Abstract
Purpose:
To assess the effect of oral re-esterified omega-3 fatty acids on tear osmolarity, matrix metalloproteinase-9 (MMP-9), tear break-up time (TBUT), Ocular Surface Disease Index (OSDI), fluorescein corneal staining, Schirmer score, meibomian gland dysfunction (MGD) stage and omega-3 index in subjects with dry eyes and confirmed MGD.
Methods:
This was a multicenter, prospective, interventional, placebo-controlled, double-masked study. Subjects were randomized to receive 4 softgels containing a total of 1680 mg of eicosapentaenoic acid/560 mg of docosahexaenoic acid or a control of 3136 mg of linoleic acid, daily for 12 weeks. Subjects were measured at baseline, week 6, and week 12 for tear osmolarity, TBUT, OSDI, fluorescein corneal staining, and Schirmer test with anesthesia. MMP-9 testing and omega-3 index were done at baseline and at 12 weeks.
Results:
One hundred five subjects completed the study. They were randomized to omega-3 (n = 54) and control group (n = 51). Statistically significant reduction in tear osmolarity was observed in the omega-3 group versus control group at week 6 (−16.8 ± 2.6 vs. −9.0 ± 2.7 mOsm/L, P = 0.042) and week 12 (−19.4 ± 2.7 vs. −8.3 ± 2.8 mOsm/L, P = 0.004). At 12 weeks, a statistically significant increase in omega-3 index levels (P < 0.001) and TBUT (3.5 ± 0.5 s vs. 1.2 ± 0.5 s, P = 0.002) was also observed. Omega-3 group experienced a significant reduction in MMP-9 positivity versus control group (67.9% vs. 35.0%, P = 0.024) and OSDI scores decreased significantly in omega-3 (−17.0 ± 2.6) versus control group (−5.0 ± 2.7, P = 0.002).
Conclusions:
Oral consumption of re-esterified omega-3 fatty acids is associated with statistically significant improvement in tear osmolarity, omega-3 index levels, TBUT, MMP-9, and OSDI symptom scores.
Key Words: dry eyes, omega-3 fatty acid, tear osmolarity, re-esterified omega-3, meibomian gland dysfunction
Dry eye disease (DED) is a common, yet complex, multifactorial progressive condition that can lead to visual loss, damage to the ocular surface, discomfort, and overall reduction in quality of life.1,2 Meibomian gland dysfunction (MGD) results in inadequate and dysfunctional lipid production, which leads to evaporative DED.3 MGD has, also, recently been shown to be a sign of hypercholesterolemia.4,5 Because MGD is associated with altered lipid composition, dietary supplementation with omega-3 fatty acids has been recommended in both the International Dry Eye Workshop and International Workshop on Meibomian Gland Dysfunction as primary therapy.2,3 With increased tear film evaporation, tear film osmolarity is elevated and results in ocular surface damage: epithelial cell desiccation, loss in glycocalyx,6 inflammation, and cell apoptosis.7 Essential fatty acids, including the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), perform numerous roles in the human body and are considered essential nutrients.8–10 They are important in the treatment and prevention of DED.11–13
The rationale for treatment with oral omega-3 supplementation in the management of meibomian gland disease may be explained by 2 different mechanisms of action. The breakdown of omega-3 fatty acids results in anti-inflammatory molecules that suppress the inflammatory pathways that are found in meibomian gland disease. In addition, the unstable tear film associated with meibomian gland disease results in evaporative dry eye. The oral supplementation of omega-3 fatty acids changes the fatty acid composition of the meibomian gland secretions resulting in a secretion that contains increased levels of unsaturated fatty acids, which are in a liquid state at body temperature preventing the blockage of the meibomian gland ducts and meibum stagnation. The increased quality of the meibomian gland secretions reduces tear film evaporation and the symptoms of DED.14
Several studies have previously shown the effectiveness of omega-3 fatty acid supplementation on DED. A large retrospective study of 32,470 women showed that women who consumed 5 to 6 servings of tuna fish per week (high in omega-3) had a 66% reduction in DED compared with women who consumed 2 or fewer servings a week.13 A randomized clinical trial investigated the effect of omega-3 fatty acids on meibomian gland secretions and found an improvement in Schirmer scores and fluorophotometry.15 Three studies have previously shown symptomatic improvement in meibomian gland disease with oral omega-3 supplementation.16–18
Substantial differences exist in the omega-3 preparations that are commercially available. Fish contains mercury and carcinogens, making it risky to consume sufficient omega-3 fatty acids by simply ingesting this food group. As an important safety precaution, almost all commercial fish oils employ a process of adding alcohol to detoxify these compounds. However, this addition of alcohol induces a chemical change in the natural triglycerides found in fish oil and converts the triglyceride to an ethyl ester compound. Our bodies have difficulties processing and absorbing the ethyl ester compound, which is not found in nature.19
Re-esterification is a process that removes the artificially induced alcohol in chemically modified ethyl ester fish oil to create a more natural form of omega-3 fatty acids that is not only better tolerated with less gastrointestinal side effects but also better absorbed than omega-3 fatty acids in the ethyl ester form.20 The great majority of commercially available omega-3 fatty acids are in the ethyl ester form, whereas a limited number of omega-3 fish oils are converted back to the more bioavailable triglyceride form. In this study, we evaluated the efficacy of a re-esterified omega-3 fatty acid in its natural triglyceride form (PRN Dry Eye Omega Benefits softgels; PRN Physician Recommended Nutraceuticals; Plymouth Meeting, PA). This study was designed to determine the effect of re-esterified omega-3 nutritional supplement versus placebo control over 12 weeks on the tear film, ocular surface, patient symptoms, and omega-3 blood levels.
