et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young. 2007;17:1–7. doi: 10.1017/S1047951107000248. [DOI] [PubMed] [Google Scholar]
- 35.Hinton RB, Andelfinger G, Sekar, et al. Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res. 2008;64:364–369. doi: 10.1203/PDR.0b013e3181827bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hangge PT, Cnota JF, Woo JG, et al. Microcephaly is associated with early adverse neurologic outcomes in hypoplastic left heart syndrome. Pediatr Res. 2013;74:61–67. doi: 10.1038/pr.2013.61. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Awate SP, Licht DJ, et al. Assessment of MRI-Based Automated Fetal Cerebral Cortical Folding Measures in Prediction of Gestational Age in the Third Trimester. Am J Neuroradiol. 2015;36:1369–1374. doi: 10.3174/ajnr.A4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinrichs AKM, Holschen A, Krings T, et al. Neurologic and psycho-intellectual outcome related to structural brain imaging in adolescents and young adults after neonatal arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg. 2014;148:2190–2199. doi: 10.1016/j.jtcvs.2013.10.087. [DOI] [PubMed] [Google Scholar]
- 40.Back SA, Luo NL, Borenstein NS, et al. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp neurol. 2002;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- 41.Lynch JM, Buckley EM, Schwab PJ, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014;148:2181–2188. doi: 10.1016/j.jtcvs.2014.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerdes M, Solot C, Wang PP, et al. Cognitive and behavior profile of preschool children with chromosome 22q11.2 deletion. Am J Med Genet. 1995;85:127–133. [PubMed] [Google Scholar]
- 43.Atallah J, Joffe AR, Robertson CMT, et al. Two-year general and neurodevelopmental outcome after neonatal complex cardiac surgery in patients with deletion 22q11.2: A comparative study. J Thor Cardiovasc Surg. 2007;134:772–779. doi: 10.1016/j.jtcvs.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Guerra G, Joffe AR, Robertson CMT, et al. Health-Related Quality of Life Experienced by Children With Chromosomal Abnormalities and Congenital Heart Defects. Pediatr Cardiol. 2014;35:536–541. doi: 10.1007/s00246-013-0820-3. [DOI] [PubMed] [Google Scholar]
- 45.Yi JJ, Tang SX, McDonald-McGinn DM, et al. Contribution of Congenital Heart Disease to Neuropsychiatric Outcome in School-Age Children with 22q11.2 Deletion Syndrome. Am J Med Genet B Neuropsychiatr Genet. 2014;2:137–147. doi: 10.1002/ajmg.b.32215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–1745. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 47.Fuller S, Nord AS, Gerdes M, et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009;36:40–47. doi: 10.1016/j.ejcts.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 48.Gaynor JW, Nord AS, Wernovsky G, et al. Apolipoprotein E genotype modifies the risk of behavior problems in preschool children following neonatal and infant cardiac surgery. Pediatrics. 2009;124:241–250. doi: 10.1542/peds.2008-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burnham N, Ittenbach RF, Stallings VA, et al. Genetic factors are important determinants of impaired growth after infant cardiac surgery. J Thoarac Cardiovasc Surg. 2010;140:144–149. doi: 10.1016/j.jtcvs.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman FM, Croughwell ND, Blumenthal JA, et al. Predictors of cognitive decline after cardiac operation. Ann ThoracThe role of apolipoprotein E in cognitive decline after cardiac operation. Ann Thorac Surg. 2001;71:823–826. doi: 10.1016/s0003-4975(00)02511-x. [DOI] [PubMed] [Google Scholar]
