Abstract
Phosphoinositides are key players in many trafficking and signaling pathways. Recent advances regarding the synthesis, location and functions of these lipids have dramatically improved our understanding of how and when these lipids are generated and what their roles are in animal physiology. In particular, phosphoinositides play a central role in insulin signaling, and manipulation of PtdIns(3,4,5)P3 levels in particular, may be an important potential therapeutic target for the alleviation of insulin resistance associated with obesity and the metabolic syndrome. In this article we review the metabolism, regulation and functional roles of phosphoinositides in insulin signaling and the regulation of energy metabolism. This article is part of a Special Issue entitled Phosphoinositides.
Keywords: Phosphatidylinositol; PtdIns(3,4,5)P3; Akt; mTORC1; Phosphorylated phosphatidylinositides; GLUT4
1. Introduction
The regulation of cellular metabolism by hormones and biogenic amines is central to normal metabolic homeostasis. Disruption of these signaling pathways is a key molecular theme during pathophysiological conditions such as metabolic syndrome and obesity [1–3]. Phosphoinositides are key molecular mediators of the anabolic functions of insulin and as such, are key regulators of metabolism. This review will discuss the role of phosphoinositides in insulin action and secretion, and how their dysregulation can provide insights into the pathogenesis of metabolic diseases.
2. How are phosphoinositide levels controlled?
Inositol phospholipids (PtdIns) can exist in one of eight molecular species, via phosphorylation at the 3, 4 or 5 position of the inositol head-group. Polyphosphoinositides are enzymatically generated by a series of phosphorylation or dephosphorylation reactions. In general, phosphoinositide kinases and phosphatases are able to specifically phosphorylate or dephosphorylate only one position on the inositol ring, though the substrate (and therefore product) may vary. The local levels of phosphoinositide species are regulated by the activity of these kinases and phosphatases, as well as the subcellular restriction of the enzymes. In most cases, this is accomplished via adaptor proteins that can regulate the activity and direct the enzyme to the correct subcellular location. Since phosphoinositides are embedded in lipid bilayers, they cannot move through the cytoplasm unless their lipid microenvironment is changed as well. This restricts these lipids to specific locations in cellular membranes. Once generated, phosphorylated phosphoinositides often function by recruiting adaptor proteins, which propagate molecular interactions to initiate phosphorylation cascades. This review will focus on the molecular roles of these lipids in regulating pathways that control glucose, lipid and protein metabolism.
Inmost cells, PtdIns(3,4)P2 and PtdIns(3,4,5)P3 are the lowest abundance phosphoinositides, and are generated primarily by phosphorylation of PtdIns(4,5)P2 the four Class I PI3-Kinase enzymes (α, β, δ, and γ; encoded by the genes PIK3CA, PIK3CB, PIK3CD and PIK3CG). Each of these kinases interacts with subunits that regulate their activity and localization. The Class IB PI3K, p110γ, is activated by GPCR signaling and plays an important role in inflammation (reviewed in [4,5]). The other three Class I PI3K isoforms (named Class IA), are present in complexes with the regulatory subunits p55 and p85. These regulatory subunits contain phosphotyrosine-binding SH2 domains, which recruit the catalytic p110 enzymes to receptors or receptor-associated adapter proteins upon tyrosine phosphorylation [6,7]. The PI3K regulatory subunits may also be activated by other protein–protein interactions including the small GTPases Ras [8,9] and Rab5 [10], as well as the lipid phosphatase PTEN [11–13]. Once recruited to tyrosine phosphorylated receptors, the Class I PI3K enzymes phosphorylate PtdIns(4,5)P2 to produce PtdIns(3,4,5)P3 and initiate downstream signaling events.
In the case of insulin signaling, the adaptor proteins that link the regulatory subunits to the activated receptors are the Insulin Receptor Substrates (IRS1, IRS2 and IRS4). In the case of insulin resistance, due to either inflammation or mTORC1 activation, these subunits may be serine phosphorylated on several sites [14–20]. This prevents tyrosine phosphorylation of IRS proteins, reducing the recruitment of PI3K and preventing the formation of PtdIns(3,4,5)P3 [21–25]. Importantly, this means that the pathophysiological states associated with insulin resistance involve reduced PtdIns(3,4,5)P3 synthesis.
PtdIns(3,4,5)P3 increases are typically transient in nature as the phospholipid is rapidly degraded following its synthesis. There are three mechanisms by which levels of PtdIns(3,4,5)P3 can be reduced, each of which depends on the activity of a lipid phosphatase acting at the 3, 4 or 5 position of the inositol ring. The best studied negative regulator of PtdIns(3,4,5)P3 is PTEN, a 3-phosphatase that catalyzes the conversion of PtdIns(3,4,5)P3 to PtdIns(4,5)P2. PTEN was first identified as a tumor suppressor in 1997 [26,27], then as a PtdIns(3,4,5)P3 3-phosphatase in 1998 [28]. PtdIns(3,4,5)P3 levels can be also reduced by the 5-phosphatases such as SHIP-2 (encoded by INPPL1) or SKIP (INPP5K) [29]. In contrast to PTEN, the 5-phosphatases generate PtdIns(3,4)P2 rather than PtdIns(4,5)P2 from PtdIns(3,4,5)P3. Depletion of either SHIP or INPP5K results in increased PtdIns(3,4,5)3 levels, while enhancing Akt signaling in both cultured cells and animals [30–36].
Although PtdIns(3,4)P2 is a degradation product of PtdIns(3,4,5)P3, some data indicates that this lipid can also support Akt activation via interactions with the PH domain of the kinase [37]. Furthermore, PtdIns(3,4)P2 can itself be degraded by the 4-phosphatases INPP4A and INPP4B [38,39]. Work by several groups has shown that like PTEN, INPP4B functions as a tumor suppressor, and that depletion of this enzyme leads to increased Akt signaling [40,41]. These data suggest that sustained levels of PtdIns(3,4)P2 are able to maintain Akt signaling, though whether this is functionally different from PtdIns(3,4,5)P3-supported Akt activity has not yet been established. Notably, in the case of sustained Akt activation in the absence of INPP4B, the end product of this phosphoinositide pathway is PtdIns(3)P rather than PtdIns(4,5)P2. It remains uncertain whether negative regulation of Akt by 4-phosphatases differs from negative regulation by 5-phosphatases, and this point is important for insulin-sensitizing interventions that aim to inhibit these catalytic activities.
3. Regulation of Akt signaling pathways by PtdIns(3,4,5)P3
The protein kinase Akt was first identified as the homolog of a viral oncogene v-Akt [42], and has three isoforms in mammalian genomes. In addition to a carboxy-terminal AGC kinase domain, each isoform of Akt has an amino-terminal Pleckstrin homology (PH) domain which is able to bind PtdIns(3,4)P2 or PtdIns(3,4,5)P3 [37]. Correspondingly, the activation and phosphorylation of Akt by growth factors are dependent on the synthesis of these phospholipids, and can be blocked by inhibitors of PtdIns(3,4,5)P3 synthesis such as wortmannin [43–45].
