Abstract
Rapid detection and reporting of third generation cephalosporine resistance (3GC-R) and of extended spectrum betalactamases in Enterobacteriaceae (ESBL-E) is a diagnostic and therapeutic priority to avoid inefficacy of the initial antibiotic regimen. In this study we evaluated a commercially available chromogenic screen for 3GC-R as a predictive and/or confirmatory test for ESBL and AmpC activity in clinical and veterinary Enterobacteriaceae isolates. The test was highly reliable in the prediction of cefotaxime and cefpodoxime resistance, but there was no correlation with ceftazidime and piperacillin/tazobactam minimal inhibitory concentrations. All human and porcine ESBL-E tested were detected with exception of one genetically positive but phenotypically negative isolate. By contrast, AmpC detection rates lay below 30%. Notably, exclusion of piperacillin/tazobactam resistant, 3GC susceptible K1+ Klebsiella isolates increased the sensitivity and specificity of the test for ESBL detection. Our data further imply that in regions with low prevalence of AmpC and K1 positive E. coli strains chromogenic testing for 3GC-R can substitute for more time consuming ESBL confirmative testing in E. coli isolates tested positive by Phoenix or VITEK2 ESBL screen. We, therefore, suggest a diagnostic algorithm that distinguishes 3GC-R screening from primary culture and species-dependent confirmatory ESBL testing by βLACTATM and discuss the implications of MIC distribution results on the choice of antibiotic regimen.
Introduction
Penicillins and cephalosporines are among the most common antibiotic substances used in human and veterinary medicine. Despite the availability of alternative classes of antibiotics up to today the betalactams are first choice substances due to their high efficacy. However, widespread use of betalactams has led to the emergence of betalactam resistance in both Gram positive and Gram negative bacteria. Enzmyes termed “βtermed “erm” cleave the βhe ave “ermed “es termed “myes termethereby prevent their interference with the transpeptidase activity of the tidase ase h hydrolysis and [1]. Over the last decades point mutations in the " \o "Witte W, 20have changed the active site and extended the substrate spectrum [2,3,4]. The arising extended-spectrum beta-lactamases (ESBL) are not only able to hydrolyze narrow-spectrum antibiotics such as penicillins and first and second generation cephalosporins but also inactivate broad-spectrum antibiotics such as aztreonam and third, fourth and fifth generation cephalosporins [5,6]. The spread of these enzymes is facilitated by their encoding on plasmids and represents the major cause for the increased resistance to broad-spectrum esistance ncoding on in enterobacteriaceae [6]. Therapeutic failure of first line antibiotics due to production of ESBL is associated with prolonged hospitalization, increased patient mortality and increased medical costs [7,8,9,10,11].
The methods routinely used for detection of ESBL in clinical isolates are mostly based on phenotypical diagnosis of ESBL involving bacterial culture in the presence or absence of antibiotics and betalactamase inhibitors. These include selective media, ESBL screening algorithms in automated susceptibility testing such as VITEK2 or Phoenix and confirmatory testing with discs or E-test stripes containing betalactam antibiotics with and without supplementation of betalactamase inhibitors [12]. Although genetic proof of ESBL is most convincing, due to the high cost and only few CE-certified commercial assays available, most microbiological laboratories do not routinely use molecular tests for ESBL detection. Nevertheless, false positive and false negative results in automated ESBL screening algorithms require additional confirmatory testing, which is time consuming.
Lately, both EUCAST and CLSI guidelines have lowered their breakpoints for cephalosporine susceptibility testing and switched to the paradigm that results obtained should be reported as measured in the microbiology laboratory [13,14,15,16]. The formerly employed interpretative approach corrected all cephalosporines tested as susceptible to resistant if the isolate was tested positive for ESBL. With the newly defined lower and more sensitive break points this is no longer viewed as necessary [14,17].
It is, however, an ongoing matter of debate whether the presence of ESBL could lead to in vivo inefficacy of betalactam antibiotics, in particular third generation cephalosporines (3GC), despite in vitro susceptibility of the infecting strains [18,19,20,21]. Nevertheless, confidence in the in vitro susceptibility results could open new therapeutic options and reduce the use of broad-spectrum reserve antibiotics such as carbapenems [22,23,24]. In views of the rapid emergence of carbapenemases any therapeutic alternative is an option to be taken under serious consideration.
Obviously, therapeutic decisions based on in vitro testing must apply the necessary caution until clinical studies provide sufficient evidence for the efficacy of 3GC in infections with ESBL-E. It is, therefore, an important duty for the microbiologist to provide as much information as possible, i.e. perform ESBL testing of suspected isolates. Since the routine methods currently employed require an additional 18–24 hours for definite diagnosis of ESBL activity, the development of faster methods for reliable prediction of ESBL activity is a diagnostic challenge and a therapeutic priority.
In this study we evaluated a rapid commercially available test for its prediction of ESBL expression in enterobacteriaceae cultured from patient materials and pigs. The principle of the ßrom pTM test is based on the cleavage of the substrate HMRZ-86*, a chromogenic cephalosporine [25,26]. This substrate, initially yellow, turns red in the presence of mogenic cephalosporine in n in sion in d-spectrum-beta-lactamase, AmpC, and Carbapenemase issues<linases (e.g. SHV-1, TEM-1) but processed by ESBL, acquired AmpC and carbapenemases (KPC and metallobetalactamases) [26,27]. Thus, the βLACTATM test offers a rapid method for the identification of strains with resistance to third-generation cephalosporins (3GC-R), e.g. if loss in susceptibility is due to the production of β-lactamases. In the present study we evaluated the utility of this assay as an ESBL confirmatory test in the routine clinical microbiology laboratory.
