Abstract
Purpose of review
The paracellular pathway through the tight junction provides an important route for chloride reabsorption in the collecting duct of the kidney. This review describes recent findings of how defects in paracellular chloride permeation pathway may cause kidney diseases and how such a pathway may be regulated to maintain normal chloride homeostasis.
Recent findings
The tight junction in the collecting duct expresses two important claudin genes – claudin-4 and claudin-8. Transgenic knockout of either claudin gene causes hypotension, hypochloremia, and metabolic alkalosis in experimental animals. The claudin-4 mediated chloride permeability can be regulated by a protease endogenously expressed by the collecting duct cell – Cap1. Cap1 regulates the intercellular interaction of claudin-4 and its membrane stability. KLHL3, previously identified as a causal gene for Gordon’s syndrome, also known as pseudohypoaldosteronism II (PHA-II), directly interacts with claudin-8 and regulates its ubiquitination and degradation. The dominant PHA-II mutation (R528H) in KLHL3 abolishes claudin-8 binding, ubiquitination, and degradation.
Summary
The paracellular chloride permeation pathway in the kidney is an important but understudied area in nephrology. It plays vital roles in renal salt handling and regulation of extracellular fluid volume and blood pressure. Two claudin proteins – claudin-4 and claudin-8 contribute to the function of this paracellular pathway. Deletion of either claudin protein from the collecting duct causes renal chloride reabsorption defects and low blood pressure. Claudins can be regulated on post-translational levels by several mechanisms involving protease and ubiquitin ligase. Deregulation of claudins may cause human hypertension as exemplified in the Gordon’s syndrome.
Keywords: claudin, tight junction, blood pressure, chloride, ion channel, kidney
Introduction
The tight junction (TJ) is composed of a series of direct membrane contacts of adjacent cells in polarized epithelia [1]. Freeze-fracture electron microscopy has revealed the tight junction as a branching and anastomosing reticulum of “fibrils” or “strands” on the P fracture face [2]. These fibrils have been demonstrated to comprise integral membrane proteins that not only mediate cell-cell interactions but also create an ionic pathway between the cells, known as the parachellular pathway [3]. The known integral membrane proteins of the tight junction include occludin [4], the Junctional Adhesion Molecules (JAMs) [5], and the claudins [6,7]. Among them, the claudins selectively regulate the TJ permeability, presumably through electrostatic interaction of permeating ions with the charged residues located in the extracellular domains of claudins [8].
The physiologic role of paracellular Cl− permeability in the collecting duct
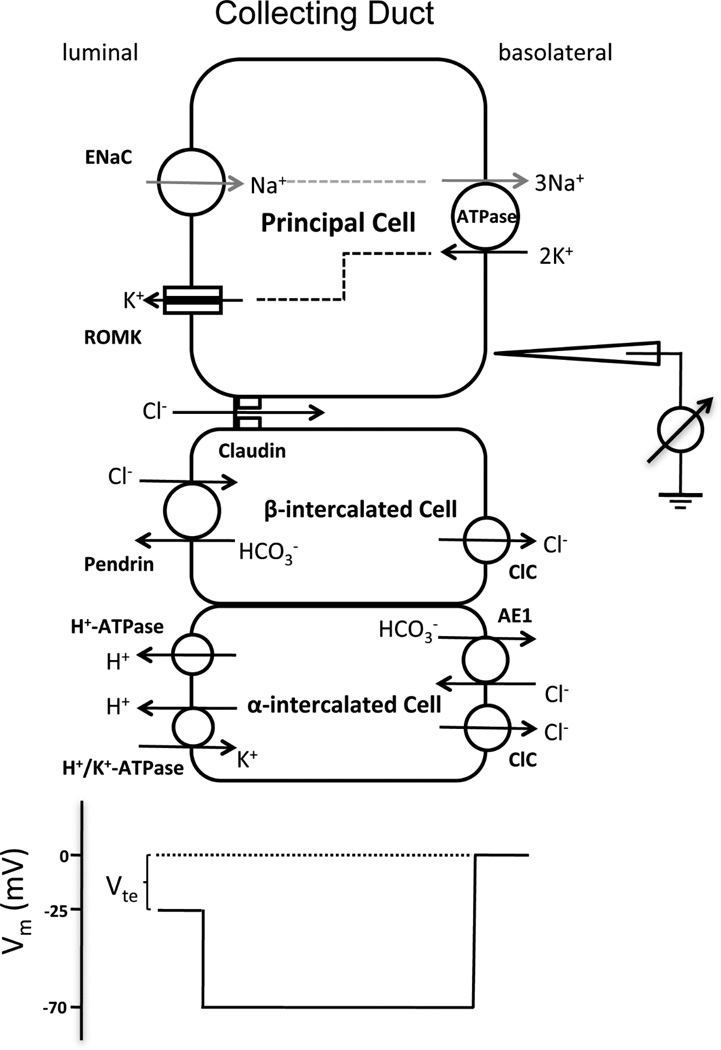
The renal collecting duct is different from many other NaCl absorbing epithelia in that its Na+ transport process is separated from its Cl− transport process. The Na+ reabsorption occurs in the principal cell via the epithelial sodium channel (ENaC), while the Cl− reabsorption is mediated predominantly (~70%) through the paracellular pathway and to a lesser extent through the β-type intercalated cell via the Cl−/HCO− exchanger – pendrin [9]. The driving force for paracellular Cl− reabsorption is the lumen-negative transepithelial potential (Vte: ~ −25mV; Figure 1). Vte is generated by the unidirectional transepithelial Na+ current (lumen to bath) and is sufficient to overcome the transepithelial Cl− concentration gradient that would otherwise favor Cl− secretion. The paracellular Cl− conductance in the collecting duct has been recorded primarily by ex-vivo studies using microdissected and perfused renal tubules from rodents [9–12]. Its conductance level is between 1.1–1.2 mS/cm2 [12]. The ENaC mediated Na+ conductance, on the other hand, is around 0.6 mS/cm2 under basal condition [13]. In theory, the paracellular pathway has provided an efficient route for coupling Cl− reabsorption with Na+ reabsorption so as to maintain luminal fluid electroneutrality.