- 51.Tagarakis GI, Tsolaki-Tagaraki F, Tsolaki M, et al. The Role of Apolipoprotein E in Cognitive Decline and Delirium after Bypass Heart Operations. Am J Alzheimers Dis Other Demen. 2007;22:223–228. doi: 10.1177/1533317507299415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav A, Radhakrishnan A, Bhanot G, et al. Differential regulation of antagonistic pleiotropy in synthetic and natural populations suggests its role in adaptation. G3 (Bethesda) 2015;5:699–709. doi: 10.1534/g3.115.017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DS, Kim JH, Burt AA, et al. Patient genotypes impact survival after surgery for isolated congenital heart disease. Ann Thorac Surg. 2014;98:104–110. doi: 10.1016/j.athoracsur.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 55.Bellinger DC, Wypij D, Kuban KCK, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 56.Newburger JW, Jonas RA, Wernovsky G, et al. Comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 57.Bellinger DC, Wypij D, du Plessis AJ, et al. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2001;121:374–383. doi: 10.1067/mtc.2001.111206. [DOI] [PubMed] [Google Scholar]
- 58.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow bypass and circulatory arrest. Circulation. 1995;92:2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 59.Skaryak LA, Lodge AJ, Kirshbom PM, et al. Low-flow cardiopulmonary bypass produces greater pulmonary dysfunction than circulatory arrest. Ann Thorac Surg. 1996;62:1284–1288. doi: 10.1016/0003-4975(96)00602-9. [DOI] [PubMed] [Google Scholar]
- 60.Du Plessis AJ, Jonas RA, Wypij D, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1000. doi: 10.1016/S0022-5223(97)70013-8. [DOI] [PubMed] [Google Scholar]
- 61.Bellinger DC, Wypij D, du Plessis AJ, et al. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2001;121:374–383. doi: 10.1067/mtc.2001.111206. [DOI] [PubMed] [Google Scholar]
- 62.Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765–1774. doi: 10.1016/j.jtcvs.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Newburger JW, Jonas RA, Soul J, et al. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135:347–354. doi: 10.1016/j.jtcvs.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 64.Wypij D, Jonas RA, Bellinger DC, et al. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg. 2008;135:355–360. doi: 10.1016/j.jtcvs.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 65.Hirsch JC, Jacobs ML, Andropoulos D, et al. Protecting the infant brain during cardiac surgery: a systematic review. Ann Thorac Surg. 2012;94:1365–1373. doi: 10.1016/j.athoracsur.2012.05.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naim MY, Gaynor JW, Chen J, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–178. doi: 10.1016/j.jtcvs.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kornilov IA, Sinelnikov YS, Soinov IA, et al. Outcomes after aortic arch reconstruction for infants: deep hypothermic circulatory arrest versus moderate hypothermia with selective antegrade cerebral perfusion. Eur J Cardiothorac Surg. 2015;48:e45–50. doi: 10.1093/ejcts/ezv235. [DOI] [PubMed] [Google Scholar]
- 68.Hannan RL, Ybarra MA, Ojito JW, et al. Complex neonatal single ventricle palliation using antegrade cerebral perfusion. Ann Thorac Surg. 2006;82:1278–1284. doi: 10.1016/j.athoracsur.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 69.Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133:880–887. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Visconti KJ, Rimmer D, Bauvreau K, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one-year neurodevelopmental outcome. Ann Thorac Surg. 2006;82:2207–2211. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 71.Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 72.Bellinger DC, Jonas RA, Rappaport LA, et al. A comparison of the developmental and neurologic status at one year of children who underwent heart surgery using hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 73.Bellinger DC, Rappaport LA, Wypij D, et al. Patterns of developmental dysfunction after surgery during infancy to correct transposition of the great arteries. J Dev Behav Pediatr. 1997;18:75–83. doi: 10.1097/00004703-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Circulation. 1998;97:773–779. doi: 10.1161/01.cir.97.8.773. [DOI] [PubMed] [Google Scholar]
