Abstract
Objectives
Patients without voluntary finger extension early post-stroke are suggested to have a poor prognosis for regaining upper limb capacity at 6 months. Despite this poor prognosis, a number of patients do regain upper limb capacity. We aimed to determine the time window for return of voluntary finger extension during motor recovery and identify clinical characteristics of patients who, despite an initially poor prognosis, show upper limb capacity at 6 months post-stroke.
Methods
Survival analysis was used to assess the time window for return of voluntary finger extension (Fugl-Meyer Assessment hand sub item finger extension≥1). A cut-off of ≥10 points on the Action Research Arm Test was used to define return of some upper limb capacity (i.e. ability to pick up a small object). Probabilities for regaining upper limb capacity at 6 months post-stroke were determined with multivariable logistic regression analysis using patient characteristics.
Results
45 of the 100 patients without voluntary finger extension at 8 ± 4 days post-stroke achieved an Action Research Arm Test score of ≥10 points at 6 months. The median time for regaining voluntary finger extension for these recoverers was 4 weeks (lower and upper percentile respectively 2 and 8 weeks). The median time to return of VFE was not reached for the whole group (N = 100). Patients who had moderate to good lower limb function (Motricity Index leg≥35 points), no visuospatial neglect (single-letter cancellation test asymmetry between the contralesional and ipsilesional sides of <2 omissions) and sufficient somatosensory function (Erasmus MC modified Nottingham Sensory Assessment≥33 points) had a 0.94 probability of regaining upper limb capacity at 6 months post-stroke.
Conclusions
We recommend weekly monitoring of voluntary finger extension within the first 4 weeks post-stroke and preferably up to 8 weeks. Patients with paresis mainly restricted to the upper limb, no visuospatial neglect and sufficient somatosensory function are likely to show at least some return of upper limb capacity at 6 months post-stroke.
Introduction
Voluntary finger extension (VFE) is an important early predictor of recovery of upper limb capacity at 6 months post-stroke[1;2]. Patients without VFE within the first days post-stroke have been suggested to have a poor prognosis for regaining some upper limb capacity at 6 months[1–3]. Absence of VFE reflects the loss of functional corticospinal tract integrity, acknowledging that the hand muscles are almost solely innervated by contralateral corticospinal pathways[4]. Indirect bilateral innervation of the hand muscles by the reticulospinal tract may also contribute to hand motor control after stroke[5]. However, it remains unclear if the reticulospinal system can influence the digital extensor muscles of the paretic hand[6].
Despite an initially poor prognosis, some patients without VFE within the first days after stroke do regain upper limb capacity at 6 months[2]. In view of the lack of evidence-based therapies for patients without VFE[7;8], this return of VFE seems most likely to be driven by spontaneous neurobiological processes such as alleviation of diaschisis[9]. Unfortunately, the clinical characteristics as well as the optimal time window for recovery of VFE are unknown, due to lack of prospective cohort studies in which patients are assessed serially at fixed times post-stroke[10;11]. More knowledge regarding this time window is important for future prognostic algorithm development. Up till now, the most optimal timing and added value of neurophysiological and neuroimaging measurements with respect to clinical measurements like VFE are unclear.
The aims of the present study were therefore (1) to determine the clinical time window for return of VFE in ischemic stroke patients without VFE in the first days post-stroke, and (2) to identify clinical characteristics for the return of some upper limb capacity in these patients within the first 6 months after stroke. We hypothesized that return of VFE would occur within the purported time window of spontaneous neurobiological recovery between 0 and 10 weeks after stroke onset[10;12]. We also hypothesized that patients with lesions affecting upper limb function who exhibit no other neurological impairments such as visuospatial neglect and somatosensory dysfunction would have a high probability of regaining some upper limb capacity at 6 months[13–15].
Materials and Methods
Recruitment
Data from the EXPLICIT-stroke program was used[16;17]. EXPLICIT-stroke was a Dutch translational research program including two multi-center single blinded randomized trials and an intensive repeated measurements design up to 6 months post-stroke. Between October 2008 and November 2013 a total number of 159 patients were included. For the present study only patients in the EMG-triggered neuromuscular stimulation (EMG-NMS) trial were selected (N = 101). Patients were recruited within the first 2 weeks post-stroke and allocated to control treatment (usual care) or experimental treatment focused on regaining VFE (EMG-NMS). Details of the EMG-NMS treatment protocol have been described elsewhere.[16;17] EXPLICIT-stroke was approved by the ethics committee of all participating centers (Leiden University Medical Center: No. P08.035; Dutch Central Committee on Research Involving Human Subjects [CCMO]: No. NL21396.058.08) and was registered at the Netherlands Trial Register (TC1424).