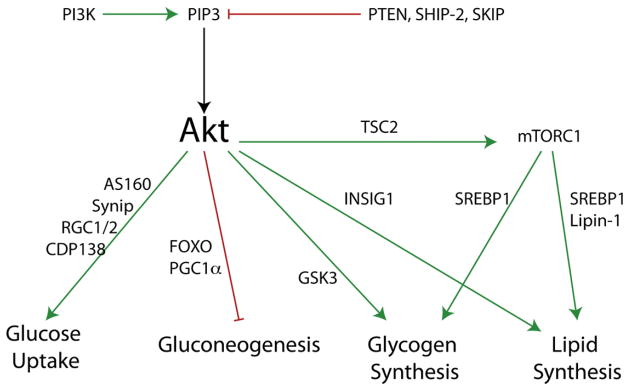
The activation of Akt is a multi-step process involving the recruitment of Akt to the plasma membrane via direct binding to PtdIns(3,4,5)P3 or PtdIns(3,4)P2, bringing the enzyme within proximity of two activating protein kinases, PDK1 and mTORC2 (mTOR Complex 2), which phosphorylate Akt on Threonine 308 and Serine 473, respectively. Phosphorylation of both of these residues is required for complete activation of Akt [46,47]. The role of phosphatidylinositol-3-kinases in mTORC2 activity is unclear, and the degree to which mTORC2 function is increased by insulin remains uncertain. However, PDK1 has a PH domain which is itself able to bind PtdIns(3,4,5)P3, PtdIns(3,4)P2, and more weakly PtdIns(3)P and PtdIns(4,5)P2 [48]. Ablation of the PDK1 lipid binding activity results in a complete blockade in Akt phosphorylation in embryonic fibroblasts [49]. Once activated, Akt is able to phosphorylate substrates essential for suppressing gluconeogenesis, enhancing glucose uptake, glycogen and lipid synthesis (Fig. 1).
Fig. 1.
Roles of the PtdIns(3,4,5)P3/Akt pathway on insulin signaling in liver fat and muscle tissue with representative substrates indicated.
4. Regulation of mTORC1 signaling by PI(3,4,5)P3 and PI(3,5)P2
The nutrient- and growth factor-responsive protein kinase complex TOR complex 1 (TORC1) is regulated by phosphoinositides via at least two separate mechanisms. Early studies revealed that the PI3K inhibitor wortmannin blocks activation of mTORC1 by insulin [50,51], and it was later shown that this inhibition was at least in part due to the Akt-mediated inactivation of the Rheb GAP complex TSC1/2 [52–56].
The localization of mTORC1 to the lysosome is also as an important component of activation. A second set of small GTPases, the Rag GTPases, along with the vacuolar ATPase are also important regulators of TORC1 activation and lysosomal localization [57–61]. In addition to this, recent work has supported the hypothesis that the TSC1/2 complex also must be recruited to the lysosome [62]. The localization of mTORC1 to the lysosome is inhibitory, and its release from the lysosome is essential for its activation [63]. The molecular events that modulate the binding and release of TORC1 are still not completely understood, but work by our group and others has implicated PtdIns(3)P and PtdIns(3,5)P2 as playing a role in this process [64–69]. The loss of PtdIns(3,5)P2 disrupts the localization of TORC1 to the lysosome in both yeast and mammalian systems potentially via direct binding of Raptor to this lipid [64,66]. The loss of either PtdIns(3)P or PtdIns(3,5)P2 has been associated with reduced TORC1 activity in both yeast and mammalian systems [64–69]. Alternately, the PtdIns(3)P binding protein phospholipase D has also been identified as another activator of TORC1 [68,70–73]. The precise molecular events by which these lipids modulate the activation cascade of TORC1 are still under intense investigation. Once activated, mTORC1 plays an essential role in the regulation of protein, lipid and glycogen synthesis (see below).
5. Regulation of GLUT4 trafficking by multiple phosphoinositides
Insulin stimulates glucose uptake into peripheral tissues, primarily fat and muscle. In adipocytes and muscle cells, this is largely dependent on the facilitative glucose transporter GLUT4 (encoded by SLC2A4; [74,75]). This transporter is normally sequestered in internal compartments, but upon insulin stimulation, these vesicles fuse with the plasma membrane, allowing glucose to travel down a concentration gradient into cells (this was recently reviewed in [76–78]). The recycling, translocation and fusion of GLUT4-containing vesicles in response to insulin can be broken down into several general steps, each of which is regulated by several distinct phosphoinositide species.
Studies from the early 1990s showed that the PI3-Kinase inhibitor wortmannin blocks insulin stimulated glucose uptake and GLUT4 translocation in multiple cell types [79–81]. Further work employing Akt inhibitors and knockdowns has suggested that the effects of wortmannin may be mediated through a PtdIns(3,4,5)P3–Akt signaling pathway [82–86]. Once activated by PtdIns(3,4,5)P3, Akt has several important targets in GLUT4 trafficking. The Rab10 family GTPase activating protein (GAP) AS160 (encoded by TBC1D4) is phosphorylated and inactivated by insulin, allowing for translocation of GLUT4 storage vesicles (reviewed in [76,78,87,88]). Inactivation of AS160 leads to the increased activity of its targets, the Rab10 family GTPases. However, the direct activation of these G proteins by insulin has not been demonstrated, and the downstream effectors of Rab10 have not been defined [89,90].
Another recently described Akt substrate in GLUT4 translocation is the RGC1/2 complex [91–94]. Akt works in concert with 14–3–3 proteins to inhibit this RalGAP allowing for activation of that GTPase on Glut4-containing vesicles [94]. Once activated, RalA targets these vesicles to the plasma membrane by interacting with the exocyst complex, which is assembled in response to insulin and is required for efficient fusion of GLUT4 vesicles [93,95,96].
The final fusion step of GLUT4 trafficking has been proposed to be regulated by two other Akt substrates. Synip (encoded by STXBP4), a masking protein for the targeting SNARE syntaxin-4 has been proposed to be phosphorylated and dis-inhibited by Akt as an important positive regulator of GLUT4 fusion [97,98], but this finding has been controversial [99]. A more recent report has implicated CDP138 (CDCD5) in the regulation of GLUT4 fusion. Loss of this Akt substrate appears to have no effect on translocation of vesicles, but blocks fusion events [100]. Interestingly, the C2 domain of this protein has been shown to interact with mixed intracellular lipids, but whether it interacts with specific phosphoinositides is currently unknown.
Aside from PtdIns(3,4,5)P3-dependent Akt substrates, PtdIns(4,5)P2 has also been proposed to be necessary for vesicle exocytosis. This phospholipid is essential for clustering and activation of t-SNAREs in a variety of exocytic contexts [101–103], and also interacts directly with a number of exocyst components. Both Exo70 [104,105] and Sec3 [106] interact with PtdIns(4,5)P2, but the relevance of these interactions to insulin-stimulated GLUT4 trafficking has not yet been examined.