MATERIALS AND METHODS
This was a multicenter, prospective, interventional, placebo-controlled, double-masked study. Subjects over 18 years of age were included in the study if they had a previously confirmed diagnosis of DED. Subjects with MGD stage 1 or 2, as defined by the Meibomian Gland Workshop,3 were included. Subjects with more advanced MGD and significant dropout of their meibomian glands (stage 3) would not be expected to respond to the study protocol and were therefore excluded from the study. The second requirement was a tear osmolarity of 312 mOsm/L or greater, in at least one eye using the TearLab Osmolarity System (TearLab, San Diego, CA) on 2 separate visits scheduled 1 week apart. Subjects were excluded from the study if: they were using topical cyclosporine 0.05%, topical corticosteroids, nonsteroidal anti-inflammatory drugs, glaucoma medications, or oral omega-3 fatty acids within 3 weeks of screening and anytime during their participation in the study. Subjects were instructed to discontinue contact lenses within 12 hours of any study visit. Subjects who underwent LASIK or PRK surgery within 1 year of screening visit or who were currently using a systemic medication that might affect the ocular surface also were also excluded. Computer use and artificial tears were allowed during the study. This study was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice and was approved by Western Institutional Review Board (Puyallup, WA).
Subjects were randomly assigned using a random number-generated sequence to ingest 4 softgels daily with meals containing a total of either 1680 mg of EPA/560 mg of DHA re-esterified omega-3 group or 3136 mg linoleic acid safflower oil as the control group for 12 weeks. Both active and control softgels seemed identical and were supplied in identical containers for masking purposes. Subjects were instructed to follow their usual diet and to record daily study softgel ingestion in a diary. Subjects were provided a daily reminder by text/email/voicemail to take the study supplements to encourage compliance. A window period of ±7 days was allowed for follow-up visits at week 6 and week 12. At the end of the week 12/exit visit, the softgel containers and subject logs were collected and remaining softgels counted as a measure of compliance to treatment.
Tear osmolarity was measured on a 50-nL sample collected from the inferior lateral meniscus at baseline and 6 and 12 weeks after treatment before any ocular examination using the TearLab Osmolarity System. Tear osmolarity was tested in both eyes, and the higher value from the 2 eyes was recorded for analysis. In all cases, tear osmolarity was performed before any examination of the patient or the application of eye drops. Subjects were instructed not to use any artificial tears for at least 2 hours before testing. They were also instructed to continue any over-the-counter habitual artificial tears at the same frequency and were instructed not to change the brand of drops being used. At baseline, 6 and 12 weeks posttreatment, slit-lamp examination included TBUT after instillation of sodium fluorescein solution, MGD assessment of the lid margin on a scale of 0 to 3 (0: normal; 1: mild stenosis +/or mild telangiectasia; 2: moderate stenosis +/or telangiectasia involving less than half of lid margin no notching; 3: Absolute stenosis +/or telangiectasia involving more than half lid margin +/or notching). Corneal staining was evaluated with fluorescein using Oxford staining scale 0 to5 (0: no staining, 5: severe staining). Symptoms were assessed using the Ocular Surface Disease Index (OSDI) questionnaire, and Schirmer test with anesthesia was performed at baseline and 6 and 12 weeks posttreatment. Matrix metalloproteinase-9 (MMP-9) level was assessed using the InflammaDry test (Rapid Pathogen Screening Inc, Sarasota, FL) at baseline and at week 12. Omega index blood samples were collected for omega-3 fatty acid profiling at baseline and week 12.