Subjects
Patients were included when they met the following criteria upon screening: (1) first-ever ischemic middle cerebral artery stroke verified by CT and/or MRI scan; (2) upper limb paresis according to National Institutes of Health Stroke Scale item 5 (score >0); (3) no VFE at baseline according to Fugl-Meyer Assessment hand sub question FE (score = 0); (4) mini mental state examination ≥23; (5) 18–80 years of age; (6) no upper limb musculoskeletal impairments; (7) no botulinum toxin treatment, as this may distort the measurement of upper limb capacity; (8) able to sit independently for 30 seconds, i.e. sufficient sitting balance to facilitate clinical measurements; (9) written informed consent.
Serial Assessments
Eight repeated assessments were performed using the Fugl-Meyer Assessment hand sub question FE to monitor return of VFE (0 = no movement, 1 = partial movement, 2 = full movement)[18]. Assessments were performed weekly in the first 5 weeks post-stroke and at 8, 12 and 26 weeks.
Dependent Variable
The Action Research Arm Test (ARAT) served as outcome measure at 6 months post-stroke. The ARAT is a capacity test in the activities domain of the International Classification of Function, Disability and Health framework[19] and includes 19 tasks divided into 4 subdomains: grasp, grip, pinch and gross movement (maximum = 57 points). The ARAT has a maximal score of 57 points and good clinimetric properties[20;21].
Independent Variables
The following independent baseline variables were identified based on previous literature[22]: (1) Sex; (2) Age; (3) Hemisphere of stroke; (4) Bamford classification[23]; (5) Time between stroke and baseline assessment; (6–7) Hemianopia and facial palsy (National Institutes of Health Stroke Scale)[24]; (8) Visuospatial neglect (Letter Cancellation Test)[25], the presence of visuospatial neglect being defined as an asymmetry between the contralesional and ipsilesional body sides of ≥2 omissions[14]; (9) Somatosensory function of the upper limb (touch, sharp-blunt discrimination and proprioception of the Erasmus MC modified Nottingham Sensory Assessment)[26]; and (10–12) Range of motion and strength of the elbow, shoulder, and lower limb (Motricity Index)[27]. In addition, we added the EXPLICIT-stroke randomization group as independent variable: experimental (EMG-NMS) versus control group.
Data Analysis
We used survival analysis on the repeated assessments to determine the time until patients regained VFE for the first time (i.e. partial or full movement according to the Fugl-Meyer Assessment hand sub item FE). For those patients with missing data we took the first assessment where VFE was measured as ‘time to return of VFE’. Possibly, these patients could have regained VFE earlier in time. Recovery was defined as an ARAT score of ≥10 points, representing at least some upper limb capacity, i.e. patients should at least be able to pick up a small object against gravity (dichotomization: 0 = ARAT<10 and 1 = ARAT≥10)[2;28]. A Kaplan-Meier [29] cumulative ‘event’ curve was constructed for the whole group and for the patients with 10 or more points on the ARAT at 6 months post-stroke.
For our second objective, statistical analyses were performed on subjects with complete baseline and 6-month assessments. Dichotomization of independent variables was preferably based on clinical reasoning or previous literature[2]. Otherwise, we used the Youden index to determine the cut-off point with the highest sensitivity and specificity[30]. Bivariable logistic regression analysis was used to preselect independent variables when the Wald statistic was p<0.05. Subsequently, collinearity diagnostics between preselected variables was applied using two-way contingency tables. If the Phi correlation coefficient between two variables was ≥0.8, the variable with the lowest Wald statistic was excluded from further analysis. Thereafter, we used a backward stepwise multivariable logistic regression analysis on the selected variables to form the prediction model (entry criteria = p<0.05; removal criteria = p≥0.10). In view of the large number of preselected variables relative to the number of patients included in the study, we applied a forward stepwise approach to test model stability. The Hosmer-Lemeshow test and the c-statistic (i.e. area under the receiver-operating characteristic curve) were used to quantify the goodness-of-fit of the logistic regression model, and two-way contingency tables to calculate sensitivity, specificity, positive predictive value, and negative predictive value, including the corresponding 95%CI. Statistical analyses were two-tailed with an alpha of 0.05 (SPSS version 20).