- 75.Bellinger DC, Wypij D, Kuban KCK, et al. Developmental and neurologic status of children at four years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 76.Newburger JW, Wypij D, Bellinger DC, et al. Length of stay after infant heart surgery is related to cognitive outcomes at age 8 years. J Pediatr. 2003;143:67–73. doi: 10.1016/S0022-3476(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 77.Bellinger DC, Wypij D, duPlesssis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 78.Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 79.Bellinger DC. Are children with congenital cardiac malformations at increased risk of deficits in social cognition? Cardiol Young. 2008;18:3–9. doi: 10.1017/S104795110700176X. [DOI] [PubMed] [Google Scholar]
- 80.Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-Transposition of the Great Arteries Corrected with the Arterial Switch Procedure: Neuropsychological Assessment and Structural Brain Imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rivkin MJ, Watson CG, Scoppettuolo LA, et al. Adolescents with D-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. J Thorac Cardiovasc Surg. 2013;146:543–549. doi: 10.1016/j.jtcvs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panigrahy A, Schmithorst VM, Wisnowski JL, et al. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. Neuroimage Clin. 2015;7:438–448. doi: 10.1016/j.nicl.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rollins CK, Watson CG, Asaro LA, et al. White Matter Microstructure and Cognition in Adolescents with Congenital Heart Disease. J Pediatrics. 2014;165:936–944. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Von Rhein M, Kugler J, Liamlahi R, et al. Persistence of visuo-constructional and executive deficits in adolescents after open-heart surgery. Research in Developmental Disabilities. 2015;36:303–310. doi: 10.1016/j.ridd.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 85.Shum-Tim D, Nagashima M, Shinoka T, et al. Postischemic hyperthermia exacerbates neurologic injury after deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1998;116:780–792. doi: 10.1016/s0022-5223(98)00449-8. [DOI] [PubMed] [Google Scholar]
- 86.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of Shunt Types in the Norwood Procedure for Single-Ventricle Lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris MC, Ittenbach RF, Godinez RI, et al. Risk factors for mortality in 137 pediatric cardiac intensive care unit patients managed with extracorporeal membrane oxygenation. Crit Care Med. 2004;32:1061–1069. doi: 10.1097/01.ccm.0000119425.04364.cf. [DOI] [PubMed] [Google Scholar]
- 88.Ortmann L, Prodham P, Gossett J, et al. Outcomes After In-Hospital Cardiac Arrest in Children With Cardiac Disease. A Report From Get With the Guidelines–Resuscitation. Circulation. 2011;124:2329–2337. doi: 10.1161/CIRCULATIONAHA.110.013466. [DOI] [PubMed] [Google Scholar]
- 89.Gaies MG, Clarke NS, Donohue JE, et al. Personnel and unit factors impacting outcome after cardiac arrest in a dedicated pediatric cardiac intensive care unit. Pediatr Crit Care Med. 2012;13:583–588. doi: 10.1097/PCC.0b013e318238b272. [DOI] [PubMed] [Google Scholar]
- 90.Newburger JW, Sleeper LA, Bellinger DC, et al. Early Developmental Outcome in Children With Hypoplastic Left Heart Syndrome and Related Anomalies - The Single Ventricle Reconstruction Trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bassan H, Gauvreau K, Newburger JW, et al. Identification of Pressure Passive Cerebral Perfusion and Its Mediators after Infant Cardiac Surgery. Pediatric Research. 2005;57:35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 92.Hoffman TM, Wernovsky G, Atz AM, et al. The efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 93.Charpie JR, Dekeon MK, Goldberg CS, et al. Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. J Thorac Cardiovasc Surg. 2000;120:73–80. doi: 10.1067/mtc.2000.106838. [DOI] [PubMed] [Google Scholar]