All categorical variables were summarized by treatment group with the frequency and percentage of subjects in each category and the continuous variables summarized descriptively with the number of subjects, mean, standard deviation, and minimum, maximum, and median values. Repeated measures analysis of variance model was used to compare the treatment differences for tear osmolarity, omega index variables, TBUT, OSDI, corneal staining, and MGD. The χ2 test was used to assess the treatment differences for MMP-9-positive enzyme biomarker categorical variable. All statistical analyses were conducted using SAS 9.4 (SAS, Cary, NC). P-value of less than 0.05 was considered statistically significant.
RESULTS
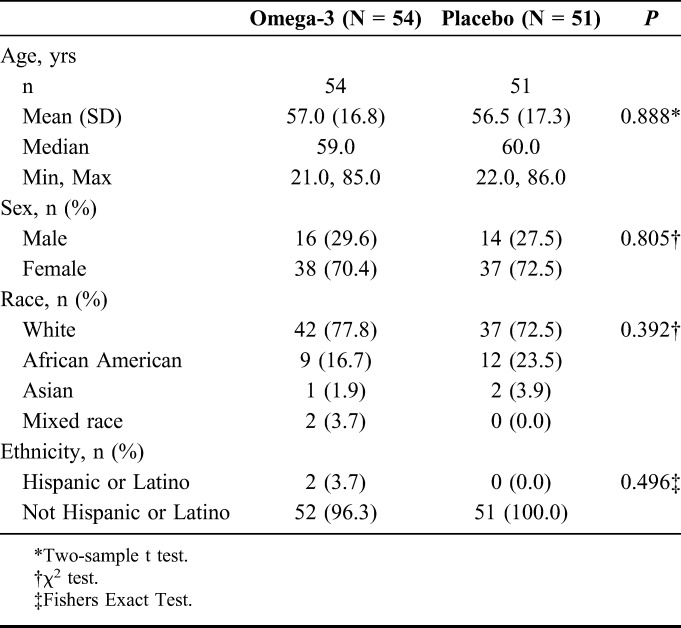
A total of 122 subjects were screened eligible for the study. Seventeen subjects dropped out over the course of 12 weeks, 7 in the omega-3 group and 10 in the control group. The mean age of 105 subjects who completed the study was 56.8 ± 17.0 years. Of these, 54 (51%) (mean age: 57.0 ± 16.8 yrs) were randomized to the omega-3 group and 51 (49%) (mean age: 56.5 ± 17.3 yrs) to the control group. The majority of subjects were female (n = 75) (71.4%) and the remaining baseline characteristics were similar among groups (Table 1).
TABLE 1.
Summary of Demographic Characteristics
The primary objective was to determine the effect of re-esterified omega-3 fatty acids on tear osmolarity. Tear osmolarity was similar at baseline in both omega-3 (326.2 ± 15.8 mOsm/L) and control groups (326.0 ± 15.4 mOsm/L, Table 2). At the 6-week follow-up, a significant reduction from baseline was measured in tear film osmolarity in the omega-3 group (−16.8 ± 2.6 mOsm/L) versus control group (−9.0 ± 2.7 mOsm/L, P = 0.042), which further decreased from baseline at 12 weeks in the omega-3 group (−19.4 ± 2.7 mOsm/L) versus control group (−8.3 ± 2.8 mOsm/L, P = 0.004) (Table 3, Fig. 1).
TABLE 2.
DED Parameters at Baseline and at the 6- and 12-wk Visits
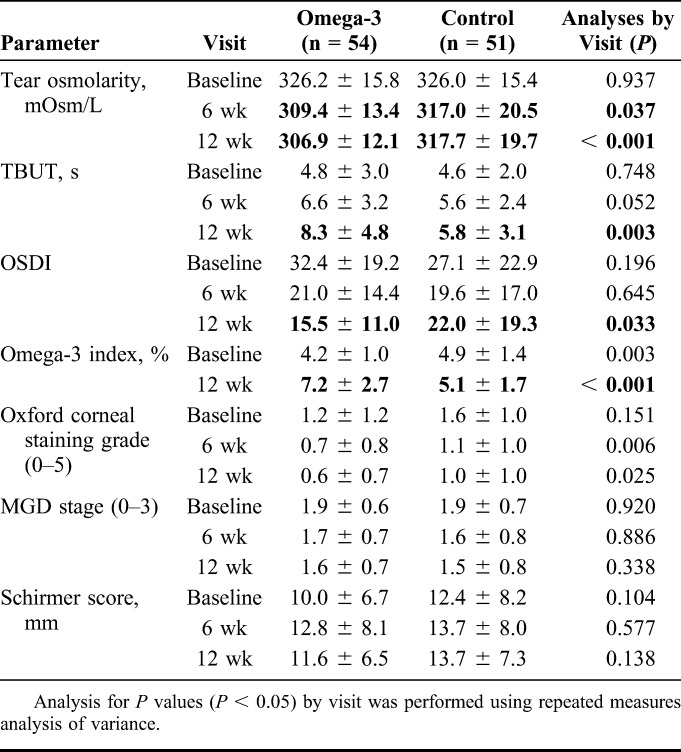
TABLE 3.