Results
One hundred one first-ever ischemic stroke patients were recruited for the EXPLICIT-stroke EMG-NMS trial. In the present study, 1 patient was excluded for further analyses due to presence of some VFE. Patient characteristics of the 100 patients eligible for further analysis are shown in Table 1. At 6 months post-stroke, 45 of the 100 patients achieved an ARAT score of ≥10 points. This group of patients had a baseline ARAT score ranging from 0 points to 7 points, with 30 patients scoring 0 points, 11 patients scoring between 1 and 5 points, and 4 patients scoring 6 or 7 points. At 6 months post-stroke, this group had a median ARAT score of 34 points (interquartile range [IQR] = 19.50–45.00; range = 10–57); 4 patients scored 10 points, and 5 of them attained the maximum score of 57 points. In comparison, the median 6-month ARAT score for the other group was 0 (IQR = 0–3; range = 0–4).
Table 1. Patient characteristics.
| Baseline | N = 100 |
|---|---|
| Sex: female/male, %male | 36/64, 64% |
| Age, years† | 58.67 (11.72) |
| Hemisphere of stroke: left/right, %right | 31/69, 69% |
| Bamford classification: LACI/PACI/TACI | 55/39/6 |
| Global disability (NIHSS, range:0–40) (N = 99)* | 9 (7–11) |
| Hemianopia (NIHSS): no/yes, %yes | 92/8, 8% |
| Facial palsy (NIHSS): no/yes, %yes | 15/85, 85% |
| Extinction and inattention (NIHSS): no/yes, %yes | 68/32, 32% |
| Sensation (NIHSS): no/yes, %yes | 52/48, 48% |
| Visuospatial neglect (LCT): no/yes, %yes (N = 94) | 36/58, 58% |
| Somatosensory function (EmNSA): good/poor, %poor (N = 99) | 57/42, 42% |
| Upper limb function (MI, range:0–100)* | 0 (0–23) |
| Upper limb function (FMA, range:0–66)* | 5 (4–8) |
| Lower limb function (MI, range:0–100) (N = 99)* | 32 (9–47) |
| Upper limb capacity (ARAT, range:0–57)* | 0 (0–0) |
| Time between stroke and baseline assessment, days† | 8.26 (4.10) |
| 6 months post-stroke | |
| Upper limb capacity (ARAT, range:0–57, N = 97)* | 4 (0–30.5) |
| Time between stroke and 6-month assessment, days† | 189.90 (14.10) |
Data from all 100 subjects, unless otherwise indicated. LACI = Lacunar Anterior Cerebral Infarction; PACI = Partial Anterior Cerebral Infarction; TACI = Total Anterior Cerebral Infarction; NIHSS = National Institutes of Health Stroke Scale; LCT = Letter Cancelation Test; EmNSA = Erasmus MC modified Nottingham Sensory Assessment; MI = Motricity Index; FMA = Fugl-Meyer Assessment. Data presents number of patients (N)
*median (IQR)
†mean (SD).
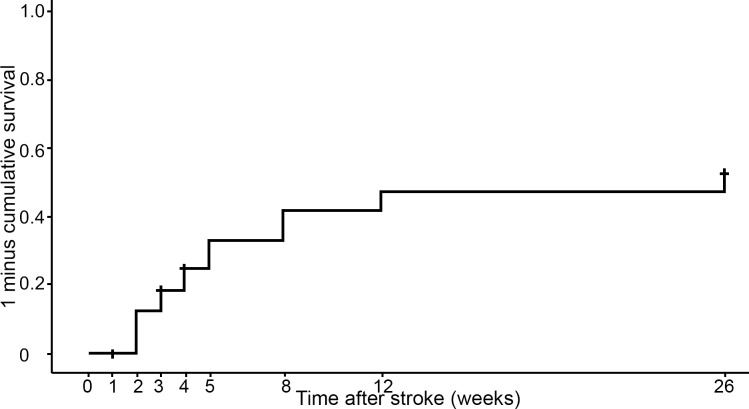
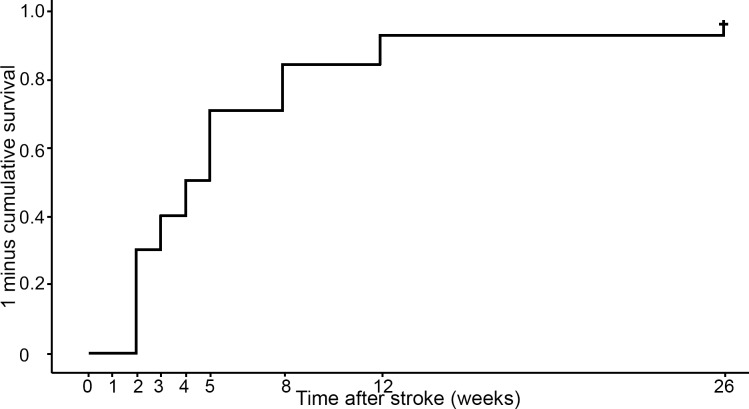