In addition to its role in GLUT4 exocytosis, PtdIns(4,5)P2 is also an essential mediator of endocytosis (reviewed in [107]). Since GLUT4 must be retrieved from the plasma membrane and sorted, endocytosis is an essential compartment of GLUT4 biology. Cargo is internalized first into compartments that are PtdIns(3,4,5)P3 and PtdIns(4,5)P2 positive. These very early endosomal structures are also positive for the small GTPase Rab5 and the adaptor protein APPL1. As these vesicles mature, the levels of PtdIns(3,4,5)P3 and PtdIns(4,5)P2 decrease, where as the levels of PtdIns(3)P increase [108,109]. In most cells, PtdIns(3)P exists primarily on early endosomal structures. Along with Rab5, PtdIns(3)P serves as a coincidence detector for EEA1 and plays an essential role in homotypic endosomal fusion. This aggregation process feeds into the recycling endosomeand is important for the internalization and sorting of membrane components, including GLUT4.
PtdIns(3)P is likely generated by the recruitment of the two Rab5 PI3-Kinase effectors, p110β and Vps34 [110,111]. These very early, APPL1 positive endosomal structures also serve as signaling platforms for Akt and EGF signaling [109,112–114]. The effect of these transient structures appears to be context dependent. In some instances, the loss of APPL1 leads to decreased Akt signaling [113,114], whereas in other instances, such as phagocytosis and EGF signaling, APPL1 serves as a negative regulator of signaling [109,115]. These studies have been largely performed in undifferentiated dividing cells, and it will be interesting to test the role of APPL1 and PtdIns(3)P positive endosomal structures in GLUT4 internalization. Ablation of APPL1 is inhibitory towards insulin-stimulated glucose uptake in cultured adipocytes, but it is difficult to decouple the effects of this knockdown on Akt signaling from other potential trafficking effects [114]. GLUT4 continuously traffics between the plasma membrane and various pools of internalized, recycled and pre-exocytic vesicles. Therefore maintenance of internal pools of PtdIns(3)P is likely essential for the efficient movement of GLUT4 through these stages.
In addition to the roles of PtdIns(3)P in endosomal trafficking, cultured myocytes and adipocytes also have a second major pool of this lipid at or near the plasma membrane [116–119]. The precise role of this PtdIns(3)P pool is currently unclear, but reductions in this pool are co-incident with reductions in insulin-stimulated GLUT4 trafficking and glucose transport [116–120]. Furthermore, exogenous transfection of this lipid into cultured cells results in increased translocation, but not fusion of GLUT4 with the plasma membrane [121]. These data suggest that in insulin responsive tissues, PtdIns(3)P may play a role in the recruitment of GLUT4 to a location proximal to sites of fusion whereas PtdIns(3,4,5)P3 may play a more dominant role in vesicle fusion.
In addition to the well documented roles of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 in GLUT4 trafficking, there have also been reports of positive roles of PtdIns(3,5)P2 or PtdIns(5)P in the regulation of insulin-stimulated glucose transport. Synthesis of PtdIns(3,5)P2 and PtdIns(5)P obligatorily requires a single kinase PIKFYVE [122,123]. Overexpression of dominant interfering mutants, and knockdown experiments suggests a correlation between reduced PtdIns(3,5)P2 or PtdIns(5)P levels with reduced insulin-stimulated GLUT4 translocation and glucose uptake [124–128]. A muscle-specific Pikfyve knockout mouse exhibited modest insulin resistance and reduced glucose disposal in muscle tissues, and potentially secondary to that, increased adiposity on normal chow diet [129]. These data implicate PIKFYVE as a positive regulator of GLUT4 trafficking, though the exact stages of GLUT4 trafficking and the specific molecular targets of PtdIns(3,5)P2 or PtdIns(5)P are still not yet known.
6. Insulin secretion
Phosphoinositides also play a role in the glucose-dependent secretion of insulin from pancreatic beta cells. Similar to its role in t-SNARE assembly for GLUT4 exocytosis, PtdIns(4,5)P2 has also been shown to be essential for insulin release from pancreatic beta cells. Direct application of PtdIns(4)P or PtdIns(4,5)P2 to cultured beta cells promotes the priming of insulin granules [130,131]. Furthermore, either sequestration of PtdIns(4,5)P2 by overexpression of the PtdIns(4,5)P2-binding GFP-PLCδ1 [131,132], or by anti-PtdIns(4,5)P2 antibodies [130] reduces insulin secretion from beta cells. Finally siRNA-mediated depletion of either PI4Kβ (which generates PtdIns(4)P) or PIP5Kγ (which generates PtdIns(4,5)P2 from PtdIns(4)P) also reduces insulin secretion in cultured beta cells [131]. In addition to this, PtdIns(3,4,5)P3-dependent Akt signaling also plays a positive feedback role in insulin secretion, both via beta cell proliferation, and direct effects on insulin release [133–135]. Finally, PtdIns(3)P may also play a role in regulated exocytosis of insulin vesicles. Recent work has uncovered a positive role for PI3K-C2α, which generates PtdIns(3)P, in insulin exocytosis [136], perhaps reflecting a general pro-exocytic role of this phospholipid, such as has been described in neurosecretory cells [137,138].
7. Akt-dependent regulation of gluconeogenesis
The major function of insulin in the liver is to suppress gluconeogenesis. This is accomplished primarily via PtdIns(3,4,5)P3/Akt-dependent regulation of the pro-gluconeogenic transcription factor FOXO1 [139–142]. Once this transcription factor is phosphorylated, it exits the nucleus and is not transcriptionally active. Interestingly, in cases of obesity-induced insulin resistance, gluconeogenesis is impaired but lipogenesis is still active, implying either differential sensitivity of these Akt-dependent processes, or alternative Akt-independent mechanisms that are activated in obese states [139,143,144].
8. Glycogen and lipid synthesis
Both glycogen and lipid storage in metabolically-responsive tissues are largely dependent on Akt signaling pathways. Inmuscle and adipose tissue, both substrate availability (via glucose uptake), and lipid and glycogen synthesis are sensitive to wortmannin [145,146]. The direct pathways by which PtdIns(3,4,5)P3 regulates lipid synthesis involve both Akt and mTORC1 dependent effects, as well as transcriptional and post-translational changes [147–151]. For example, Akt inactivates GSK3, which is a negative regulator of glycogen synthase [152,153]. However, recent models wherein Akt is unable to inhibit GSK3 have called into question the physiological relevance of GSK inhibition on glycogenesis [154–156]. Our group and others have implicated mTORC1-dependent activation of SREBP1 as a novel regulator in liver glycogen synthesis [151,157]. Similarly, a mTORC1/SREBP1 pathway plays an important role in lipogenesis in concert with the effects of mTORC1 on the phosphatidic acid phosphatase Lipin [143,148,158–160].
9. In vivo studies regarding the role of phosphoinositides in metabolism
Due to the key role of phosphoinositides in several metabolically relevant biological processes, there have been intensive studies directed towards understanding their roles in whole-animal physiology. This has been done primarily using knockout mouse or inhibitor studies as detailed below.