Material and Methods
Bacterial isolates
A total of 245 strains of Enterobacteriaceae were retrospectively analyzed. 173 members of the 200 bacterial isolates used for evaluation were collected from patient samples in the routine microbiology laboratory of the University Hospital Bonn from July 2012 to June 2014. The remaining 72 isolates were derived from fecal swabs obtained from pigs subjected to a hygiene monitoring program between June and September 2012 [28]. Species identification was performed with VITEK-MS (bioMh VITx S.A., Nuertingen, Germany). Enterobacteriaceae isolates were pre-selected based on VITEK2 susceptibility results compatible with an ESBL phenotype, growth on ESBL screening agar (ChromIDTM, bioMmIDing S.A.) selective agar or unbiased collection from single pathogen urinary tract infections with E. coli or Klebsiella spp. over a time period of two weeks. Replicates from the same patient were excluded from the analysis.
Susceptibility testing and detection of ESBL-E
VITEK2 (bioMK2 (bi S.A., NS.A., Nu, Germany) AST-N214 (REF 413064) and Phoenix 100 (Becton Dickinson, Heidelberg, Germany) UNMIC/ID-87 panel (REF 448771) were used for automated antibiotic susceptibility testing using EUCAST criteria.
Several methods were used in parallel to detect ESBL positive bacterial isolates: culture on ChromIDTM, bioMoMD on S.A., Nuertingen,Germany) selective agar, VITEK2 (bioMioMivx S.A., NS.A., NN, Germany) and Phoenix 100 (Becton Dickinson, Heidelberg, Germany) ESBL for screens. Third generation cephalosporine resistance (3GC-R) was further screened by βLACTATM test following the manufactureridelberg, Ge(Bio-Rad, Marnes-la-Coquette, France).
For molecular typing bacterial DNA was isolated using UltraClean®lMicrobial DNA Isolation Kit (MO BIO Laboratories, Carlsbad, California, USA). The PCR was carried out using the PN-Mix (GenID®eGmbH, Strassberg, Germany) and Taq DNA Polymerase (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) on a Labcycler (SensoQuest GmbH, GGmbH, Gs, Germany). Reverse hybridization was performed using the respective biotinylated amplicons using the protocol from GenID®eGmbH, Strarberg, Germany with sequence-specific oligonucleotides for betalactamases and controls immobilized on nitrocellulose membranes.
In those isolates tested negative in the molecular ESBL screen ESBL activity was confirmed using the disc diffusion method using AmpC&ESβmpC&EScreen ESBL activCefpodoxim ESpodoxim ESESBL activity was conDiagnostica GmbH) and E-Test ESBL from bioMH) and S.A.
Statistics
Statistical analysis was performed using iWork, Numbers software version 3.5.3 (Apple Inc., Cupertino, CA, USA). Sensitivity and specificity were calculated as true positive / total positive and true negative / total negative.
Results
Positive βLACTATM results correlate with cefotaxime and cefpodoxime resistance
In the present study we evaluated the βLACTATM test for detection and confirmation of ESBL. To this end we used a collection of 245 Enterobactericeae (170 E. coli, 58 Klebsiella spp, 17 other species) with either negative or suspected ESBL, AmpC or K1 activity based on VITEK2 analysis (Table 1). The isolates were derived from hospitalized patients or from pigs screened for ESBL-E on farms in the nearby region. Among these isolates 72.2% (177/245) were tested as positive for 3GC-R by βLACTATM, 26.5% (65/245) were negative and 1.2% (3/245) of test results were non-interpretable (Table 2). Testing of samples was fast (2 to 5 minutes) and easy-to-handle and could, therefore, represent an interesting alternative to conventional ESBL testing.
Table 1. Species-specific distribution of ESBL, AmpC, K1 and KPC in this study.
| ESBL | AmpC | K1* | |||||
|---|---|---|---|---|---|---|---|
| Species | # | pos | neg | pos | neg | pos | neg |
| Escherichia coli | 170 | 122 | 48 | 4 | 166 | 5 | 165 |
| Klebsiella pneumoniae | 40 | 31** | 9 | 1 | 39 | 4 | 36 |
| Klebsiella oxytoca | 18 | 2 | 16 | 0 | 18 | 15 | 3 |
| Enterobacter cloacae | 4 | 0 | 4 | 2 | 2 | 0 | 4 |
| Enterobacter aerogenes | 2 | 0 | 2 | 1 | 1 | 0 | 2 |
| Serratia marcescens | 3 | 0 | 3 | 2 | 1 | 0 | 3 |
| Proteus mirabilis | 3 | 0 | 3 | 2 | 1 | 0 | 3 |
| Morganella morganii | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Citrobacter freundii | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Pantoea agglomerans | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Kluyvera cryocrescens | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Citrobacter amalonaticus | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Total | 245 | 158 | 87 | 13 | 232 | 24 | 221 |
* Piperacillin/Tazobactam-resistant (K1- suspicious) isolates
** one strain also contained KPC
Table 2. βLACTATM, VITEK2 and PHOENIX100 results in the strain collective.