Approximately 7% of the repeated assessments of VFE were missing. The Kaplan-Meier curve for the whole group of 100 patients is shown in Fig 1. Three patients were lost to follow-up due to death, recurrent stroke and withdrawal. The median time to return of VFE was not reached within the first 26 weeks post-stroke. Visually, we do see that return of VFE occurs primarily within the first 8 weeks. Note that the cumulative ‘event’ curve is 1 minus survival, and resembles the probability of an event. In other words, a high and/or rapidly rising curve is a favorable outcome (i.e. return of FE). An additional analysis was performed on the group of patients who did regain some upper limb capacity (i.e. ARAT ≥ 10 points) at 6 months post-stroke (N = 45, Fig 2). The Kaplan-Meier curve in Fig 2 shows an initial sharp rise and reaches a median time to return of VFE at 4 weeks post-stroke (SE = 0.52; 95%CI = 2.99–5.01; Fig 2). The lower (25th) and upper (75th) percentiles were respectively 2 weeks and 8 weeks post-stroke. Twenty-three patients (51%) of the recoverers had regained VFE at 4 weeks after stroke, and at 8 weeks this number had increased to 38 patients (84%).
Fig 1. Kaplan-Meier cumulative ‘event’ curve for recovery of VFE (N = 100).
The numbers represent the number of patients with VFE at each time point (Fugl-Meyer Assessment hand sub item FE≥1). Three patients were lost to follow-up.
Fig 2. Kaplan-Meier cumulative ‘event’ curve for recovery of VFE in the group of patients who regain some upper limb capacity at 6 months post-stroke (N = 45).
The numbers represent the number of patients with VFE at each time point (Fugl-Meyer Assessment hand sub item FE≥1).
Logistic regression analyses were only performed on patients with complete baseline and 6-month assessments (N = 91); we excluded 3 drop-outs as described before and another 6 patients due to missing baseline data. Bivariable logistic regression analysis showed that 7 of the 13 baseline variables were significantly related to recovery of upper limb capacity (ARAT≥10 points) at 6 months post-stroke (p<0.05, Table 2). Collinearity diagnostics between independent variables showed no correlation coefficients ≥0.8. The subsequent backward multivariable logistic regression analysis, performed on the 7 preselected baseline variables, yielded a prediction model for return of upper limb capacity despite initial lack of VFE consisting of 3 variables: (1) moderate to good lower limb motor function, i.e. Motricity Index-leg≥35 points; (2) absence of visuospatial neglect, i.e. a letter cancellation test asymmetry between the contralesional and ipsilesional sides of <2 omissions; and (3) sufficient somatosensory function, i.e. Erasmus MC modified Nottingham Sensory Assessment≥33 points. Forward stepwise analysis confirmed these results. Table 3 displays all predicted probabilities of the model, ranging from 0.04–0.94, with a Hosmer-Lemeshow chi-square of 3.41(p = 0.637) and a c-statistic of 0.89. Two-way contingency tables showed a sensitivity of 0.88 (95%CI = 0.74–0.96), specificity of 0.73 (95%CI = 0.60–0.85), positive predictive value of 0.74 (95%CI = 0.60–0.85) and negative predictive value of 0.88 (95%CI = 0.74–0.96) for the final model.
Table 2. Candidate baseline determinants associated with regaining some upper limb capacity at 6 months post-stroke.