10. Decreases in PtdIns(3,4,5)P3 and PtdIns(3,4)P2 levels lead to insulin resistance
The majority of animal models describing phosphoinositide disruption of enzymes involve the synthesis and degradation of PtdIns(3,4,5)P3 and PtdIns(3,4)P2, both of which are generated by Class IA PI3-kinases. Ablation of the most widely expressed catalytic isoforms (α and β) both result in embryonic lethality [161,162]. Somewhat surprisingly, a kinase-dead knock-in for Pik3cb (p110β) is not embryonic lethal, suggesting that it is the absence of the protein rather than the loss of catalytic activity that leads to the lethality [163]. Transgenic mice expressing kinase-dead Pik3cb have mild peripheral insulin resistance. Expression of an inactivating kinase domain mutation in Pik3ca is embryonic lethal when homozygous, and peripherally insulin resistant as a heterozygote [164]. While heterozygotes mutants of Pik3ca and Pik3cb have not been shown to have a metabolic phenotype, compound double Pik3ca/Pik3c heterozygotes have mild glucose and insulin intolerance [165]. Together, these data suggest that global reductions of Pik3ca, and to a lesser extent Pik3cb lead to peripheral insulin resistance, likely due to an inability of insulin to activate Akt-dependent processes.
To test tissue-specific roles of these isoforms, floxed alleles have been generated for both Pik3ca and Pik3cb. Liver-specific knockout of Pik3ca, either through use of albumin-Cre or transfection with adenoviral Cre ablates insulin-stimulated PI3K activity, with associated reductions in Akt phosphorylation in the liver. Based on both insulin-tolerance tests, and hyperinsulinemic/euglycemic-clamp studies, insulin suppression of endogenous glucose production is impaired [166]. This is consistent with the phenotype of hepatic insulin resistance for both acute and chronic depletion of Pik3ca in the liver. Interestingly, chronic reductions of liver Pik3ca on a normal chow diet does not cause significant reductions in the serum lipid profile [166,167], as is the case for acute reduction of liver Pik3ca or liver insulin-receptor knockout mice [166,168]. There were, however, reductions in high-fat diet induced hypercholesterolemia and hepatosteatosis [167].
Although p110β is reported to provide only a small fraction of insulin-stimulated PtdIns(3,4,5)P3, liver-specific knockout of this enzyme also resulted in substantial hepatic insulin resistance, but surprisingly has only very limited effects on Akt phosphorylation [167,169]. A single nucleotide polymorphism in the promoter of PIK3CB is associated with improved hepatic but not peripheral insulin sensitivity [170]. Liver-specific Pik3cb knockout mice had no detectable differences in serum or hepatic lipid profiles, even when challenged by a high fat diet [167]. Together these data strongly implicate PtdIns(3,4,5)P3 and/or PI(3,4)P2 in the regulation of gluconeogenesis, but the physiological relevance of this pathway in lipid steatosis is not as clear.
11. Increasing the levels of PtdIns(3,4,5)P3 causes insulin sensitization
Pathophysiological conditions such as insulin resistance cause impaired production of PtdIns(3,4,5)P3. Since decreased PtdIns(3,4,5)P3 reduces insulin signaling, insulin resistance could be potentially ameliorated by increasing PtdIns(3,4,5)P3 levels. Therefore, inhibition of PtdIns(3,4,5)P3 degradation has been explored as a means by which the deleterious effects of high fat diet-induced insulin resistance may be corrected. Converse to the PtdIns(3,4,5)P3 reductions present in Class IA PI3K knockout models, Pten knockout mice have increased levels of this phosphoinositide, due to an inability to dephosphorylate PtdIns(3,4,5)P3 into PtdIns(4,5)P2.
Homozygous deletion of Pten results in embryonic lethality [171,172], but heterozygotes have reduced fasting glucose levels and elevated insulin sensitivity [173]. Antisense oligonucleotides have also been used to probe PTEN function in whole animals. Reduction of PTEN levels by 70–90% in liver and adipose tissue caused normalization of blood glucose and insulin in ob/ob mice [174]. PTEN has been extensively studied in tissue-specific knockout models (reviewed in [175]). Mice with a targeted knockout of Pten in skeletal and cardiac muscle using Ckmm-Cre were resistant to high fat diet-induced insulin resistance [176], but there were no detectable differences in body weight either under normal or high fat diet fed conditions. These mice were also characterized by enhanced Akt phosphorylation and glycogen accumulation in their soleus muscles. It is unclear in this case why enhanced insulin sensitivity of these mice is only observed after a high-fat diet challenge and not under normal diet conditions, and may underlie a role for PTEN/PtdIns(3,4,5)P3 in the muscle response to high fat feeding.
Two groups have independently generated Alb-Cre driven liver-specific Pten knockout mice [177,178]. Both groups report substantial hepatomegaly, suppressed fasting glucose levels and increased insulin sensitivity. These data are consistent with a positive role for PtdIns(3,4,5)P3 in cell division and the suppression of gluconeogenesis. One group also reported substantial hepatosteatosis and accumulation of glycogen in the liver-specific PTEN knockout mice, likely due to accelerated insulin-stimulated lipogenesis and glycogenesis [177]. Some of these mice also developed hepatocellular carcinomas, underlying the important role of PTEN both in normal physiology and as a tumor suppressor.
Insulin leads to increased glucose uptake and lipid/glycogen storage in adipose tissue. Targeted deletion of PTEN from white adipose tissue using the Ap2-Cre promoter revealed increased Akt signaling, an accumulation of lipids in white adipose tissue depots, and an increase in whole-body insulin sensitivity [179]. This phenotype is consistent with other models of increased insulin action in adipose tissue [180].
PtdIns(3,4,5)P3 and PTEN have also been implicated in the regulation of pancreatic function. Two groups have explored the phenotype of beta-cell specific knockouts of Pten, driven by RIP (Rat Insulin Promoter)-Cre [135,181]. Both groups report hyperplasia of islets, increased glucose-induced insulin secretion, decreased fasting glucose and reduced whole-animal insulin sensitivity. These data highlight a role for PTEN in suppressing islet growth and dampening glucose-induced insulin secretion from islet cells.
The increased insulin sensitivity in Pten loss of function models led to the hypothesis that pharmacological inhibition of PTEN may improve insulin resistance by elevating PtdIns(3,4,5)P3 levels. Bisperoxovanadium compounds inhibit PTEN in vitro and result in increased Akt activation in cells [182,183]. These vanadate derivatives also have dramatic positive effects on the stimulation of glucose transport in cells [184–188]. In animals, these drugs cause reductions in circulating glucose levels consistent stimulation of glucose uptake and suppression of gluconeogenesis [189,190]. Complicating these results however is the insight that bisperoxovanadium compounds increase tyrosine phosphorylation of the insulin receptor and IRS proteins [185,186]. These findings suggest that these drugs also inhibit the Protein Tyrosine Phosphatase family of tyrosine phosphatases, another important set of negative regulators of insulin signaling that work upstream of PI3K activity.