| VITEK2 | PHOENIX100 | βLACTA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | # | pos | neg | n.i. | pos | neg | n.i. | pos | neg | n.i. |
| Escherichia coli | 170 | 125 | 42 | 3 | 125 | 45 | 0 | 123 | 47 | 0 |
| Klebsiella pneumoniae | 40 | 30 | 5 | 5 | 25 | 15 | 0 | 33 | 5 | 2 |
| Klebsiella oxytoca | 18 | 16 | 1 | 1 | 13 | 4 | 1 | 15 | 3 | 0 |
| Enterobacter cloacae | 4 | 0 | 1 | 3 | 1 | 3 | 0 | 2 | 2 | 0 |
| Enterobacter aerogenes | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 1 |
| Serratia marcescens | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 3 | 0 |
| Proteus mirabilis | 3 | 0 | 0 | 3 | 0 | 3 | 0 | 1 | 2 | 0 |
| Morganella morganii | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Citrobacter freundii | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Pantoea agglomerans | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Kluyvera cryocrescens | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Citrobacter amalonaticus | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Total | 245 | 172 | 49 | 24 | 165 | 79 | 1 | 177 | 65 | 3 |
pos: positive, neg: negative, n.i.: non-interpretable
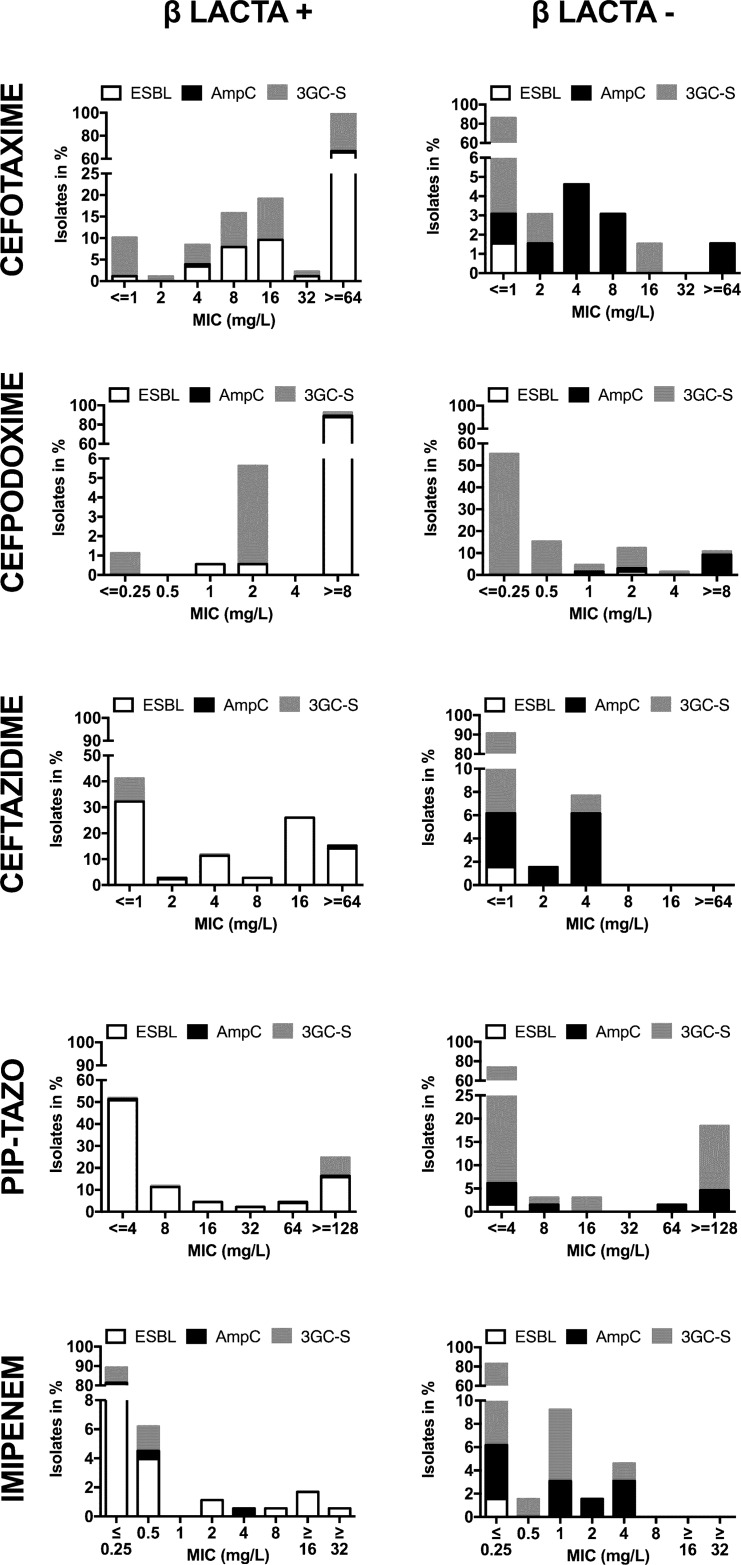
The βLACTATM test is based on chromogenic detection of 3GC-R. To evaluate the method we correlated the minimal inhibitory concentrations (MIC) obtained by VITEK2 analysis with positive and negative βLACTATM results. The results showed that the fraction of isolates tested positive by βLACTATM had high MIC values for cefotaxime and cefpodoxime. 66.7% had a MIC for cefotaxime ≥64 mg/L and 89.8% were categorized as resistant to cefotaxime according to EUCAST breakpoints, e.g. cefotaxime MIC >2 (Fig 1). 92.7% of βLACTA positive isolates had a MIC of ≥8 mg/L for cefpodoxime and 98.3% were classified as resistant according to EUCAST breakpoints (MIC >1) (Fig 1). On the contrary, the negative fraction displayed low MIC values for cefotaxime (86.2% susceptible according to EUCAST breakpoints, e.g. ≤1 mg/L) and a variable MIC distribution for cefpodoxime with 75.4% susceptible and 24.6% resistant isolates (Fig 1).