- 94.Hannan RL, Yvarra MA, White JA, et al. Patterns of Lactate Values after Congenital Heart Surgery and Timing of Cardiopulmonary Support. Ann Thorac Surg. 2005;80:1468–1474. doi: 10.1016/j.athoracsur.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 95.Li J, Schulze-Neick I, Lincoln C, et al. Oxygen consumption after cardiopulmonary bypass surgery in children: Determinants and implications. J Thorac Cardiovasc Surg. 2000;119:525–533. doi: 10.1016/s0022-5223(00)70132-2. [DOI] [PubMed] [Google Scholar]
- 96.Li J, Zhang G, McCrindle BW, et al. Profiles of hemodynamics and oxygen transport derived by using continuous measured oxygen consumption after the Norwood procedure. J Thorac Cardiovasc Surg. 2007;133:441–448. doi: 10.1016/j.jtcvs.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 97.McHoney M, Eaton S, Pierro A. Metabolic response to surgery in infants and children. European Journal of Pediatric Surgery. 2009;19:275–285. doi: 10.1055/s-0029-1241192. [DOI] [PubMed] [Google Scholar]
- 98.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 99.Samanta B, Bird GL, Kuijpers M, et al. Prediction of periventricular leukomalacia. Part I: Selection of hemodynamic features using logistic regression and decision tree algorhythms. Artif Intell Med. 2009;46:201–215. doi: 10.1016/j.artmed.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Samanta B, Bird GL, Kuijpers M, et al. Prediction of periventricular leukomalacia. Part II: Selection of hemodynamic features using computational intelligence. Artif Intell Med. 2009;46:217–231. doi: 10.1016/j.artmed.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krueger JJ, Brotschi B, Balmer C, et al. Postoperative Hyperglycemia and 4-Year Neurodevelopmental Outcome in Children Operated for Congenital Heart Disease. J Pediatr. 2015 Jul 30; doi: 10.1016/j.jpeds.2015.07.007. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 102.Ballweg JA, Wernovsky G, Ittenbach RF, et al. Hyperglycemia after infant cardiac surgery does not adversely impact neurodevelopmental outcome. Ann Thorac Surg. 2007;84:2052–2058. doi: 10.1016/j.athoracsur.2007.06.099. [DOI] [PubMed] [Google Scholar]
- 103.CDC Morbitity and Mortality Weekly Report. Hospital Stays, Hospital Charges, and In-Hospital Deaths among Infants with Selected Birth Defects — United States. 2003 Availlable at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5602a1.htm. [PubMed]
- 104.Slonim AD, LaFleur BJ, Ahmed W, et al. Hospital-reported medical errors in children. Pediatrics. 2003;111:617–621. doi: 10.1542/peds.111.3.617. [DOI] [PubMed] [Google Scholar]
- 105.Majnemer A, Limperopoulos C, Shevell M, et al. Health and well-being of children with congenital cardiac malformations, and their families, following open-heart surgery. Cardiol Young. 2006;16:157–164. doi: 10.1017/S1047951106000096. [DOI] [PubMed] [Google Scholar]
- 106.Franck LS, Mcquillan A, Wray J, et al. Parent Stress Levels During Children’s Hospital Recovery After Congenital Heart Surgery. Pediatr Cardiol. 2010;31:961–968. doi: 10.1007/s00246-010-9726-5. [DOI] [PubMed] [Google Scholar]
- 107.Tabbutt S, Ghanayem N, Ravishankar C, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–895. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Limperopoulos C, Majnemer A, Shevell MI, et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. 2002;141:51–58. doi: 10.1067/mpd.2002.125227. [DOI] [PubMed] [Google Scholar]
- 109.Forbess JF, Visconti KJ, Hancock-Friesen C, et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106(suppl1):I95–I102. [PubMed] [Google Scholar]