Change From Baseline (Using Least Squares Estimate)
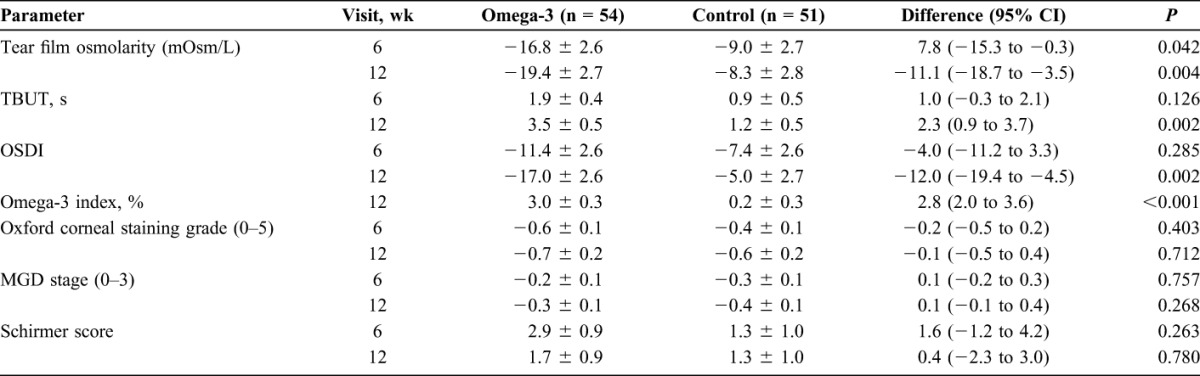
FIGURE 1.
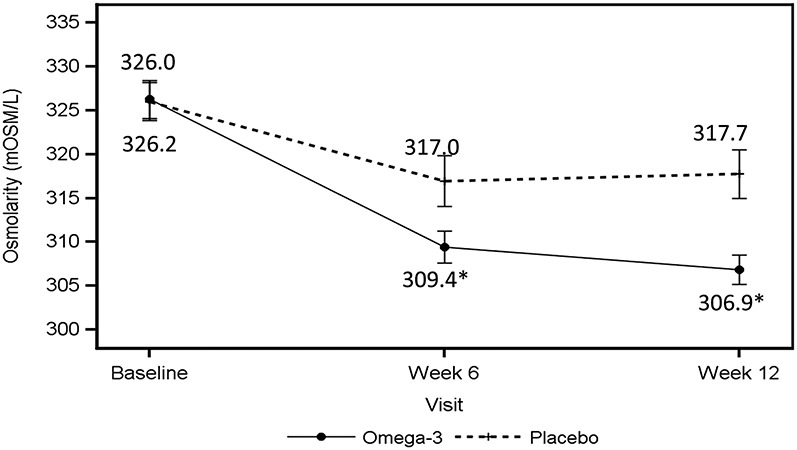
Tear osmolarity data at baseline and at the 6- and 12-week visits. The solid line indicates the average tear osmolarity values in the worst eye for the omega-3 group; the dashed line indicates the average tear osmolarity values in the worst eye for the control group. *P value < 0.05.
Secondary objectives assessed the effect of re-esterified omega-3s on omega-3 index scores, TBUT, MMP-9, symptom scores, corneal staining, Schirmer scores with anesthesia, and MGD stage. At baseline, omega-3 index score was slightly better in the control group versus the omega-3 group. At the 12-week follow-up visit, there was a statistically significant change (P < 0.001) in omega-3 index score in the omega-3 group increasing by 3.0 from 4.2 ± 1.0 to 7.2 ± 2.7 at week 12; no significant change was measured in omega-3 index score in the control group from baseline (4.9 ± 1.4 to 5.1 ± 1.7) at week 12 (Tables 2 and 3).
TBUT showed a significant improvement during the 12 weeks in the omega-3 group. At 6 weeks, there was a trend toward TBUT improvement in the omega-3 group, which was not statistically significant (P = 0.126), but the difference between the groups was significant at the 12-week visit (P = 0.002, Table 3 and Fig. 2). TBUT increased from 4.8 ± 3.0 seconds to 8.3 ± 4.8 seconds, a change from baseline of 3.5 ± 0.5 seconds in the omega-3 group versus 4.6 ± 2.0 seconds to 5.8 ± 3.1 seconds, a change from baseline of 1.2 ± 0.5 seconds in the control group (P = 0.002, Tables 2 and 3).