Fig 1. βLACTATM results in regards to distribution of MICs and ESBL or AmpC.
The graphs depict the MIC of cefotaxime (A), cefpodoxime (B), ceftazidime (C), piperacillin/tazobactam (D) and imipenem (E) in the positive (left) and negative (right) βLACTATM fractions. Information on ESBL activity (white), AmpC expression (black) and 3GC susceptibility (3GC-S, grey) is provided.
Further analyses revealed that there was no obvious correlation with MIC for ceftazidime, e.g. isolates with MIC ≤ 1 mg/L were found in both fractions. MIC >4 mg/L, however, were confined to the positive fraction (Fig 1). Similarly, we observed no correlation with piperacillin/tazobactam MIC values in any of the fractions, e.g. MIC ≤ 8 and ≥6 were found in both fractions (Fig 1). Lastly, βLACTATM fractions did not differ in the MIC for imipenem (Fig 1). Altogether, the βLACTATM results showed the clearest discrimination between cefotaxime susceptible and resistant isolates. Automated susceptibility testing with PhoenixTM provided comparable results (S1 Fig).
Reliable detection of ESBL with βLACTATM
Next, we assessed whether the βLACTATM assay could be used to detect ESBL as suggested by the manufacturer. To this end we analyzed the correlation of βLACTATM test results with ESBL genotyping by PCR. Among the isolates tested positive with the βLACTATM assay 156 (88.1%) were ESBL positive (TEM (1), SHV (3), CTX-M (147); 5 isolates were double positive: CTX-M+SHV+ (3), CTX-M+TEM+ (1) and KPC+SHV+ (1)) and 21 (11.9%) were negative by molecular testing. In these negative isolates, phenotypical ESBL and AmpC activity were analyzed by E-test and disc diffusion. One isolate displayed ESBL-activity, 3 were AmpC positive and 17 were negative in regards to both.
The 65 isolates of the βLACTATM negative fraction as well as the 3 non-interpretable results were all ESBL negative by PCR except for one CTX-M positive isolate. This isolate lacked ESBL activity in E-test and phenotypic disc diffusion tests. The PCR negative fraction contained 10 AmpC+ isolates and 58 isolates tested negative for AmpC and ESBL by disc diffusion test and E-test.
Of note, when using the manufacturer test and and ion 171 isolates positive for either ESBL, AmpC or KPC were βLACTATM positive (93.6%), 9 (5.3%) were negative and 2 (1.1%) non-interpretable. This resulted in an overall sensitivity of 94.7% for undifferentiated detection of ESBL, AmpC and/or KPC. Among the 74 isolates genotypically and phenotypically negative for ESBL, AmpC and KPC 56 (75.7%) were tested βLACTATM negative, 17 (23%) positive and 1 (1.3%) non-interpretable, providing a specificity of 76.7%. When limiting our analysis to ESBL prediction we found that the sensitivity was 99.4% and specificity 76.2%, e.g. from 158 ESBL positive isolates 157 were βLACTATM positive (99.4%) and one (0.6%) was negative, and, from 84 ESBL negative isolates 64 (76.2%) were βLACTATM negative, 20 (23.8%) βLACTATM positive and 3 (3.6%) results were non-interpretable.
βLACTA results in AmpC-expressing strains
We, next, wanted to assess whether the βLACTATM test can identify AmpC producers as indicated by the manufacturer. However, in our strain collection the βLACTA testing only detected 3 out of 13 AmpC-expressing strains (Table 1). Thus, the sensitivity and specificity for AmpC detection were low, e.g. 27.3% and 24.7%, respectively.
Sensitivity of the βLACTATM test is superior to that of selective ESBL agar plates
Screening of patients for ESBL-E carriage is routinely performed with selective media containing cephalosporines. Comparison of βLACTATM results to growth on ChromIDTM ESBL agar showed comparable sensitivities for detection of ESBL-E, e.g. 99.4% and 100%, respectively. The specificity was higher for βLACTATM, e.g. 76.2% versus 10.3% for ChromIDTM ESBL agar.
The specificity of the βLACTATM test is superior to that of VITEK2 and Phoenix ESBL screens
In infection, ESBL activity is usually suspected based on an ESBL screening algorithm employed during automated susceptibility testing of the clinical isolate. We, thus, compared βLACTATM testing to the ESBL screens provided on VITEK2 and Phoenix systems. Among the βLACTATM positive fraction 91.5% (162/177) of isolates were ESBL positive in the VITEK2 screen (Table 2), 1.7% (3/177) were negative and 6.8% (12/177) not interpretable by VITEK2. In the βLACTATM negative fraction 11 isolates were positive, 10 not interpretable and only 44 were negative by VITEK2 analysis. Sensitivity (98.7%) and specificity (64.8%) of the VITEK2 ESBL screen were, thus, lower than those achieved by βLACTATM. Despite lower sensitivity (90.5%) the Phoenix ESBL test was superior to the VITEK2 ESBL screen with 74.4% specificity for ESBL-E (Table 2). However, it remained below the specificity level of βLACTATM.
Species-specific differences in ESBL detection with βLACTATM
A more detailed analysis revealed that the ESBL-negative isolates tested false positive by βLACTATM mainly belonged to the Klebsiella spp., while the majority of isolates in the βLACTATM positive fraction were E. coli. The βLACTATM negative fraction displayed a higher diversity in regards to species variation (Table 2).