PtdIns(3,4,5)P3 levels are also negatively regulated by a 5-phosphatases such as SHIP-2 or SKIP. Whole-body Inppl1 (SHIP-2) knockout mice are viable although animals were smaller than littermate controls [32,191]. On normal chow diet, these mice had no apparent improvements in glucose homeostasis, but these animals were resistant to high-fat diet induced hyperglycemia and hyperinsulinemia [32]. An inhibitor of SHIP-2 was developed by Astellas Pharmaceuticals and has shown some promise, as it also increases Akt phosphorylation and glucose uptake in cells, as well as reducing glucose levels in mice [192].
Another 5-phosphatase that has been suggested to negatively regulate PtdIns(3,4,5)P3 levels is Inpp5k (also known as SKIP). Whereas homozygous deletion of this enzyme is embryonic lethal, heterozygotes are insulin sensitive with modest resistance to diet-induced obesity. These data are consistent with a negative role of INPP5K in Akt signaling [30,31,34,193]. In each of these cases (for PTEN, SHIP-2 or INPP5K) the presumed effect of loss or inhibition is through hyperactivation of Akt, but this has not been formally established, as other PtdIns(3,4,5)P3 or PtdIns(3,4)P2 dependent processes may also play a role. The PtdIns(3,4)P2 phosphatases INPP4A and INPP4B have been identified as playing a role in Akt signaling in the context of tumor growth, but their role in regulating metabolism has not yet been explored.
Related to this point, the benefits of inhibiting PTEN or other PtdIns(3,4,5)P3 phosphatases, either genetically or pharmacologically should be cautioned due to the important roles of PTEN and INPP4A/B potent tumor suppressors [26,40,41,171]. Careful balancing of the insulin-sensitizing effects associated with PtdIns(3,4,5)P3 phosphatase inhibition with the potential transformation potential of this idea would be necessary for this to be effective.
12. Key questions in the field
Since their discovery as major modulators of insulin action, phosphoinositides have been under intensive investigation. The study of these lipids has led to major advances in our understanding of intracellular trafficking, signal transduction and organelle biogenesis.
Blockade of phosphoinositide synthesis in insulin resistance is an important, yet underappreciated aspect of our understanding of the pathophysiology of obesity. The majority of insulin-resistance inducing interventions are associated with serine phosphorylation of IRS, which occurs upstream of PtdIns(3,4,5)P3 synthesis. This makes the re-establishment of PtdIns(3,4,5)P3 levels an important insulin-sensitizing and glucose lowering possibility. This idea though should be taken with caution, for two reasons. One point is that promoting glucose storage in an obese person may reduce circulating glucose levels, but has the confounding problem of exacerbating lipid synthesis and other obesity associated complications. This has been a concern with several insulin sensitizing interventions including insulin therapy [194–197], thiazolidinediones [198–200] and sulfonylureas [195].
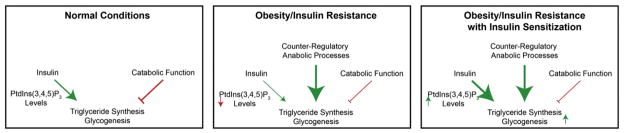
Complicating this problemis the observation that in insulin resistant states, alternative mechanisms of nutrient disposition are upregulated including mTORC1 activation [143,151,201], counter-inflammatory processes [202,203] and a downregulation of catabolic signaling processes [204–207]. This is especially true for glycogenic and lipogenic processes, which are not substantially suppressed in obesity-associated insulin resistance [2,144]. These data suggest that re-sensitization of PtdIns(3,4,5)P3 dependent processes may not alleviate obesity-associated comorbidities if these other counter-regulatory processes are not also suppressed (see Fig. 2). It is not clear from current studies the role that phosphoinositides play in these important counter-regulatory actions associated with obesity but understanding these mechanisms are extremely important to developing better treatments for obesity/insulin resistance.
Fig. 2.
Inter-relationship between sensitization of insulin action, counter-regulatory actions and lipid deposition in obesity.
That being said, the extent to which re-sensitization of PtdIns(3,4,5)P3 dependent signaling is associated with global improvements in energy balance has not been thoroughly explored. As obesity is associated with dramatic decreases in peripheral energy expenditure, the role of phosphoinositides, especially in muscle and brown adipose tissue, associated with these decreases is a ripe area for investigation. The in vivo tools for the manipulation of phosphoinositides in a temporal and tissue specific manner are just starting to emerge and we expect that these studies will shed light on the complex inter-relationships involved in the balance between anabolic and catabolic action at an organismal level. Understanding the effects of manipulation of these lipids in health and disease will play a key role in combating the co-morbidities associated with obesity and Type 2 diabetes.
Acknowledgments
We would like to thank members of the Bridges and Saltiel laboratories for insightful comments on this manuscript. This work was supported by a Le Bonheur Grant # 650700 (DB) and NIH RO1DK061618 and DK060591 (ARS).
Footnotes
This article is part of a Special Issue entitled Phosphoinositides.