FIGURE 2.
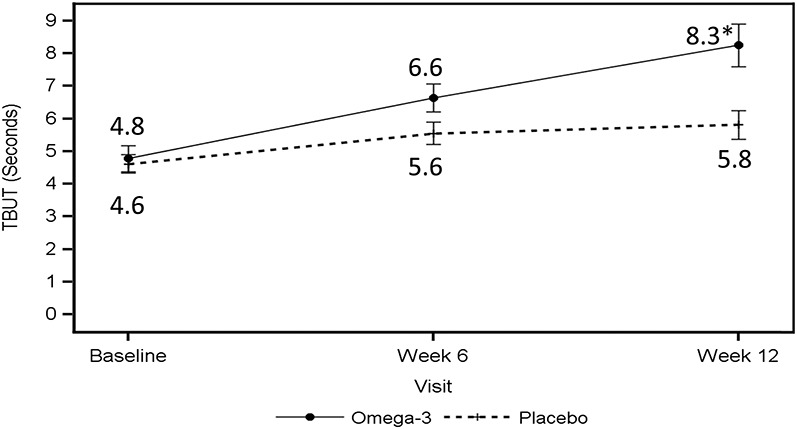
TBUT at baseline and at the 6- and 12-week visits. The solid line indicates the average TBUT in the worst eye for the omega-3 group; the dashed line indicates the average TBUT in the worst eye for the control group. *P value < 0.05.
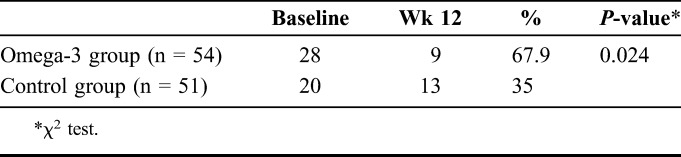
MMP-9 testing showed that the number of subjects with positive MMP-9 in the omega-3 group decreased from 28 positive subjects to 9 subjects (67.9%) over 12 weeks compared with a decrease from 20 subjects to 13 subjects (35.0%) in the control group (P = 0.024) (Table 4). There was no significant difference in the number of subjects testing MMP-9 negative between the 2 groups.
TABLE 4.
Frequency and Percentage of Patients With Positive Enzyme Biomarker at Wk 12 By Baseline Status
Both groups had similar OSDI symptom scores at baseline (Table 2 and Fig. 3). At 12 weeks, OSDI symptom scores in the omega-3 group dropped from 32.4 ± 19.2 to 15.5 ± 11.0 versus 27.1 ± 22.9 to 22.0 ± 19.3 in the control group (P = 0.002, Table 2 and 3). Both groups showed a decrease in symptoms at 6 weeks, but this change between the 2 groups was not significant (P = 0.285) at 6 weeks. The mean corneal fluorescein staining grade (measured by Oxford staining scale) at baseline was 1.2 ± 1.2 for the omega-3 group versus 1.6 ± 1.0 for the control group. Eighty-six of 105 subjects who completed the study had a staining grade ≤2 at baseline. At 6 weeks follow-up, it decreased to 0.7 ± 0.8 in the omega-3 group versus 1.1 ± 1.0 in the placebo group. It further decreased at week 12 to 0.6 ± 0.7 in the omega-3 group versus 1.0 ± 1.0 in the placebo group (Table 2). No significant difference was measured in the change from baseline for Schirmer score (P = 0.263 at week 6, P = 0.780 at wk 12; omega-3 vs. control group) or MGD stage (P = 0.757 at week 6, P = 0.268 at week 12; omega-3 vs. control group) at any of the study visits (Tables 2 and 3).
FIGURE 3.
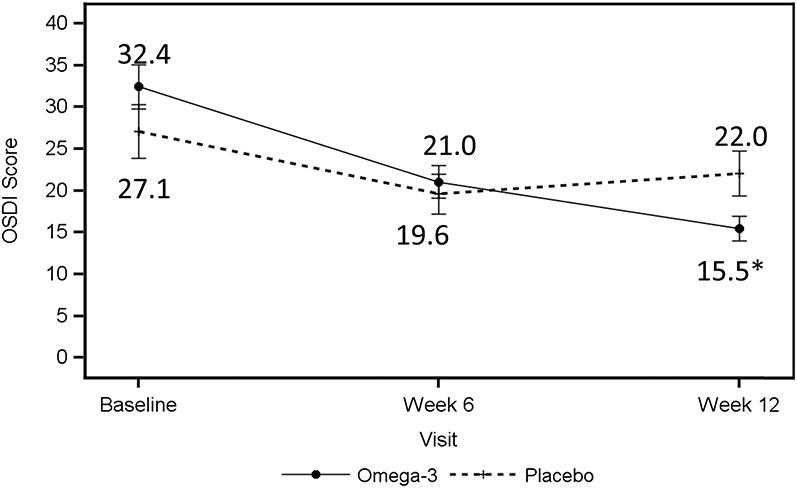
OSDI symptom scores reported at baseline and at the 6- and 12-week visits. The solid line indicates the average OSDI scores in the omega-3 group; the dashed line indicates the average OSDI scores in the control group. *P value < 0.05.