Since false positive results were mainly obtained with Klebsiella spp. isolates we compared the βLACTATM results of E. coli and Klebsiella spp. isolates. Differential analysis of the Klebsiella spp. isolates revealed that among the 48 βLACTATM positive isolates only 33 were ESBL positive and 15 were ESBL negative (Table 3). 8 isolates were concordantly negative for βLACTATM and ESBL (Table 3). For detection of ESBL, this provided a sensitivity of 100% but a specificity of only 34.8% when testing Klebsiella spp. (Table 3). Our analysis of the E. coli isolates showed that among the strains tested 122/170 were ESBL positive and 48/170 were ESBL negative (Table 1). In this subcategory of isolates all isolates were classified correctly by βLACTATM with one exception, an AmpC+ strain, which was tested positive. This resulted in 100% sensitivity and 97.9% specificity for ESBL detection in the E. coli strains tested (Table 3).
Table 3. Sensitivity and specificity for βLACTATM for 3-GCR, ESBL and AmpC in Enterobacteriaceae and for ESBL detection in E. coli and Klebsiella spp.
| # n.i. | # false / total positive | # false / total negative | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Entero-bacteriaceae | |||||
| 3GC-R | 3 (2 3GC-R) | 17/177 | 9/65 | 94.7 | 76.7 |
| ESBL+ | 3 (3 ESBL-) | 20/177 | 1/65 | 99.4 | 76.2 |
| AmpC | 3 (2 AmpC+) | 174/177 | 8/65 | 27.3 | 24.7 |
| E. coli | |||||
| ESBL+ | 0 | 1/123 | 0/47 | 100 | 97.9 |
| Klebsiella spp. | |||||
| ESBL+ | 2 (2 ESBL-) | 15/48 | 0/8 | 100 | 34.8 |
n.i. = non-interpretable
Comparison with the species-specific reliability of VITEK2 and Phoenix in regards to ESBL prediction in E. coli our results showed that VITEK2 and Phoenix reached sensitivities of 100% and 97.5%, respectively, and a specificity of 87.5%, which lay markedly below that obtained by βLACTATM (Table 4). For Klebsiella species sensitivities where lower than those achieved with the βLACTA test, e.g. 93.3 and 69.7%, respectively (Table 4). The specificity of ESBL detection in VITEK2 was lower than that obtained by the βLACTATM test, e.g. 18.2% (Table 4). However, with Klebsiella spp. the Phoenix analysis was slightly better than the βLACTATM test with 37.5% compared to 34.8% specificity (Table 4).
Table 4. Sensitivity and specificity obtained by VITEK2 and Phoenix100 ESBL algorythms in Enterobacteriaceae, E. coli and Klebsiella spp.
| # n.i. | # false / total positive | # false / total negative | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| VITEK2 | |||||
| Enterobacteriaceae | 24 (8) | 25/173 | 2/48 | 99 | 64.8 |
| E. coli | 3 (3) | 6/125 | 0/42 | 100 | 87.5 |
| Klebsiella spp. | 6 (3) | 18/46 | 2/6 | 93.3 | 18.2 |
| Phoenix 100 | |||||
| Enterobacteriaceae | 1 (0) | 22/165 | 15/79 | 90.5 | 74.4 |
| E. coli | 0 | 6/125 | 3/45 | 97.5 | 87.5 |
| Klebsiella spp. | 1 (0) | 15/38 | 10/19 | 69.7 | 37.5 |
n.i. = non-interpretable (# of n.i. ESBL+ isolates)
Klebsiella K1 isolates reduce βLACTA specificity
More profound analysis revealed that from the 17 false positive βLACTATM results in the total enterobacteriaceae collective 14 could be attributed to Klebsiella spp. isolates resistant to both piperacillin/tazobactam (MIC ≥128 mg/L) and cefpodoxime (two with non-interpretable AmpC test) and to one piperacillin/tazobactam resistant but 3GC susceptible Klebsiella spp. isolate. The residual two false positive results were found in a piperacillin/tazobactam susceptible (MIC = 8), cefpodoxime and cefotaxime resistant Enterobacter cloacae isolate and a 3GC susceptible Citrobacter amalonaticus isolate.
Upon exclusion of phenotypical K1+ Klebsiella spp. isolates we achieved a sensitivity and specificity of 100% for ESBL-E detection. However, the absolute number of piperacillin/tazobactam susceptible Klebsiella spp. isolates was low (3 ESBL-E, 3 non-ESBL-E). Moreover, 4 piperacillin/tazobactam resistant Klebsiella spp. and 5 piperacillin/tazobactam resistant ESBL/AmpC-negative E. coli isolates were tested negative by βLACTATM, indicating that, in turn, βLACTATM test does not necessarily react with phenotypical K1+ isolates.
Taken together, the results obtained demonstrated that upon exclusion of suspected K1+ Klebsiella spp. isolates the βLACTATM test displays high reliability for prediction of true ESBL-E.
Discussion
Presently, EUCAST and CLSI guidelines recommend reporting of betalactam susceptibility testing results as measured omitting secondary ESBL testing. However, in vitro susceptibility to cephalosporines might not always predict their in vivo efficacy in infections with Enterobacteriaceae expressing extended spectrum betalactamases (ESBL-E) and bears the risk of recurrence or prolonged and more severe disease [29]. This therapeutic risk has raised an intense debate among microbiologists and infectious disease specialists [18,21] and defined a new need for the detection of ESBL in the routine diagnostic laboratory. The study presented here was performed on this background.