References
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. http://dx.doi.org/10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.Saltiel AR. Insulin resistance in the defense against obesity. Cell Metab. 2012;15:798–804. doi: 10.1016/j.cmet.2012.03.001. http://dx.doi.org/10.1016/j.cmet.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev Mol Med. 2012;14:e1. doi: 10.1017/S1462399411002109. http://dx.doi.org/10.1017/S1462399411002109. [DOI] [PubMed] [Google Scholar]
- 4.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 5.Wymann MP, Björklöf K, Calvez R, Finan P, Thomast M, Trifilieff a, et al. Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochem Soc Trans. 2003;31:275–280. doi: 10.1042/bst0310275. http://dx.doi.org/10.1042/ [DOI] [PubMed] [Google Scholar]
- 6.Mellor P, Furber La, Nyarko JNK, Anderson DH. Multiple roles for the p85α isoform in the regulation and function of PI3K signalling and receptor trafficking. Biochem J. 2012;441:23–37. doi: 10.1042/BJ20111164. http://dx.doi.org/10.1042/BJ20111164. [DOI] [PubMed] [Google Scholar]
- 7.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. http://dx.doi.org/10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. http://dx.doi.org/10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 9.Khwaja A, Rodriguez-Viciana P, Wennström S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. http://dx.doi.org/10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain M, Berry T, Pastor M, Anderson DH. The p85{alpha} subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. J Biol Chem. 2004;279:48607–48614. doi: 10.1074/jbc.M409769200. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovsky R, Pochanard P, McNear C, Brachmann SM, Duke-Cohan JS, Garraway LA, et al. p85 associates with unphosphorylated PTEN and the PTEN-associated complex. Mol Cell Biol. 2009;29:5377–5388. doi: 10.1128/MCB.01649-08. http://dx.doi.org/10.1128/MCB.01649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber DF, Alvarado-Kristensson M, González-García A, Pulido R, Carrera AC. PTEN regulation, a novel function for the p85 subunit of phosphoinositide 3-kinase. Sci STKE. 2006;2006:e49. doi: 10.1126/stke.3622006pe49. http://dx.doi.org/10.1126/stke.3622006pe49. [DOI] [PubMed] [Google Scholar]
- 13.Chagpar RB, Links PH, Pastor MC, Furber LA, Hawrysh AD, Chamberlain MD, et al. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2010;107:5471–5476. doi: 10.1073/pnas.0908899107. http://dx.doi.org/10.1073/pnas.0908899107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. http://dx.doi.org/10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology. 2005;146:1328–1337. doi: 10.1210/en.2004-0777. http://dx.doi.org/10.1210/en.2004-0777. [DOI] [PubMed] [Google Scholar]
- 16.Um S, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GkS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha-and obesity-induced insulin resistance. Science. 1996;271:665–670. doi: 10.1126/science.271.5249.665. http://dx.doi.org/10.1126/science.271.5249.665 (80) [DOI] [PubMed] [Google Scholar]
- 18.Cai D, Yuan M, Frantz DF, Melendez Pa, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting fromhepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. http://dx.doi.org/10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White MF, Takayama S, Kahn CR. Differences in the sites of phosphorylation of the insulin receptor in vivo and in vitro. J Biol Chem. 1985;260:9470–9478. [PubMed] [Google Scholar]
- 20.Takayama S, White MF, Lauris V, Kahn CR. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984;81:7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saad MJ, Araki E, Miralpeix M, Rothenberg PL, White MF, Kahn CR. Regulation of insulin receptor substrate-1 in liver andmuscle of animalmodels of insulin resistance. J Clin Invest. 1992;90:1839–1849. doi: 10.1172/JCI116060. http://dx.doi.org/10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadowaki T, Kasuga M, Akanuma Y, Ezaki O, Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984;259:14208–14216. [PubMed] [Google Scholar]
- 23.Block NE, Komori K, Robinson KA, Dutton SL, Lam CF, Buse MG. Diabetes-associated impairment of hepatic insulin receptor tyrosine kinase activity: a study of mechanisms. Endocrinology. 1991;128:312–322. doi: 10.1210/endo-128-1-312. [DOI] [PubMed] [Google Scholar]
- 24.Heydrick SJ, Jullien D, Gautier N, Tanti J, Giorgetti S. Defect in skeletal muscle phosphatidylinositol-3-kinase in obese insulin-resistant mice. 1993 doi: 10.1172/JCI116337. http://dx.doi.org/10.1172/JCI116337.Defect. [DOI] [PMC free article] [PubMed]
- 25.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes central role of tumor necrosis factor-a. J Clin Invest. 1994;94:1543–1549. doi: 10.1172/JCI117495. http://dx.doi.org/10.1172/JCI117495.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 27.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. http://dx.doi.org/10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 28.Maehama T, Dixon J. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 29.Ooms LM, Horan KA, Rahman P, Seaton G, Gurung R, Kethesparan DS, et al. The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem J. 2009;419:29–49. doi: 10.1042/BJ20081673. http://dx.doi.org/10.1042/BJ20081673. [DOI] [PubMed] [Google Scholar]
- 30.Ijuin T, Takenawa T. Regulation of insulin signalling and glucose transporter 4 (GLUT4) exocytosis by the phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, SKIP. J Biol Chem. 2012;4 doi: 10.1074/jbc.M111.335539. http://dx.doi.org/10.1074/jbc.M111.335539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ijuin T, Takenawa T. SKIP negatively regulates insulin-induced GLUT4 translocation and membrane ruffle formation. Mol Cell Biol. 2003;23:1209–1220. doi: 10.1128/MCB.23.4.1209-1220.2003. http://dx.doi.org/10.1128/MCB.23.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleeman MW, Wortley KE, Lai K-MV, Gowen LC, Kintner J, Kline WO, et al. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat Med. 2005;11:199–205. doi: 10.1038/nm1178. http://dx.doi.org/10.1038/nm1178. [DOI] [PubMed] [Google Scholar]
- 33.Clément S, Krause U, Desmedt F, Tanti JF, Behrends J, Pesesse X, et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature. 2001;409:92–97. doi: 10.1038/35051094. http://dx.doi.org/10.1038/35051094. [DOI] [PubMed] [Google Scholar]
- 34.Ijuin T, Yu YE, Mizutani K, Pao A, Tateya S, Tamori Y, et al. Increased insulin action in SKIP heterozygous knockout mice. Mol Cell Biol. 2008;28:5184–5195. doi: 10.1128/MCB.01990-06. http://dx.doi.org/10.1128/MCB.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada T, Sasaoka T, Funaki M, Hori H, Murakami S, Ishiki M, et al. Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via its 5′-phosphatase catalytic activity. Mol Cell Biol. 2001;21:1633–1646. doi: 10.1128/MCB.21.5.1633-1646.2001. http://dx.doi.org/10.1128/MCB.21.5.1633-1646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rommel C, Bodine SC, Clarke Ba, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. http://dx.doi.org/10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 37.James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315(Pt 3):709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris FA, Atkins RC, Majerus PW. The cDNA cloning and characterization of inositol polyphosphate 4-phosphatase type II. Evidence for conserved alternative splicing in the 4-phosphatase family. J Biol Chem. 1997;272:23859–23864. doi: 10.1074/jbc.272.38.23859. [DOI] [PubMed] [Google Scholar]
- 39.Norris FA, Auethavekiat V, Majerus PW. The isolation and characterization of cDNA encoding human and rat brain inositol polyphosphate 4-phosphatase. J Biol Chem. 1995;270:16128–16133. doi: 10.1074/jbc.270.27.16128. [DOI] [PubMed] [Google Scholar]