Figure 1.
Ion transport mechanism in the collecting duct. The membrane voltage (Vm) trace depicts the virtual measurement by an electrode that is pushed from the basolateral side through the cell to the luminal side. In this example, the basolateral membrane voltage is −70 mV and the luminal membrane voltage is −45 mV, resulting in a transepithelial Vte of −25 mV with respect to the basolateral side. Vte drives Cl− permeation through the tight junction.
The molecular component of the paracellular pathway in the collecting duct
Several claudins, including claudin-3, -4, -7, and -8, have been found expressed in the collecting duct of rodent kidneys [14,15]. They have been extensively studied for many extrarenal roles [16–19]. Hou and colleagues used a systematic RNA interference approach to functionally investigate these four claudins in two native collecting duct cell lines, M-1 and mIMCD3 [20]. Knockdown of claudin-4 or claudin-8 significantly decreased the paracellular Cl− permeability (PCl) without affecting the Na+ permeability (PNa). Knockdown of claudin-3 was without any noticeable effect on either PCl or PNa, while knockdown of claudin-7 led to a global loss of barrier function against both Na+ and Cl−. Consistent with the non-selective barrier function, claudin-7 knockout mice developed severe renal wasting of Na+, K+, Cl−, and water, and died within 12 days after birth [21]. Mutagenesis has identified a protruding amino acid locus (K65) in the extracellular domain of claudin-4 protein, which is critical for its Cl− permeability [20]. Claudin-4 was found interacting with claudin-8, in vitro in biochemical solution, in yeast membrane and in epithelial cell membrane [20]. Without claudin-8, claudin-4 intracellular trafficking to tight junction is interrupted, which may provide a molecular explanation for claudin-8’s effects on PCl [20].
The lesson learned from claudin-4 knockout animal
The global claudin-4 knockout (KO) mice developed a wide spectrum of renal and urologic defects, which included urinary loss of Ca++ and Cl−, hydronephrosis, urinary tract obstruction, and urothelial hyperplasia [22]. While blood pressure was not measured for these animals, their urinary volume and osmolality appeared unchanged. By co-localization with known molecular markers for different nephron segments, Gong and colleagues have determined the cellular localization of claudin-4 in the kidney [23]. Their data indicate that claudin-4 is expressed by the thin limb (TL) of the Henle’s loop, the late portion of the distal convoluted tubule (DCT2), the connecting tubule (CNT) and the collecting duct (CD). Using the Cre-loxP recombination strategy, Gong and colleagues have generated the CD specific KO mouse model of claudin-4 [23]. These animals developed hypotension, hypochloremia, and metabolic alkalosis due to renal loss of Cl− under regular condition of dietary salt intake. Natriuretic diuresis was also evident, compatible with decreased extracellular fluid volume in the KO mice. Dietary salt restriction further exacerbated hypotension and renal loss of salt and volume in these animals [23]. Notably, the fold of increase in the salt excretion level was much higher when the KO was given low salt diet instead of regular salt diet. This data may suggest that claudin-4 is physiologically regulated in response to dietary salt variation as a feedback mechanism to adjust the renal excretion of Cl− accordingly.