- 110.Atallah J, Dinu IA, Robertson CM, et al. Two-year survival and mental and psychomotor outcomes after the Norwood procedure: an analysis of the modified Blalock-Taussig shunt and right ventricle-to-pulmonary artery shunt surgical eras. Circulation. 2008;118:1410–1418. doi: 10.1161/CIRCULATIONAHA.107.741579. [DOI] [PubMed] [Google Scholar]
- 111.Garcia-Guerra G, Robertson CMT, Alton GY, et al. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr Anaesth. 2011;21:932–941. doi: 10.1111/j.1460-9592.2011.03581.x. [DOI] [PubMed] [Google Scholar]
- 112.Andropoulos DB, Ahmad HB, Haq T, et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth. 2014;24:266–274. doi: 10.1111/pan.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wise-Faberowski L, Quinonez ZA, Hammer GB. Anesthesia and the Developing Brain: Relevance to the Pediatric Cardiac Surgery. Brain Sci. 2014;4:295–310. doi: 10.3390/brainsci4020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia-Guerra G, Robertson CMT, Alton GY, et al. Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4-year follow-up. Paediatr Anaesth. 2014;24:257–265. doi: 10.1111/pan.12257. [DOI] [PubMed] [Google Scholar]
- 115.Jordan B, Franich-Ray C. Early mother-infant relationships after cardiac surgery in infancy. Arch Dis Child. 2014;99:641–645. doi: 10.1136/archdischild-2012-303488. [DOI] [PubMed] [Google Scholar]
- 116.Dulfer K, Duppen N, Van Dijk APJ, et al. Parental Mental Health Moderates the Efficacy of Exercise Training on Health-Related Quality of Life in Adolescents with Congenital Heart Disease. Pediatr Cardiol. 2015;36:33–40. doi: 10.1007/s00246-014-0961-z. [DOI] [PubMed] [Google Scholar]
- 117.Rempel GR, Ravindran V, Rogers LG, et al. Parenting under Pressure: a grounded theory of parenting young children with life-threatening congenital heart disease. J Adv Nurs. 2013;69:619–630. doi: 10.1111/j.1365-2648.2012.06044.x. [DOI] [PubMed] [Google Scholar]
- 118.Jackson AC, Frydenberg E, Liang RPT, et al. Familial Impact and Coping with Child Heart Disease: A Systematic Review. Pediatr Cardiol. 2015;36:695–712. doi: 10.1007/s00246-015-1121-9. [DOI] [PubMed] [Google Scholar]
- 119.Torowicz D, Irving SY, Hanlon AL, et al. Infant temperament and parental stress in 3-month-old infants after surgery for complex congenital heart disease. J Dev Behav Pediatr. 2010;31:202–208. doi: 10.1097/DBP.0b013e3181d3deaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCusker CG, Doherty NN, Molloy B, et al. A Randomized Controlled Trial of Interventions to Promote Adjustment in Children With Congenital Heart Disease Entering School and Their Families. J Pediatr Psychol. 2012;37:1089–1103. doi: 10.1093/jpepsy/jss092. [DOI] [PubMed] [Google Scholar]
- 121.McCusker CG, Doherty NN, Molloy B, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev. 2010;36:110–117. doi: 10.1111/j.1365-2214.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 122.Franich-Ray C, Bright MA, Anderson V, et al. Trauma reactions in mothers and fathers after their infant’s cardiac surgery. J Pediatr Psychol. 2013;38:494–505. doi: 10.1093/jpepsy/jst015. [DOI] [PubMed] [Google Scholar]
- 123.Helfricht S, Latal B, Fischer JE, et al. Surgery-related posttraumatic stress disorder in parents of children undergoing cardiopulmonary bypass surgery: a prospective cohort study. Pediatr Crit Care Med. 2001;9:217–223. doi: 10.1097/PCC.0b013e318166eec3. [DOI] [PubMed] [Google Scholar]
- 124.van der Rijken R, Hulstijn-Dirkmaat G, Kraaimaat F, et al. Open-heart surgery at school age does not affect neurocognitive functioning. Eur Heart J. 2008;29:2681–2688. doi: 10.1093/eurheartj/ehn432. [DOI] [PubMed] [Google Scholar]
- 125.Quartermain MD, Ittenbach RF, Flynn TB, et al. Neuropsychological status in children after repair of acyanotic congenital heart disease. Pediatrics. 2010;126:e351–9. doi: 10.1542/peds.2009-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]