- 40.Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. http://dx.doi.org/10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedele CG, Ooms LM, Ho M, Vieusseux J, O’Toole SA, Millar EK, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci U S A. 2010;107:22231–22236. doi: 10.1073/pnas.1015245107. http://dx.doi.org/10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staal SP, Hartley JW. Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med. 1988;167:1259–1264. doi: 10.1084/jem.167.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 44.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. http://dx.doi.org/10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 45.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas a, Morrison DK, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 46.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. S0960-9822(06)00122-9 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. http://dx.doi.org/10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 48.Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt 3):575–583. [PMC free article] [PubMed] [Google Scholar]
- 49.McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, et al. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 2004;23:2071–2082. doi: 10.1038/sj.emboj.7600218. http://dx.doi.org/10.1038/sj.emboj.7600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weng QP, Andrabi K, Kozlowski MT, Grove JR, Avruch J. Multiple independent inputs are required for activation of the p70 S6 kinase. Mol Cell Biol. 1995;15:2333–2340. doi: 10.1128/mcb.15.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petritsch C, Woscholski R, Edelmann HM, Parker PJ, Ballou LM. Selective inhibition of p70 S6 kinase activation by phosphatidylinositol 3-kinase inhibitors. Eur J Biochem. 1995;230:431–438. doi: 10.1111/j.1432-1033.1995.0431h.x. [DOI] [PubMed] [Google Scholar]
- 52.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 53.Inoki K, Li Y, Zhu T, Wu J, Guan K-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. http://dx.doi.org/10.1038/ncb839 (ncb839 [pii]) [DOI] [PubMed] [Google Scholar]
- 54.Inoki K, Li Y, Xu T, Guan K-L. Rheb GTPase is a direct target of TSC2 GAP activity and regulatesmTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. http://dx.doi.org/10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 56.Dan M, Sun HC, Yang L, Feldman RI, Sui X-M, Ou CC, et al. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. http://dx.doi.org/10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 57.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor andmediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. http://dx.doi.org/10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan K-L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. http://dx.doi.org/10.1038/ncb1753 (ncb1753 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Binda M, Péli-Gulli M-P, Bonfils G, Panchaud N, Urban J, Sturgill TW, et al. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. http://dx.doi.org/10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 62.Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. http://dx.doi.org/10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–799. doi: 10.1016/j.cell.2014.01.024. http://dx.doi.org/10.1016/j.cell.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bridges D, Ma J-T, Park S, Inoki K, Weisman LS, Saltiel AR. Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol Biol Cell. 2012;23:2955–2962. doi: 10.1091/mbc.E11-12-1034. http://dx.doi.org/10.1091/mbc.E11-12-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bridges D, Fisher K, Zolov SN, Xiong T, Inoki K, Weisman LS, et al. Rab5 proteins regulate activation and localization of target of rapamycin complex 1. J Biol Chem. 2012;287:20913–20921. doi: 10.1074/jbc.M111.334060. http://dx.doi.org/10.1074/jbc.M111.334060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin N, Mao K, Jin Y, Tevzadze G, Kauffman EJ, Park S, et al. Roles for PI(3,5)P2 in nutrient sensing through TORC1. Mol Biol Cell. 2014;25:1171–1185. doi: 10.1091/mbc.E14-01-0021. http://dx.doi.org/10.1091/mbc.E14-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. http://dx.doi.org/10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoon M-S, Du G, Backer JM, Frohman Ma, Chen J. Class III PI-3-kinase activates phospholipase D in an amino acid-sensing mTORC1 pathway. J Cell Biol. 2011 doi: 10.1083/jcb.201107033. http://dx.doi.org/10.1083/jcb.201107033. [DOI] [PMC free article] [PubMed]
- 69.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. http://dx.doi.org/10.1074/jbc.M507201200 (M507201200 [pii]) [DOI] [PubMed] [Google Scholar]
- 70.Xu L, Salloum D, Medlin PS, Saqcena M, Yellen P, Perrella B, et al. Phospholipase D mediates nutrient input to mTORC1. J Biol Chem. 2011;286:25477–25486. doi: 10.1074/jbc.M111.249631. http://dx.doi.org/10.1074/jbc.M111.249631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stahelin R, Ananthanarayanan B, Blatner N, Singh S, Bruzik K, Murray D, et al. Mechanism of membrane binding of the phospholipase D1 PX domain. J Biol Chem. 2004;279:54918–54926. doi: 10.1074/jbc.M407798200. [DOI] [PubMed] [Google Scholar]
- 72.Fang Y, Park I-H, Wu A-L, Du G, Huang P, Frohman Ma, et al. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol. 2003;13:2037–2044. doi: 10.1016/j.cub.2003.11.021. http://dx.doi.org/10.1016/j.cub.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. http://dx.doi.org/10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 74.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. http://dx.doi.org/10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 75.Zisman a, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6:924–928. doi: 10.1038/78693. http://dx.doi.org/10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 76.Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–3061. doi: 10.1021/bi2000356. http://dx.doi.org/10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 77.Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01178.x. http://dx.doi.org/10.1111/j.1600-0854.2011.01178.x. [DOI] [PubMed]
- 78.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. http://dx.doi.org/10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 79.Clarke JF, Young PW, Yonezawa K, Kasuga M, Holman GD. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994;300(Pt 3):631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 81.Kanai F, Ito K, Todaka M, Hayashi H, Kamohara S, Ishii K, et al. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem Biophys Res Commun. 1993;195:762–768. doi: 10.1006/bbrc.1993.2111. http://dx.doi.org/10.1006/bbrc.1993.2111. [DOI] [PubMed] [Google Scholar]
- 82.Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci U S A. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. http://dx.doi.org/10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Green CJ, Göransson O, Kular GS, Leslie NR, Gray A, Alessi DR, et al. Use of Akt inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/Akt in the insulin-dependent regulation of glucose and system A amino acid uptake. J Biol Chem. 2008;283:27653–27667. doi: 10.1074/jbc.M802623200. http://dx.doi.org/10.1074/jbc.M802623200. [DOI] [PubMed] [Google Scholar]
- 84.Tan S-X, Ng Y, James DE. Next generation Akt inhibitors provide greater specificity-effects on glucose metabolism in adipocytes. Biochem J. 2011 doi: 10.1042/BJ20110040. http://dx.doi.org/10.1042/BJ20110040. [DOI] [PubMed]