| N = 91 | |||
|---|---|---|---|
| Odds Ratio | 95%CI | p | |
| Sex (0 = female; 1 = male)† | 0.79 | 0.33–1.91 | 0.606 |
| Age (years)‡ | 1.00 | 0.97–1.04 | 0.950 |
| Hemisphere of stroke (0 = right; 1 = left)† | 2.40 | 0.94–6.10 | 0.066 |
| Type of stroke (Bamford classification: 0 = PACI/TACI;1 = LACI)† | 3.33 | 1.39–8.01 | 0.007* |
| Time between stroke and baseline (days)‡ | 1.07 | 0.96–1.18 | 0.222 |
| Hemianopia (NIHSS item-3:0 = yes; 1 = no)† | 0.85 | 0.16–4.44 | 0.845 |
| Facial palsy (NIHSS item-4:0 = yes; 1 = no)† | 3.99 | 1.16–13.69 | 0.028* |
| Visuospatial neglect (LCT asymmetry: 0≥2; 1<2)§ | 6.54 | 2.53–16.90 | <0.001* |
| Somatosensory function (EmNSA:0<33; 1≥33)d | 4.06 | 1.69–9.78 | 0.002* |
| Shoulder abduction (MI: 0<9; 1≥9)† | 3.68 | 1.54–8.79 | 0.003* |
| Elbow flexion (MI: 0<9; 1≥9)† | 8.00 | 2.65–24.17 | <0.001* |
| Lower limb function (MI-leg: 0<35; 1≥35)|| | 12.67 | 4.68–34.32 | <0.001* |
| Randomization (0 = control group; 1 = experimental group) † | 0.80 | 0.35–1.84 | 0.605 |
PACI = Partial Anterior Cerebral Infarction; TACI = Total Anterior Cerebral Infarction; LACI = Lacunar Anterior Cerebral Infarction; NIHSS = National Institutes of Health Stroke Scale; LCT = Letter Cancellation Test; EmNSA = Erasmus modified Nottingham Sensory Assessment; MI = Motricity Index.
*Wald statistic = p<0.05
†Based on clinical grounds
‡Not dichotomized
§Based on previous literature(14)
||Based on area under the receiver-operating characteristic curve.
Table 3. Probabilities of regaining some upper limb capacity at 6 months post-stroke in patients who initially did not show finger extension.
| LL | VSN | SSF | True Negatives (N) | False Negatives (N) | False Positives (N) | True Positives (N) | Predicted probability (0–1) |
|---|---|---|---|---|---|---|---|
| Good | No | Good | 36 | 5 | 13 | 37 | 0.94 |
| Good | No | Poor | 0.81 | ||||
| Good | Yes | Good | 0.72 | ||||
| Poor | No | Good | 0.51 | ||||
| Good | Yes | Poor | 0.39 | ||||
| Poor | No | Poor | 0.21 | ||||
| Poor | Yes | Good | 0.13 | ||||
| Poor | Yes | Poor | 0.04 |
Model: P(upper limb capacity) = 1/1+e-(-3.24+2.80xLL+1.91xVSN+1.36xSSF). LL = lower limb function (motricity index leg); VSN (letter cancellation test asymmetry); SSF = somatosensory function (Erasmus MC modified Nottingham Sensory Assessment).
Discussion
To our knowledge, this is the first study to prospectively investigate the clinical time window for spontaneous return of VFE in ischemic stroke patients who showed no VFE within the first days post-stroke. The present study shows that if patients do regain VFE, this will most likely occur within the first 8 weeks after stroke onset. However, more than half of the patients who regained some upper limb capacity at 6 months post-stroke already showed this return of VFE within the first 4 weeks post-stroke. In view of the absence of evidence-based therapies for this specific group of patients[7;8], as well as our neutral trial showing no effects of early-start EMG-NMS intervention[17], we believe this return of VFE is most likely driven by spontaneous processes of neurological recovery.
Acknowledging that most evidence-based therapies for the upper limb depend on selecting patients for their ability to voluntarily extend their fingers, we recommend that clinicians monitor these stroke patients for return of VFE weekly, at least up to 8 weeks post-stroke[31]. This recommendation is in line with the recently developed Post Stroke Arm Algorithm application (“app”) which offers clinicians an online tool to select the most appropriate evidence-based upper limb therapy for an individual patient, with an emphasis on the regular monitoring of VFE[31]. If VFE returns within the time window for neurological recovery, the prognosis changes in favor of regaining upper limb capacity, and therapy may focus on improving motor function through high-intensity repetitive exercise to prevent learned non-use of the paretic arm, for example with (modified) constraint-induced movement therapy[32].
Importantly, we do not claim that return of VFE always occurs within the first 8 weeks, as there may be exceptions due to neglect[33], acknowledging the suppressive effects of neglect on motor recovery[14;15]. However, the present study suggests that the likelihood of recovery after this time period is small. Future studies should further investigate those cases that constitute an exception to the rule that is focus on patients who show return of VFE beyond the first 8 weeks post-stroke.
Concerning our second objective, we found that patients with no initial VFE who showed moderate to good lower limb motor function, no visuospatial neglect, and sufficient somatosensory function have a high probability (0.94) of regaining at least some upper limb capacity at 6 months post-stroke (i.e. ability to pick up a small object). The model presented here may be seen as an important contribution to the prediction of upper limb capacity in patients with an initial poor prognosis early post-stroke.