The lesson learned from claudin-8 knockout animal
The renal localization pattern of claudin-8 is remarkably similar to that of claudin-4, with predominant expression in the DCT through CNT to CD [14,24]. Using the same Cre-loxP recombination strategy developed for claudin-4, Gong and colleagues have generated the CD specific KO mouse model of claudin-8 [24]. These KO animals phenocopied claudin-4 KO in many ways. For example, they developed hypotension, hypochloremia, and metabolic alkalosis. In addition to salt and volume deregulation as observed in claudin-4 KO, claudin-8 KO developed hypokalemia due to increased urinary excretion of K+. Notably, the hypotension is more severe in claudin-8 KO (reduced by 17.6 mmHg) than in claudin-4 KO animals (reduced by 6.5 mmHg), which may suggest a more complete blockade of paracellular Cl− transport in absence of claudin-8 than claudin-4. Mechanistically, deletion of claudin-8 in the collecting duct rendered nearly complete delocalization of claudin-4 from the TJ, while deletion of claudin-4 had no effect on claudin-8 localization in the TJ [24]. Claudin-7, previously shown to form a nonselective barrier to ionic movement [21], was not affected by the loss of neither claudin-4 nor claudin-8 [24]. These in vivo data are remarkably compatible with the concept derived from the in vitro assays of claudin interactions that claudin-4 and -8 form a Cl− transport channel due to their cis-interaction [20]. Deletion of claudin-8 has created a double KO on the level of tight junction because it is the exclusive interaction partner for claudin-4 [20].
Channel-activating protease 1 (Cap1) as a key regulator to adjust sodium and chloride permeability ratio in the collecting duct
Cap1 was the first of several membrane-tethered serine proteases that augment the ENaC channel conductivity by direct proteolytic cleavage of its target in the collecting duct [25,26]. The paracellular channel permeability appeared to be regulated by serine proteases too in the collecting duct. Liu and colleagues first noticed that trypsin, when added to the apical side of the mouse CD M-1 cells, increased not only the amiloride-sensitive Na+ current (Ieq) but also the transepithelial resistance (TER), an inverse indicator for paracellular channel conductance [27]. Gong and colleagues further confirmed this effect using a different mouse CD cell line – mIMCD3 [23]. Serendipitously, they found that recombinant Cap1 protein exerted a similar effect to trypsin by reducing paracellular Cl− permeability in both M-1 and mIMCD3 cells. Knockdown of claudin-4 completely abrogated the Cap1 effect in these cells, which may suggest that claudin-4 was a functional target of Cap1. Gong and colleagues addressed this possibility by developing a series of elegant biochemical assays [23]. They showed that Cap1 directly acted upon the extracellular domain of claudin-4 to disrupt its trans-interaction presumably by proteolytic cleavage at the R158 site of claudin-4 protein. The physiologic role of Cap1 now becomes particularly interesting considering its dual regulation of ENaC and claudin-4. It may fine-tune the CD function in response to hyperkalemia-triggered hyperaldosteronism. Because aldosterone increases Cap1 gene expression in the collecting duct [28], a gain of Cap1 function would increase the luminal Cl− level while at the same time transport more Na+ to the basolateral space, which inevitably lowers the already negative Vte to favor K+ secretion into the urine (Figure 1).
Paracellular Cl− transport in Gordon’s syndrome
Gordon’s syndrome, also known as pseudohypoaldosteronism II (PHA-II) or familial hyperkalemic hypertension (FHHt), features hypertension, hyperkalemia, and hyperchloremic metabolic acidosis [29]. There are four genetic loci with significant linkage to Gordon’s syndrome, which encode with no lysine kinase 1 (WNK1), WNK4, Kelch-like 3 (KLHL3) and Cullin 3 (CUL3) [30,31]. There has been a long-standing interest in how paracellular Cl− transport is deregulated in Gordon’s syndrome. Yamauchi et al and Kahle et al independently discovered that the PHA-II causing mutation (D561A) in WNK4 increased paracellular Cl− permeability in vitro in cultured MDCK cells by hyperphosphorylating claudins [32,33]. However, in vivo in a WNK4 knockin mouse model harboring the D561A mutation, there was no detectable change of paracellular Cl− permeability in microdissected and perfused collecting duct tubules [34]. Gong and colleagues turned their attention to how KLHL3 may regulate claudin-8 in the collecting duct, knowing that the CD specific claudin-8 KO mice developed the phenotypes exactly opposite to PHA-II [24]. Using a cultured CD cell model (M-1), they found that transfection of wildtype KLHL3 decreased while the PHA-II causing mutation (R528H) increased paracellular Cl− permeability [24]. Both wildtype and mutant KLHL3 effects depended upon the presence of claudin-8. The authors further elucidated the biochemical mechanism of claudin-8 regulation by KLHL3 [24]. They showed that KLHL3 was directly bound to claudin-8, and this binding led to ubiquitination and degradation of claudin-8. The PHA-II mutation (R528H) in KLHL3 played a dominant negative role by abolishing its binding, ubiquitination and degradation of claudin-8.