DISCUSSION
Oral supplementation with re-esterified omega-3 fatty acids improved both signs and symptoms of dry eyes over a 12-week period in this clinical trial. These results add to the growing volume of data demonstrating a correlation between omega-3 levels and dry eyes. One of the first studies to demonstrate the importance of omega-3 fatty acid ingestion on DED was the Harvard Women's Study, which showed a dose-dependent incidence of protection from dry eyes associated with fish consumption.13 Several other studies have shown an improvement in DED and a reduction in inflammation with omega-3 fatty acid consumption.11,12,21 Rosenberg and Asbell analyzed published literature on DED and omega-3 fatty acids and noted that a relationship between dry eye and omega-3 fatty acids exists; however, strong conclusions could not be made because of the limitations in research reported.22
Tear Osmolarity
Tear osmolarity has been reported to be a global, objective marker in both aqueous and evaporative DED and may be the most sensitive diagnostic tool for evaluating DED. Further, tear osmolarity has been shown to be significantly correlated to the severity of DED and can effectively track therapeutic response. A stable and normal osmolar tear film is critical to preventing epithelial stress and ocular surface damage.23–29 In the current study, no difference in tear osmolarity was measured between the 2 groups at baseline. The omega-3 treatment group had a significant decrease in tear film hyperosmolarity at 6 weeks (P = 0.042) relative to the control group. This decrease in tear hyperosmolarity was greater at 12 weeks (P = 0.004) in the omega-3 group. The significant reduction in osmolarity at 6 weeks preceded improvement in ocular symptoms. In this study, there was a nonsignificant improvement in tear osmolarity in the control group. This improvement, although not nearly as large as the improvement in the omega-3 group, may be because of the safflower control group, which is an omega-6 form of triglyceride, rich in linoleic and gamma-linolenic fatty acids. Previous studies have shown that omega-6 supplementation does improve dry eye signs and symptoms.30,31
Omega-3 Index
The omega-3 index measures the omega-3 level in the plasma and was very similar in both groups at the beginning of the study. The present study demonstrated a statistically significant increase in omega-3 index levels in subjects taking re-esterified omega-3 supplementation.
Tear Break-Up Time
There was a significant improvement in the TBUT scores in the omega-3 group at 12 weeks compared with control. This improvement suggests that dietary supplementation with a re-esterified formulation of omega-3 fatty acids improves the inherent stability of the tear film. It is likely that dietary supplementation of omega-3 fatty acids modifies the composition of the meibomian gland secretions, improving the quality of the meibum. We did not evaluate the characteristics of the meibum in the present trial.
The reduction in tear osmolarity and improved TBUT over the 12-week study period may have other benefits we did not measure. An improved tear film quality and stability may result in less visual fluctuation. Epitropoulos et al demonstrated that hyperosmolar eyes have inconsistent keratometry readings that can adversely impact intraocular lens selection in cataract patients.32 Other studies have reported increased variability in higher order aberrations and light scattering in DED compared with normal eyes.33–37
Matrix Metalloproteinase-9
The omega-3 group showed a significant reduction in subjects testing positive for MMP-9 bioenzyme in the tear film. MMP-9 is a proteolytic enzyme produced by stressed epithelial cells on the ocular surface in DED.38 This enzyme is elevated in mild, moderate, and severe cases of DED.39 The omega-3 group experienced a 67.9% reduction in MMP-9 positivity in a 12-week period, whereas the control group only experienced a decrease of 35.0% in the same time period. This supports the hypothesis that omega-3 fatty acids decrease the hyperosmotic stress on the ocular surface epithelium and directly reduce inflammation on the ocular surface.
Ocular Surface Disease Index
The improvement in the objective signs of DED correlate with the symptoms evaluated on OSDI. The study results show improvement in OSDI symptom scores by 17.0 ± 2.6 points in the omega-3 group at 12 weeks compared with the control group (P = 0.002), which is consistent with other studies.14,17,19,40 The improved tear film quality and decrease in inflammation could explain the improvement in OSDI symptom scores in the treatment group. The improvement in symptomatology was not significant at 6 weeks but did become statistically significant at 12 weeks. The delay in improvement in symptoms is expected, as symptoms take longer to resolve than signs.