The data obtained show that the chromogenic 3GC-R test applied in this study might represent an additional option for screening for 3GC-R in primary cultures, in particular E. coli isolates. For Klebsiella spp. prediction of 3GC-R was less reliable because of false positive testing of K1+ Klebsiella strains (Table 3). Furthermore, it was demonstrated that the βLACTA test can be used for prediction of ESBL activity in Enterobacteriaceae. While the sensitivity and specificity for ESBL was excellent in E. coli, regardless of their source, there were insecurities in regards to ESBL prediction in Klebsiella species (Table 3). This corroborates and expands the findings of Morosini et al. who reported 97.5% true positive results with the βLACTATM test in their strain collection [30].
Notably, the sensitivities achieved by the VITEK2 and Phoenix100 ESBL algorithms were comparable to the βLACTATM test in E. coli isolates (Tables 3 and 4). However, the specificities obtained by these instruments were clearly lower when compared to the βLACTATM. In Klebsiella isolates the sensitivity of the βLACTATM test for ESBL-E was comparable to that in E. coli (100%) and sensitivity of VITEK2 was only slightly lower (93.3%), at the cost of very low specificity; Phoenix analyses provided a slightly higher specificity but a number of false negative results. Altogether, the accuracy of the βLACTATM test proved to be higher than that provided by the two automated susceptibility testing systems evaluated. It could, therefore, serve as a complementary tool in the detection and confirmation of ESBL-E.
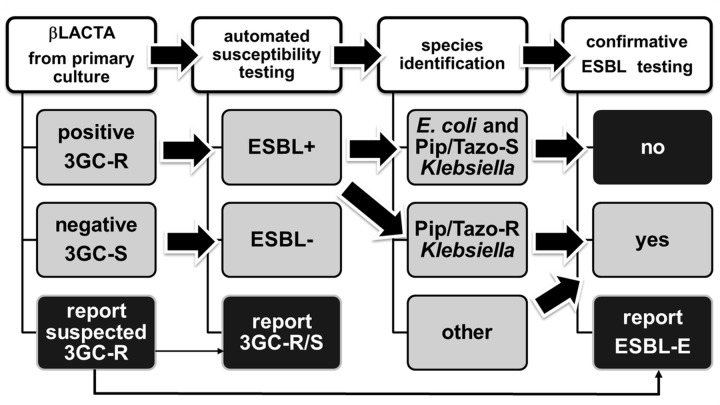
Based on our findings we propose that with E. coli isolates positivity of βLACTATM together with 3CG-R in the antimicrobial susceptibility testing omits the requirement for additional specific ESBL testing. We favor implementation of a step-wise algorithm for its use in the daily routine. Fig 2 depicts a model algorithm for the use of βLACTATM on primary Enterobacteriaceae isolates. This algorithm can easily be applied on blood culture and urine isolates. In our hands, it enables the laboratory to report suspected 3GC-R with a sensitivity of 89.7% at an early time point, thus, providing therapeutically relevant information for an early adjustment of antibiotic therapy.
Fig 2. Algorythm for diagnostic use of a chromogenic 3GC-R test in reporting of ESBL-E.
The diagram depicts the integration of the chromogenic 3GC-R test in early detection of 3GC-R in primary cultures (left): suspected 3GC-R (βLACTATM or comparative test positive (A)) is confirmed by automated susceptibility testing (AST) (B) results. It further expands the use of the chromogenic 3GC-R test to ESBL confirmatory testing: if AST (B) delivers positive result for 3GC-R and ESBL screen and isolates are E. coli or piperacillin/tazobactam susceptible (Pip/Taz-S) Klebsiella spp. (C) the βLACTATM test can be used for confirmation of ESBL (D). Reliability of the βLACTATM test is decreased in piperacillin/tazobactam resistant (Pip/Taz-R) Klebsiella spp. and, thus, not recommended. Other Enterobacteriaceae species need to be evaluated.
Recently, Gallah et al. reported 94% sensitivity and a specificity of 100% for detection of ESBL-E by βLACTATM in enterobacteriaceae isolates from urine specimen [31]. However, the present study demonstrates that phenotypical K1+ Klebsiella isolates diminished the accuracy of ESBL detection and ESBL confirmatory testing can, therefore, not be applied to these isolates. Thus, in Klebsiella species, ESBL activity can only be predicted in piperacillin/tazobactam susceptible isolates and additional analyses are necessary to confirm ESBL (and AmpC) activity in piperacillin/tazobactam resistant isolates. Moreover, method validation and routine surveillance in the local patient population should confirm the lack of interference of K1+ E. coli isolates with the βLACTATM test result before test implementation.
The chromogenic substrate HMRZ-86 is a cephalosporin originally described to be specifically cleaved by class A ESBL and class D oxacillinases, but not by penicillinases [25,26,27]. It was further suggested that addition of chelating agents can prevent activity of class B metallobetalactamases or class C cephalosporinases, including AmpC on HMRZ-86 [25,26,27]. Our results confirm that HMRZ-86 can serve as a chromogenic substrate for detection of ESBL. On the contrary, we could not confirm detection of class D oxacillinases by βLACTATM due to lack of OXA-48 positive ESBL negative Klebsiella and E. coli strains. Indeed, OXA-48-expressing strains were previously found to co-produce ESBL in 75% [32], impeding us to distinguish OXA-48 from ESBL activity in βLACTATM testing. Similarly, we could not distinguish KPC from ESBL activity in a K. pneumoniae strain bearing both KPC and SHV-ESBL. Lastly, the commercially available βLACTA test did not deliver reliable results for AmpC activity (Table 1), albeit this is suggested by the manufacturer.