Open questions
A fundamental understanding of how paracellular Cl− permeability is regulated physiologically will be crucial for establishing blood pressure disease models that may originate from deregulation in normal physiologic feedback mechanisms. It is clear from Gong and colleagues’ data that low salt-triggered hypovolemia may induce positive regulation of paracellular Cl− permeability and exacerbate the salt wasting defect and the hypotension in claudin-4 KO animals [23]. Hypovolemia will activate the renin–angiotensin–aldosterone (RAA) system. Aldosterone has been found to upregulate the transcription of claudin-8 in the distal colon [35], a known aldosterone sensitive site similar to the collecting duct. The claudin-4 protein is hyper-phosphorylated by aldosterone in the rat collecting duct RCCD2 cells [36]. Therefore, an increase in paracellular Cl− permeability will favor salt retention under hypovolemic condition. Tubular angiotensin-II signaling has long been suspected to play a vital part to adjust the collecting duct function in the face of hypovolemia versus hyperkalemia. Stimulation with angiotensin-II increases amiloride-sensitive Na+ transport while decreases luminal membrane K+ channel activity [37,38]. Although there is no direct evidence that angiotensin-II regulates paracellular Cl− permeability, Gong and colleagues have revealed a novel signaling cascade from KLHL3 to claudin-8 [24]. KLHL3, as a component of the E3 ubiquitinase mutated in PHA-II, is itself phosphorylated and activated by angiotensin-II via PKC [39]. Finally, it is important to recognize that PHA-II is a multifaceted disease altering the functions of both DCT and CD. Despite recent advances establishing the role of NCC channel phosphorylation as a key underlying mechanism for PHA-II [34,40–42], it is not known whether changes in paracellular Cl− permeability may also contribute to the pathogenesis of PHA-II. A technical challenge to consider is that such changes in the CD may be obscured by abnormalities in the DCT. The global KO strategy as in the case of Cul3 may generate perplexing phenotypes [43]. Because KLHL3 is the only recessive allele causing PHA-II, a CD-specific KO mouse model will allow delineating the transport defects through both transcellular and paracellular pathways in the collecting duct.
Conclusion
The role of tight junction permeability in causing human diseases is an important but understudied area. In the kidney, the paracellular Cl− permeability has long been suspected to play a vital role in blood pressure regulation. A series of molecular, cellular, and transgenic knockout animal studies have revealed the molecular components of the paracellular Cl− pathway and how its defect may alter the kidney’s electrolyte-handling ability, thereby causing extracellular fluid volume and systemic blood pressure deregulation.
Bullet points.
Paracellular reabsorption of Cl− is driven by a lumen negative potential through the tight junction in the collecting duct of the kidney.
Two claudin proteins – claudin-4 and claudin-8 make the paracellular pathway for Cl− permeation.
Loss of function in either claudin-4 or claudin-8 increases urinary excretion of salt and causes hypotension in transgenic animals.
Cap1 regulates paracellular Cl− permeability by disrupting claudin-4 trans-interaction.
KLHL3 regulates paracellular Cl− permeability by ubiquitinating and degrading claudin-8.
Acknowledgments
Acknowledgements: None
Financial support and sponsorship
This work was supported by the National Institutes of Health Grants RO1DK084059 and P30 DK079333, and American Heart Association Grant 0930050N.
Footnotes
Conflict of interest
The author declares no conflict of interest.