Corneal Staining, Schirmer Score, MGD Stage
Corneal staining decreased in both groups, but was not statistically significant between the 2 groups. This could be because of a staining grade of <1.6 at baseline in each group (Table 2) and 81.9% (86/105) of subjects having a staining grade ≤2. This finding on corneal staining is consistent with Schargus et al,41 who concluded that corneal staining is likely a late-stage sign that is rarely overexpressed in mild to moderate dry eye subjects. Although our findings showed no effect on tear volume after dietary supplementation as measured by Schirmer test with anesthesia and are consistent with one study, others found slight improvements in Schirmer scores but no symptomatic improvement.42–44 This reconfirms the notion that the Schirmer test is highly variable and may limit its role in DED evaluation.45 In the FDA trial of cyclosporine for the treatment of DED, there was, however, a significant improvement noted in Schirmer scores at 6 months.46 Our study showed no significant change in MGD assessment; however, as shown in Table 2, there was a trend toward more improvement. We believe that visual changes in the meibomian glands may require longer follow-up to see the effect on MGD staging.
In conclusion, the results from this study demonstrated a significant improvement in dry eye signs and symptoms from baseline with the oral ingestion of re-esterified omega-3 supplements for 12 weeks compared with those taking a control. The improvement of many of the signs was seen as early as 6 weeks, suggesting a rapid response to nutritional therapy. The increased bioavailability of the triglyceride form of omega-3 nutritional supplementation has been well established. However, the use of a re-esterified omega-3 fatty acid was not compared with the ethyl ester form of omega-3 fatty acids so the advantage of the increased bioavailability of the re-esterified omega-3 form was not assessed. The improvement in signs and symptoms for dry eyes as seen in this study support the recommendation that dietary supplementation of re-esterified omega-3 fatty acids should be included as a primary therapy for dry eyes.
Footnotes
A. T. Epitropoulos, E. D. Donnenfeld, Z. A. Shah, E. J. Holland, M. Gross, W. J. Faulkner, C. Matossian, S. S. Lane, M. Toyos, and F. A. Bucci Jr received compensation from PRN Physician Recommended Nutraceuticals for participating in the study. The remaining author has no funding or conflicts of interest to disclose.
REFERENCES
- 1.Clegg JP, Guest JF, Lehman A, et al. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13:263–274. [DOI] [PubMed] [Google Scholar]
- 2.The definition and classification of dry eye disease: report of the International Dry Eye Workshop. Ocul Surf. 2007;5:65–204. [DOI] [PubMed] [Google Scholar]
- 3.The international workshop on meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:1917–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dao AH, Spindle JD, Harp BA, et al. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am J Ophthalmol. 2010;150:371–375. [DOI] [PubMed] [Google Scholar]
- 5.Pinna A, Biasetti F, Zinellu A, et al. Meibomian gland dysfunction and hypercholesterolemia. Ophthalmology. 2013;120:2385–2389. [DOI] [PubMed] [Google Scholar]
- 6.Gilbard JP, Carter JB, Sang DN, et al. Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology. 1984;91:1205–1212. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007;26:452–460. [DOI] [PubMed] [Google Scholar]
- 8.Linscheer WG, Vergroesen AJ. Lipids. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease. 8th ed Philadelphia, PA: Lea and Febiger; 1994. [Google Scholar]
- 9.Barnard N. Foods That Fight Pain. New York, NY: Harmony Books; 1998. [Google Scholar]
- 10.Omega-3 fatty acids and depression: new data. Harv Ment Health Lett. 2003;19:7. [PubMed] [Google Scholar]
- 11.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term supplementation with n-6 and n-3 PUFAs improves moderate- to–severe keratoconjunctivitis sicca: a randomized double-blind clinical trial. Cornea. 2013;32:1297–1304. [DOI] [PubMed] [Google Scholar]
- 12.Pinna A, Piccinini P, Carta F. Effect of oral linoleic acid and gamma-linoleic acid on meibomian gland dysfunction. Cornea. 2007;26:260–264. [DOI] [PubMed] [Google Scholar]
- 13.Miljanovic B, Trivedi KA, Dana MR, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction. Trans Am Ophthalmol Soc. 2008;106:336–356. [PMC free article] [PubMed] [Google Scholar]
- 15.Wojtowicz JC, Butovich I, Uchiyama E, et al. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–314. [DOI] [PubMed] [Google Scholar]