Based on its chemical structure, which resembles cefotaxime, it was not surprising that the results obtained with HMRZ-86 correlated best with cefotaxime MICs, a cephalosporine previously reported to serve as a reliable predictor of ESBL- and AmpC-conferred resistance in E. coli and Klebsiella pneumoniae [33]. In this study, βLACTATM positivity correlated with ESBL detection by PCR and phenotypic ESBL detection. However, βLACTA positive testing and detection of ESBL did neither correlate with high MICs for ceftazidime nor with piperacillin/tazobactam resistance (Fig 1), whose clinical efficacy is currently being reevaluated for therapy of ESBL-E infections [11,16,21,22,24,34,35]. Notably, in VITEK2 analysis only 48.1% of ESBL-E displayed in vitro resistance to ceftazidime according to EUCAST criteria, e.g. MIC >4, while 39.2% of ESBL-E and 87.4% non-ESBL-E displayed MICs <4 (Fig 1) albeit an earlier study that the βLACTATM test was useful in discriminating ceftazidime-susceptible from tor of ESBLPseudomonas aeruginosa isolates [36]. Furthermore, only 24.7% of ESBL-E (and 10.7% of E. coli ESBL-E) were resistant to piperacillin/tazobactam in vitro, e.g. displayed MICs >16 (EUCAST criteria) (Fig 1), while 70.3% of ESBL-E (86.9% of E. coli ESBL-E) were susceptible to piperacillin/tazobactam in vitro. Non-ESBL isolates were found in both the piperacillin/tazobactam susceptible and resistant (i.e. K1+ Klebsiella spp.) fractions (Fig 1). Thus, due to lack of correlation the test provides no additional information in regards to the prediction of the in vivo efficacy of ceftazidime and/or piperacillin/tazobactam in the patient.
Altogether, our data determine that the βLACTATM test is a fast and reliable method to predict 3GC-R in Enterobacteriaceae and ESBL in E. coli isolated from humans and pigs. When used in combination with automated susceptibility testing it has a complementary function and can be used to predict ESBL in E. coli and preselected Klebsiella strains.
Supporting Information
The graph shows positive (left) and negative (right) βLACTATM results and the respective distribution of MIC (number of isolates for each MIC value) of cefotaxime (upper panel), ceftazidime (middle), piperacillin/tazobactam (lower panel) susceptibility testing obtained by PhoenixTM analysis.
(PDF)
Acknowledgments
The study is part of the doctoral thesis of M. R. E.-J.; AH is a member of the German Centre for Infection Research (DZIF). We would like to thank the technicians of the medical microbiology unit of the Institute for Medical Microbiology, Immunology and Parasitology (IMMIP) of the University Hospital Bonn for assistance with the collection of strains and VITEK-MS and VITEK2 analysis.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Witte W MM (2003) Betalactamases with broad activity spectrum. Principles, epidemiology and prevention of spread Bundesgesundheitsbl—Gesundheitsforsch—Gesundheitsschutz 46: 881–890. [Google Scholar]
- 2.Paterson DL, Bonomo RA (2005) Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18: 657–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeifer Y, Cullik A, Witte W (2010) Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol 300: 371–379. 10.1016/j.ijmm.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 4.Harris PN (2015) Clinical management of infections caused by Enterobacteriaceae that express extended-spectrum beta-lactamase and AmpC enzymes. Semin Respir Crit Care Med 36: 56–73. 10.1055/s-0034-1398387 [DOI] [PubMed] [Google Scholar]
- 5.Vidaillac C, Leonard SN, Sader HS, Jones RN, Rybak MJ (2009) In vitro activity of ceftaroline alone and in combination against clinical isolates of resistant gram-negative pathogens, including beta-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 53: 2360–2366. 10.1128/AAC.01452-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Bano J, Navarro MD, Romero L, Muniain MA, Perea EJ, et al. (2006) Clinical and molecular epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli as a cause of nosocomial infection or colonization: implications for control. Clin Infect Dis 42: 37–45. [DOI] [PubMed] [Google Scholar]
- 7.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO (2001) Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32: 1162–1171. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Bano J, Pascual A (2008) Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther 6: 671–683. 10.1586/14787210.6.5.671 [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Bano J, Navarro MD, Romero L, Muniain MA, de Cueto M, et al. (2006) Bacteremia due to extended-spectrum beta -lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Dis 43: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 10.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, et al. (2007) Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 51: 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, et al. (2015) Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis 60: 1319–1325. 10.1093/cid/civ003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson KS (2010) Extended-spectrum-beta-lactamase, AmpC, and Carbapenemase issues. J Clin Microbiol 48: 1019–1025. 10.1128/JCM.00219-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclercq R, Canton R, Brown DF, Giske CG, Heisig P, et al. (2013) EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect 19: 141–160. 10.1111/j.1469-0691.2011.03703.x [DOI] [PubMed] [Google Scholar]
- 14.Kahlmeter G (2008) Breakpoints for intravenously used cephalosporins in Enterobacteriaceae—EUCAST and CLSI breakpoints. Clin Microbiol Infect 14 Suppl 1: 169–174. [DOI] [PubMed] [Google Scholar]