Severity of lower limb paresis was previously suggested to be an important predictor of regaining upper limb function[13;28;34] and may reflect the extent of the lesion and as such the severity of initial neurological damage. The substantial negative impact of visuospatial neglect on upper extremity motor recovery which was found in other studies[15;35;36] may reflect the suppressive effects of the dysfunctional site of injury on anatomically and functionally related brain areas at remote distance from the location of the infarct[37]. Our model also suggests that somatosensory dysfunction is a crucial factor that prevents upper limb recovery [14;37]. Repeated, systematic monitoring of neurological impairments within the first 8 weeks after stroke onset may give more insight in the underlying logistic pattern of spontaneous neurobiological recovery.
Study Limitations & Future Research
It should be noted that half of the patients in the current study received 3-weeks of additional therapy with EMG-NMS focused at regaining VFE, in comparison to the other group that solely received usual care according to evidence-based guidelines[7]. We postulate that this did not affect our results, as the EXPLICIT-stroke trial did not show any significant interaction effects of time with EMG-NMS on the likelihood of return of upper limb capacity within this time window[17]. High quality randomized clinical trials are needed to determine if the likelihood and timing of this return of VFE can be improved and accelerated by innovative therapies that may enhance neuronal activity and restore activity homeostasis, such as transcranial direct-current stimulation, repetitive transcranial magnetic stimulation or pharmaceuticals combined with exercise therapy.
Second, the present study shows that in particular patients with a paresis of the arm without somatosensory dysfunction and neglect are more likely to have some return of upper limb capacity. The sensitivity of the current model was quite good (0.88, 95%CI = 0.74–0.96), however, the specificity was somewhat lower (0.73, 95%CI = 0.60–0.85). This misclassification of patients as recoverers was also observed in previous studies using transcranial magnetic stimulation (TMS)[38;39]. Moreover, the presence of a motor evoked potential does not necessarily mean that patients will show recovery of hand function[38;39]. Preliminary results from the PREP-algorithm, which sequentially combines clinical measurements of shoulder abduction and finger extension (SAFE) with TMS and diffusion tensor imaging (DTI), also showed a specificity and sensitivity of respectively 0.88 and 0.73[40]. However, these values were for the ‘complete recovery’ subcategory (i.e. ARAT score of 50 points or higher). Sensitivity and specificity values for the other categories (i.e. notable, limited and no recovery) were not provided. We therefore cannot directly compare the preliminary results from the PREP-algorithm with our results as the cut-off values are different. The current results emphasize the importance of repeated clinical assessment of VFE in the first weeks post-stroke for clinical decision making. Within the PREP-algorithm, SAFE was however not reassessed when TMS and DTI were performed at about 2 weeks post-stroke[40]. Determination of confidence intervals reflecting precision of this algorithm, as well as cross-validation are needed to underpin the robustness of propagated models. Future cohort studies are needed to refine our clinical prediction model by exploring the added value of neuroimaging, such as stroke volume or localization (e.g. cortical or subcortical)[41;42] and DTI[43], TMS[39] and other biomarkers associated with the recovery of upper limb capacity after stroke[11].
Third, the sample size of the present study was relatively small in relation to the number of variables. However, model stability and robustness was confirmed with an additional forward stepwise logistic regression analysis.
Fourth, although models with dichotomized outcomes are often used within clinical practice, they do limit the understanding of individual recovery profiles. Moreover, with the current model we cannot differentiate between patients who regain some upper limb capacity (ARAT = 10 points) and those who regain full upper limb capacity (ARAT = 57 points). The cut-off value of 10 or more points on the ARAT was used in previous literature as it represents the recovery of some dexterity[2;28]. Future studies are needed to identify subgroups of patients achieving notable or limited dexterity at 3 and 6 months post-stroke. To achieve this goal, larger prospective cohorts are needed to test the precision and with that the robustness of current models for identifying these subgroups with limited and notable recovery.
Finally, the present study was restricted to patients with a first-ever ischemic middle cerebral artery stroke and did not include cross-validation of the logistic regression model.
Acknowledgments
The authors would like to thank the EXPLICIT-stroke physicians, therapists and nurses at the stroke units of the participating centers for providing the intervention and monitoring rehabilitation, and the patients who participated in the study (www.explicit-stroke.nl). In addition to the authors of the present study, the consortium consists of Frans C. van der Helm, Erwin de Vlugt and Asbjørn Klomp, from the Delft University of Technology, Delft, The Netherlands; Carel G.M. Meskers and Joost van Kordelaar, from the VU University Medical Center, Amsterdam, The Netherlands; J. Hans Arendzen, Jurriaan de Groot and Hanneke J. van der Krogt, from the Leiden University Medical Center, Leiden, The Netherlands; Anne-Visser-Meily and Floor E. Buma, from the Utrecht University, Utrecht, The Netherlands; and Alexander C.H. Geurts, Annette A.A. van Kuijk and Chantal D. Bakker, from the Radboud University Medical Center, Nijmegen, The Netherlands. Gert Kwakkel (corresponding author of the present manuscript) is the lead author for this group.