- 16.Olenik A. Effectiveness and tolerability of dietary supplementation with a combination of omega-3 polyunsaturated fatty acids and antioxidants in the treatment of dry eye symptoms: results of a prospective study. Clin Ophthalmol. 2014;8:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhargava R, Kumar P, Kumar M, et al. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olenik A, Jimenez-Alfaro I, Alejandre-Alba N, et al. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creuzot C, Passemard M, Viau S, et al. Improvement in dry eye symptoms with polyunsaturated fatty acids. J Fr Ophthalmol. 2006;29:868–873. [DOI] [PubMed] [Google Scholar]
- 20.Dyerberg J, Madsen P, Moller JM, et al. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot Essent Fatty Acids. 2010;83:137–141. [DOI] [PubMed] [Google Scholar]
- 21.Gumus K, Cavanagh DH. The role of inflammation and anti-inflammation therapies in keratoconjunctivitis sicca. Clin Ophthalmol. 2009;3:57–67. [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul Surf. 2010;8:18–28. [DOI] [PubMed] [Google Scholar]
- 23.Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792 e1–798 e1. [DOI] [PubMed] [Google Scholar]
- 24.Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35:553–564. [DOI] [PubMed] [Google Scholar]
- 25.Tomlinson A, Khanal S, Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. [DOI] [PubMed] [Google Scholar]
- 26.Jacobi C, Jacobi A, Kruse FE, et al. Tear film osmolarity measurements in dry eye disease using electrical impedance technology. Cornea. 2011;30:1289–1292. [DOI] [PubMed] [Google Scholar]
- 27.Nelson JD, Farris RL, Sodium hyaluronate and polyvinyl alcohol artificial tear preparations. A comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol. 1988;106:484–487. [DOI] [PubMed] [Google Scholar]
- 28.Larmo PS, Järvinen RL, Setälä NL, et al. Oral sea buckthorn oil attenuates tear film osmolarity and symptoms in individuals with dry eye. J Nutr. 2010;140:1462–1468. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan BD, Crews LA, Sonmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31:1000–1008. [DOI] [PubMed] [Google Scholar]
- 30.Barabino S, Rolando M, Camicione P, et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22:97–101. [DOI] [PubMed] [Google Scholar]
- 31.Aragona P, Bucolo C, Spinella R, et al. Systemic Omega-6 essential fatty acid treatment and PGE1 tear content in Sjogren's syndrome patients. Invest Ophthalmol Vis Sci. 2005;46:4474–4479. [DOI] [PubMed] [Google Scholar]
- 32.Epitropoulos AE, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672–1677. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Xu J, Sun X, et al. Dynamic wavefront aberrations and visual acuity in normal and dry eyes. Clin Exp Optom. 2009;92:267–273. [DOI] [PubMed] [Google Scholar]
- 34.Montes-Mico R, Alio JL, Charman WN. Dynamic changes in the tear film in dry eyes. Invest Ophthalmol Vis Sci. 2005;46:1615–1619. [DOI] [PubMed] [Google Scholar]
- 35.Denoyer A, Rabut G, Baudouin C. Tear film aberration dynamics and vision-related quality of life in patients with dry eye disease. Ophthalmology. 2012;119:1811–1818. [DOI] [PubMed] [Google Scholar]
- 36.Himebaugh NI, Nam J, Bradley A, et al. Scale and spatial distribution of aberrations associated with tear breakup. Optom Vis Sci. 2012;89:1590–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh S, Maeda N, Ikeda C, et al. Ocular Forward light scatter and Corneal backward light scattering in patients with dry eye. Invest Ophthalmol Vis Sci. 2014;55:6601–6606. [DOI] [PubMed] [Google Scholar]
- 38.Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambursky R, Davitt WF, III, Friedberg M, et al. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014;33:812–818. [DOI] [PubMed] [Google Scholar]
- 40.Kangari H, Eftekhari MH, Sardari S, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmol. 2013;120:2191–2196. [DOI] [PubMed] [Google Scholar]
- 41.Schargus M, Ivanova S, Kakkssery V, et al. Correlation of tear film osmolarity and 2 different MMP-9 tests with common dry eye tests in a cohort of non-dry eye patients. Cornea. 2015;34:739–744. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–6130. [DOI] [PubMed] [Google Scholar]
- 43.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms of dry eye puzzle. Ocul Surf. 2012;10:2–14.22330055 [Google Scholar]
- 44.Begley C, Caffery B, Chalmers R, et al. Use of the Dry Eye Questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficiency. Cornea. 2002;2:664–670. [DOI] [PubMed] [Google Scholar]
- 45.Cedarstaff TH, Tomlinson A. Human tear volume, quality and evaporation: a comparison of Schirmer, tear break-up time and resistance hygrometry techniques. Ophthalmic Physiol Opt. 1983;3:239–245. [PubMed] [Google Scholar]
- 46.Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA phase 3 study group. Ophthalmology. 2000;107:631–639. [DOI] [PubMed] [Google Scholar]