- 15.Curello J, MacDougall C (2014) Beyond Susceptible and Resistant, Part II: Treatment of Infections Due to Gram-Negative Organisms Producing Extended-Spectrum beta-Lactamases. J Pediatr Pharmacol Ther 19: 156–164. 10.5863/1551-6776-19.3.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson LR (2008) Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect 14 Suppl 1: 181–184. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Bano J, Picon E, Navarro MD, Lopez-Cerero L, Pascual A (2012) Impact of changes in CLSI and EUCAST breakpoints for susceptibility in bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli. Clin Microbiol Infect 18: 894–900. 10.1111/j.1469-0691.2011.03673.x [DOI] [PubMed] [Google Scholar]
- 18.Livermore DM, Andrews JM, Hawkey PM, Ho PL, Keness Y, et al. (2012) Are susceptibility tests enough, or should laboratories still seek ESBLs and carbapenemases directly? J Antimicrob Chemother 67: 1569–1577. 10.1093/jac/dks088 [DOI] [PubMed] [Google Scholar]
- 19.Ku NS, Chung HS, Choi JY, Yong D, Lee K, et al. (2015) Clinical usefulness of the 2010 clinical and laboratory standards institute revised breakpoints for cephalosporin use in the treatment of bacteremia caused by Escherichia coli or Klebsiella spp. Biomed Res Int 2015: 831074 10.1155/2015/831074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, et al. (2013) Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis 56: 488–495. 10.1093/cid/cis916 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen HM, Shier KL, Graber CJ (2014) Determining a clinical framework for use of cefepime and beta-lactam/beta-lactamase inhibitors in the treatment of infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 69: 871–880. 10.1093/jac/dkt450 [DOI] [PubMed] [Google Scholar]
- 22.Harris PN, Tambyah PA, Paterson DL (2015) beta-lactam and beta-lactamase inhibitor combinations in the treatment of extended-spectrum beta-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis 15: 475–485. 10.1016/S1473-3099(14)70950-8 [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Bano J (2015) The times they are a-changin': carbapenems for extended-spectrum-beta-lactamase-producing bacteria. Antimicrob Agents Chemother 59: 5095–5096. 10.1128/AAC.01333-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levasseur P, Girard AM, Miossec C, Pace J, Coleman K (2015) In vitro antibacterial activity of the ceftazidime-avibactam combination against enterobacteriaceae, including strains with well-characterized beta-lactamases. Antimicrob Agents Chemother 59: 1931–1934. 10.1128/AAC.04218-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanaki H, Kubo R, Nakano T, Kurihara M, Sunagawa K (2004) Characterization of HMRZ-86: a novel chromogenic cephalosporin for the detection of extended-spectrum beta-lactamases. J Antimicrob Chemother. England. pp. 888–889. [DOI] [PubMed] [Google Scholar]
- 26.Livermore DM, Warner M, Mushtaq S (2007) Evaluation of the chromogenic Cica-beta-Test for detecting extended-spectrum, AmpC and metallo-beta-lactamases. J Antimicrob Chemother 60: 1375–1379. [DOI] [PubMed] [Google Scholar]
- 27.Hanaki H, Koide Y, Yamazaki H, Kubo R, Nakano T, et al. (2007) Substrate specificity of HMRZ-86 for beta-lactamases, including extended-spectrum beta-lactamases (ESBLs). J Infect Chemother 13: 390–395. [DOI] [PubMed] [Google Scholar]
- 28.Schmithausen RM, Schulze-Geisthoevel SV, Stemmer F, El-Jade M, Reif M, et al. (2015) Analysis of Transmission of MRSA and ESBL-E among Pigs and Farm Personnel. PLoS One 10: e0138173 10.1371/journal.pone.0138173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CH, Su LH, Chen FJ, Tang YF, Chien CC, et al. (2015) Clinical and microbiologic characteristics of adult patients with recurrent bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae. Clin Microbiol Infect 21: 10. [DOI] [PubMed] [Google Scholar]
- 30.Morosini MI, Garcia-Castillo M, Tato M, Gijon D, Valverde A, et al. (2014) Rapid detection of beta-lactamase-hydrolyzing extended-spectrum cephalosporins in Enterobacteriaceae by use of the new chromogenic betaLacta test. J Clin Microbiol 52: 1741–1744. 10.1128/JCM.03614-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallah S, Decre D, Genel N, Arlet G (2014) The beta-Lacta test for direct detection of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in urine. J Clin Microbiol 52: 3792–3794. 10.1128/JCM.01629-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potron A, Poirel L, Rondinaud E, Nordmann P (2013) Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro Surveill 18. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura Y, Yamamoto M, Matsushima A, Nagao M, Ito Y, et al. (2012) Cefotaxime for the detection of extended-spectrum beta-lactamase or plasmid-mediated AmpC beta-lactamase and clinical characteristics of cefotaxime-non-susceptible Escherichia coli and Klebsiella pneumoniae bacteraemia. Eur J Clin Microbiol Infect Dis 31: 1931–1939. 10.1007/s10096-011-1523-4 [DOI] [PubMed] [Google Scholar]
- 34.Frakking FN, Rottier WC, Dorigo-Zetsma JW, van Hattem JM, van Hees BC, et al. (2013) Appropriateness of empirical treatment and outcome in bacteremia caused by extended-spectrum-beta-lactamase-producing bacteria. Antimicrob Agents Chemother 57: 3092–3099. 10.1128/AAC.01523-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavin PJ, Suseno MT, Thomson RB Jr., Gaydos JM, Pierson CL, et al. (2006) Clinical correlation of the CLSI susceptibility breakpoint for piperacillin- tazobactam against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella species. Antimicrob Agents Chemother 50: 2244–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurent T, Huang TD, Bogaerts P, Glupczynski Y (2013) Evaluation of the betaLACTA test, a novel commercial chromogenic test for rapid detection of ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates. J Clin Microbiol 51: 1951–1954. 10.1128/JCM.00524-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The graph shows positive (left) and negative (right) βLACTATM results and the respective distribution of MIC (number of isolates for each MIC value) of cefotaxime (upper panel), ceftazidime (middle), piperacillin/tazobactam (lower panel) susceptibility testing obtained by PhoenixTM analysis.
(PDF)
Data Availability Statement
All relevant data are within the paper.