Data Availability
At this moment, additional papers are being prepared using part of the data presented in this manuscript. In line with existing contracts with participating universities in the EXPLICIT consortium that will publish on the basis of the current dataset reflecting 1271 assessments as well as agreement with the local regulations of the funding committee ZonMw, full open access availability of the data is restricted and disclosed preventing publication of these manuscripts. The data will be available upon request. This statement is now added to the manuscript on page 14. Requests for this unique data set can be submitted to the corresponding author, Gert Kwakkel (g.kwakkel@vumc.nl), who will evaluate the request for its purposes in line with the funding organization (ZonMW; grant no. 98000001) as well as the EXPLICIT-stroke consortium (ethics committee Leiden University Medical Center; no. P08.035).
Funding Statement
The research leading to these results has received funding from the EXPLICIT-stroke grant from the Dutch Organization for Health Research and Development (ZonMw grant No.89000001), supported by the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013) / ERC Grant Agreement no. 291339-4D-EEG. The funding organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
References
- 1.Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, et al. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke 2007;38:1088–1090. [DOI] [PubMed] [Google Scholar]
- 2.Nijland RH, Van Wegen EE, Harmeling-Van der Wel BC, Kwakkel G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke 2010;41:745–750. 10.1161/STROKEAHA.109.572065 [DOI] [PubMed] [Google Scholar]
- 3.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol 2010;9:1228–1232. 10.1016/S1474-4422(10)70247-7 [DOI] [PubMed] [Google Scholar]
- 4.Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol 1992;448:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol 2011;589:5603–5612. 10.1113/jphysiol.2011.215160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 2009;29:4993–4999. 10.1523/JNEUROSCI.3720-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veerbeek JM, Van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 2014;9:e87987 10.1371/journal.pone.0087987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009;8:741–754. 10.1016/S1474-4422(09)70150-4 [DOI] [PubMed] [Google Scholar]
- 9.Feeney DM, Baron JC. Diaschisis. Stroke 1986;17:817–830. [DOI] [PubMed] [Google Scholar]
- 10.Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke 2006;37:2348–2353. [DOI] [PubMed] [Google Scholar]
- 11.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 2008;63:272–287. 10.1002/ana.21393 [DOI] [PubMed] [Google Scholar]
- 12.Van Kordelaar J, van Wegen EEH, Kwakkel G. Impact of time on quality of motor control of the paretic upper limb after stroke. Arch Phys Med Rehabil 2014;95:338–344. 10.1016/j.apmr.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 13.Winters C, Van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the Proportional Recovery Model for the Upper Extremity After an Ischemic Stroke. Neurorehabil Neural Repair 2015;29:614–622. 10.1177/1545968314562115 [DOI] [PubMed] [Google Scholar]
- 14.Nijboer TC, Kollen BJ, Kwakkel G. Time course of visuospatial neglect early after stroke: a longitudinal cohort study. Cortex 2013;49:2021–2027. 10.1016/j.cortex.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 15.Nijboer TC, Kollen BJ, Kwakkel G. The impact of recovery of visuo-spatial neglect on motor recovery of the upper paretic limb after stroke. PLoS One 2014;9:e100584 10.1371/journal.pone.0100584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwakkel G, Meskers CG, Van Wegen EE, Lankhorst GJ, Geurts AC, van Kuijk AA, et al. Impact of early applied upper limb stimulation: the EXPLICIT-stroke programme design. BMC Neurol 2008;8:49 10.1186/1471-2377-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwakkel G, Winters C, Van Wegen EE, Nijland RH, van Kuijk AA, Visser-Meily A, et al. Effects of Unilateral Upper Limb Training in Two Distinct Prognostic Groups Early After Stroke: The EXPLICIT-Stroke Randomized Clinical Trial. [Epub ahead of print Jan 7, 2016]. Neurorehabil Neural Repair 2016. [DOI] [PubMed] [Google Scholar]
- 18.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 19.WHO. International classification of functioning, disability and health. Geneva. 2001.