Reference
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970;45:272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou J, Rajagopal M, Yu AS. Claudins and the kidney. Annu Rev Physiol. 2013;75:479–501. doi: 10.1146/annurev-physiol-030212-183705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 6.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and-2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or-2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sansom SC, Weinman EJ, O'Neil RG. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984;247:F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- 10.O'Neil RG, Boulpaep EL. Ionic conductive properties and electrophysiology of the rabbit cortical collecting tubule. Am J Physiol. 1982;243:F81–F95. doi: 10.1152/ajprenal.1982.243.1.F81. [DOI] [PubMed] [Google Scholar]
- 11.O'Neil RG, Sansom SC. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol. 1984;82:281–295. doi: 10.1007/BF01871637. [DOI] [PubMed] [Google Scholar]
- 12.Warden DH, Schuster VL, Stokes JB. Characteristics of the paracellular pathway of rabbit cortical collecting duct. Am J Physiol. 1988;255:F720–F727. doi: 10.1152/ajprenal.1988.255.4.F720. [DOI] [PubMed] [Google Scholar]
- 13.Stoos BA, Naray-Fejes-Toth A, Carretero OA, Ito S, Fejes-Toth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 1991;39:1168–1175. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- 14.Li WY, Huey CL, Yu AS. Expression of claudin-7 and-8 along the mouse nephron. Am J Physiol Renal Physiol. 2004;286:F1063–F1071. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2011;301:L40–L49. doi: 10.1152/ajplung.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R, Cao J, Chen YH. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 2012;142:305–315. doi: 10.1053/j.gastro.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem. 2003;278:17350–17359. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatum R, Zhang Y, Salleng K, Lu Z, Lin JJ, Lu Q, Jeansonne BG, Ding L, Chen YH. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol. 2010;298:F24–F34. doi: 10.1152/ajprenal.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita H, Hamazaki Y, Noda Y, Oshima M, Minato N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS One. 2012;7:e52272. doi: 10.1371/journal.pone.0052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Y, Yu M, Yang J, Gonzales E, Perez R, Hou M, Tripathi P, Hering-Smith KS, Hamm LL, Hou J. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc Natl Acad Sci U S A. 2014;111:E3766–E3774. doi: 10.1073/pnas.1406741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci U S A. 2015;112:4340–4345. doi: 10.1073/pnas.1421441112. ∙∙ This report described the collecting duct specific knockout model of claudin-8 and revealed how KLHL3 regulated claudin-8 protein ubiquitination and paracellular Cl− permeability.
- 25.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 26.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus Oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Hering-Smith KS, Schiro FR, Hamm LL. Serine protease activity in m-1 cortical collecting duct cells. Hypertension. 2002;39:860–864. doi: 10.1161/01.hyp.0000013055.48885.8d. [DOI] [PubMed] [Google Scholar]
- 28.Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, Nonoguchi H, Chen LM, Chai KX, Chao J, et al. Regulation of prostasin by aldosterone in the kidney. J Clin Invest. 2002;109:401–408. doi: 10.1172/JCI13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon RD. Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension. 1986;8:93–102. doi: 10.1161/01.hyp.8.2.93. [DOI] [PubMed] [Google Scholar]
- 30.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 31.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci U S A. 2004;101:4690–4694. doi: 10.1073/pnas.0306924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, et al. Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc Natl Acad Sci U S A. 2004;101:14877–14882. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, et al. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun. 2009;378:45–50. doi: 10.1016/j.bbrc.2008.10.164. [DOI] [PubMed] [Google Scholar]
- 36.Le Moellic C, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol. 2005;289:C1513–C1521. doi: 10.1152/ajpcell.00314.2005. [DOI] [PubMed] [Google Scholar]
- 37.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 38.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem. 2007;282:6455–6462. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibata S, Arroyo JP, Castaneda-Bueno M, Puthumana J, Zhang J, Uchida S, Stone KL, Lam TT, Lifton RP. Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci U S A. 2014;111:15556–15561. doi: 10.1073/pnas.1418342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. ∙∙ This study elegantly showed how serum K+ levels regulated NCC phosphorylation and systemic blood pressure via changes in membrane potential, intracellular Cl− and WNK4 activity.
- 41.Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, et al. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014;23:5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 42. Araki Y, Rai T, Sohara E, Mori T, Inoue Y, Isobe K, Kikuchi E, Ohta A, Sasaki S, Uchida S. Generation and analysis of knock-in mice carrying pseudohypoaldosteronism type II-causing mutations in the cullin 3 gene. Biol Open. 2015;4:1509–1517. doi: 10.1242/bio.013276. ∙∙ This report described a novel knockin model to mimic human PHA-II mutation in Cul3 and provided important insights of how PHA-II causing allele may regulate the kidney function.
- 43.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, et al. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest. 2014;124:4723–4736. doi: 10.1172/JCI76126. [DOI] [PMC free article] [PubMed] [Google Scholar]