- 20.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res 1981;4:483–492. [DOI] [PubMed] [Google Scholar]
- 21.Van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med 2001;33:110–113. [DOI] [PubMed] [Google Scholar]
- 22.Coupar F, Pollock A, Rowe P, Weir C, Langhorne P. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil 2012;26:291–313. 10.1177/0269215511420305 [DOI] [PubMed] [Google Scholar]
- 23.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521–1526. [DOI] [PubMed] [Google Scholar]
- 24.Brott T, Adams HP Jr., Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 25.Plummer P, Morris ME, Dunai J. Assessment of unilateral neglect. Phys Ther 2003;83:732–740. [PubMed] [Google Scholar]
- 26.Stolk-Hornsveld F, Crow JL, Hendriks EP, van der Baan R, Harmeling-Van der Wel BC. The Erasmus MC modifications to the (revised) Nottingham Sensory Assessment: a reliable somatosensory assessment measure for patients with intracranial disorders. Clin Rehabil 2006;20:160–172. [DOI] [PubMed] [Google Scholar]
- 27.Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry 1990;53:576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181–2186. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan E, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 1958;53:457–481. [Google Scholar]
- 30.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 31.Wolf S, Kwakkel G, Bayley M, McDonnell M. Best practice for arm recovery post stroke: an international application. Phys Ther 2015;101:e22–e23. [DOI] [PubMed] [Google Scholar]
- 32.Kwakkel G, Veerbeek JM, Van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol 2015;14:224–234. 10.1016/S1474-4422(14)70160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farne A, Buxbaum LJ, Ferraro M, Frassinetti F, Whyte J, Veramonti T, et al. Patterns of spontaneous recovery of neglect and associated disorders in acute right brain-damaged patients. J Neurol Neurosurg Psychiatry 2004;75:1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke 2001;32:107–12. [DOI] [PubMed] [Google Scholar]
- 35.Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch Phys Med Rehabil 1999;80:379–384. [DOI] [PubMed] [Google Scholar]
- 36.Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, et al. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology 2004;62:749–756. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci 2005;8:1603–1610. [DOI] [PubMed] [Google Scholar]
- 38.Hendricks HT, Pasman JW, van LJ, Zwarts MJ. Motor evoked potentials in predicting recovery from upper extremity paralysis after acute stroke. Cerebrovasc Dis 2003;16:265–71. [DOI] [PubMed] [Google Scholar]
- 39.Van Kuijk AA, Pasman JW, Hendricks HT, Zwarts MJ, Geurts AC. Predicting hand motor recovery in severe stroke: the role of motor evoked potentials in relation to early clinical assessment. Neurorehabil Neural Repair 2009;23:45–51. 10.1177/1545968308317578 [DOI] [PubMed] [Google Scholar]
- 40.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012;135:2527–2535. 10.1093/brain/aws146 [DOI] [PubMed] [Google Scholar]
- 41.Schiemanck SK, Kwakkel G, Post MW, Kappelle LJ, Prevo AJ. Predicting long-term independency in activities of daily living after middle cerebral artery stroke: does information from MRI have added predictive value compared with clinical information? Stroke 2006;37:1050–1054. [DOI] [PubMed] [Google Scholar]
- 42.Cheng B, Forkert ND, Zavaglia M, Hilgetag CC, Golsari A, Siemonsen S, et al. Influence of stroke infarct location on functional outcome measured by the modified rankin scale. Stroke 2014;45:1695–1702. 10.1161/STROKEAHA.114.005152 [DOI] [PubMed] [Google Scholar]
- 43.Kumar P, Kathuria P, Nair P, Prasad K. Prediction of Upper Limb Motor Recovery after Subacute Ischemic Stroke Using Diffusion Tensor Imaging: A Systematic Review and Meta-Analysis. J Stroke. 2016;18:50–9. 10.5853/jos.2015.01186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
At this moment, additional papers are being prepared using part of the data presented in this manuscript. In line with existing contracts with participating universities in the EXPLICIT consortium that will publish on the basis of the current dataset reflecting 1271 assessments as well as agreement with the local regulations of the funding committee ZonMw, full open access availability of the data is restricted and disclosed preventing publication of these manuscripts. The data will be available upon request. This statement is now added to the manuscript on page 14. Requests for this unique data set can be submitted to the corresponding author, Gert Kwakkel (g.kwakkel@vumc.nl), who will evaluate the request for its purposes in line with the funding organization (ZonMW; grant no. 98000001) as well as the EXPLICIT-stroke consortium (ethics committee Leiden University Medical Center; no. P08.035).