- 85.Ng Y, Lopez Ja, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 2008;7:348–356. doi: 10.1016/j.cmet.2008.02.008. http://dx.doi.org/10.1016/j.cmet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 86.Jiang ZY, Zhou QL, Coleman Ka, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci U S A. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. http://dx.doi.org/10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. http://dx.doi.org/10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 88.Eguez L, Lee A, Chavez Ja, Miinea CP, Kane S, Lienhard GE, et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. http://dx.doi.org/10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Sun Y, Bilan PJ, Liu Z, Klip A. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc Natl Acad Sci U S A. 2010;2010:6–11. doi: 10.1073/pnas.1009523107. http://dx.doi.org/10.1073/pnas.1009523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez Ja, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. http://dx.doi.org/10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 91.Chen X-W, Leto D, Xiong T, Yu G, Cheng A, Decker S, et al. A Ral GAP complex links PI 3-kinase/Akt signaling to RalA activation in insulin action. Mol Biol Cell. 2011;22:141–152. doi: 10.1091/mbc.E10-08-0665. http://dx.doi.org/10.1091/mbc.E10-08-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gridley S, Chavez Ja, Lane WS, Lienhard GE. Adipocytes contain a novel complex similar to the tuberous sclerosis complex. Cell Signal. 2006;18:1626–1632. doi: 10.1016/j.cellsig.2006.01.002. http://dx.doi.org/10.1016/j.cellsig.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Chen XW, Leto D, Chiang S-HH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. http://dx.doi.org/10.1016/j.devcel.2007.07.007 (S1534-5807(07)00268-7 [pii]) [DOI] [PubMed] [Google Scholar]
- 94.Leto D, Uhm M, Williams A, Chen X, Saltiel AR. Negative regulation of the RalGAP complex by 14–3–3. J Biol Chem. 2013;288:9272–9283. doi: 10.1074/jbc.M112.426106. http://dx.doi.org/10.1074/jbc.M112.426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inoue M, Chiang S-HH, Chang L, Chen XW, Saltiel AR. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell. 2006;17:2303–2311. doi: 10.1091/mbc.E06-01-0030. http://dx.doi.org/10.1091/mbc.E06-01-0030 (E06-01-0030 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inoue M, Chang L, Hwang J, Chiang S-HH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. http://dx.doi.org/10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 97.Okada S, Ohshima K, Uehara Y, Shimizu H, Hashimoto K, Yamada M, et al. Synip phosphorylation is required for insulin-stimulated Glut4 translocation. Biochem Biophys Res Commun. 2007;356:102–106. doi: 10.1016/j.bbrc.2007.02.095. http://dx.doi.org/10.1016/j.bbrc.2007.02.095. [DOI] [PubMed] [Google Scholar]
- 98.Yamada E, Okada S, Saito T, Ohshima K, Sato M, Tsuchiya T, et al. Akt2 phosphorylates Synip to regulate docking and fusion of GLUT4-containing vesicles. J Cell Biol. 2005;168:921–928. doi: 10.1083/jcb.200408182. http://dx.doi.org/10.1083/jcb.200408182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sano H, Kane S, Sano E, Lienhard GE. Synip phosphorylation does not regulate insulin-stimulated GLUT4 translocation. Biochem Biophys Res Commun. 2005;332:880–884. doi: 10.1016/j.bbrc.2005.05.027. http://dx.doi.org/10.1016/j.bbrc.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 100.Xie X, Gong Z, Mansuy-Aubert V, Zhou QL, Tatulian Sa, Sehrt D, et al. C2 domain-containing phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane. Cell Metab. 2011;14:378–389. doi: 10.1016/j.cmet.2011.06.015. http://dx.doi.org/10.1016/j.cmet.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman Ma, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci U S A. 2006;103:14761–14766. doi: 10.1073/pnas.0606881103. http://dx.doi.org/10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mima J, Wickner W. Phosphoinositides and SNARE chaperones synergistically assemble and remodel SNARE complexes for membrane fusion. Proc Natl Acad Sci U S A. 2009;106:16191–16196. doi: 10.1073/pnas.0908694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.James DJ, Khodthong C, Kowalchyk Ja, Martin TFJ. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. http://dx.doi.org/10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. http://dx.doi.org/10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4, 5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell. 2007;18:4483–4492. doi: 10.1091/mbc.E07-05-0461. http://dx.doi.org/10.1091/mbc.E07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamashita M, Kurokawa K, Sato Y, Yamagata A, Mimura H, Yoshikawa A, et al. Structural basis for the Rho- and phosphoinositide-dependent localization of the exocyst subunit Sec3. Nat Struct Mol Biol. 2010;17:180–186. doi: 10.1038/nsmb.1722. http://dx.doi.org/10.1038/nsmb.1722. [DOI] [PubMed] [Google Scholar]
- 107.Di Paolo G, de Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. http://dx.doi.org/10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 108.Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, de Camilli P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. http://dx.doi.org/10.1016/j.cell.2009.01.032 (S0092-8674(09)00080-4 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bohdanowicz M, Balkin DM, De Camilli P, Grinstein S. Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol Biol Cell. 2012;23:176–187. doi: 10.1091/mbc.E11-06-0489. http://dx.doi.org/10.1091/mbc.E11-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip S-C, et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 111.Kurosu H, Katada T. Association of phosphatidylinositol 3-kinase composed of p110beta-catalytic and p85-regulatory subunits with the small GTPase Rab5. J Biochem. 2001;130:73–78. doi: 10.1093/oxfordjournals.jbchem.a002964. [DOI] [PubMed] [Google Scholar]
- 112.Tan Y, You H, Wu C, Altomare Da, Testa JR. Appl1 is dispensable for mouse development, and loss of Appl1 has growth factor-selective effects on Akt signaling in murine embryonic fibroblasts. J Biol Chem. 2010;285:6377–6389. doi: 10.1074/jbc.M109.068452. http://dx.doi.org/10.1074/jbc.M109.068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. http://dx.doi.org/10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 114.Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem. 2007;282:32280–32287. doi: 10.1074/jbc.M704150200. http://dx.doi.org/10.1074/jbc.M704150200. [DOI] [PubMed] [Google Scholar]
- 115.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, et al. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 116.Lodhi IJ, Bridges D, Chiang S-H, Zhang Y, Cheng A, Geletka LM, et al. Insulin stimulates phosphatidylinositol 3-phosphate production via the activation of Rab5. Mol Biol Cell. 2008;19:2718–2728. doi: 10.1091/mbc.E08-01-0105. http://dx.doi.org/10.1091/mbc.E08-01-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maffucci T, Brancaccio A, Piccolo E, Stein RC, Falasca M. Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBO J. 2003;22:4178–4189. doi: 10.1093/emboj/cdg402. http://dx.doi.org/10.1093/emboj/cdg402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Falasca M, Hughes WE, Dominguez V, Sala G, Fostira F, Fang MQ, et al. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J Biol Chem. 2007;282:28226–28236. doi: 10.1074/jbc.M704357200. http://dx.doi.org/10.1074/jbc.M704357200. [DOI] [PubMed] [Google Scholar]
- 119.Kong AM, Horan KA, Sriratana A, Bailey CG, Collyer LJ, Nandurkar HH, et al. Phosphatidylinositol 3-phosphate [PtdIns3P] is generated at the plasma membrane by an inositol polyphosphate 5-phosphatase: endogenous PtdIns3P can promote GLUT4 translocation to the plasma membrane. Mol Cell Biol. 2006;26:6065–6081. doi: 10.1128/MCB.00203-06. http://dx.doi.org/10.1128/MCB.00203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaussade C, Pirola L, Bonnafous S, Blondeau F, Brenz-Verca S, Tronchère H, et al. Expression of myotubularin by an adenoviral vector demonstrates its function as a phosphatidylinositol 3-phosphate [PtdIns(3)P] phosphatase in muscle cell lines: involvement of PtdIns(3)P in insulin-stimulated glucose transport. Mol Endocrinol. 2003;17:2448–2460. doi: 10.1210/me.2003-0261. http://dx.doi.org/10.1210/me.2003-0261. [DOI] [PubMed] [Google Scholar]
- 121.Ishiki M, Randhawa VK, Poon V, Jebailey L, Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J Biol Chem. 2005;280:28792–28802. doi: 10.1074/jbc.M500501200. http://dx.doi.org/10.1074/jbc.M500501200. [DOI] [PubMed] [Google Scholar]
- 122.Zolov SN, Bridges D, Zhang Y, Lee W, Riehle E, Verma R, et al. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci U S A. 2012;109:17472–17477. doi: 10.1073/pnas.1203106109. zoloberna. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gary JD, Wurmser AE, Bonangelino C, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ikonomov OC, Sbrissa D, Dondapati R, Shisheva A. ArPIKfyve–PIKfyve interaction and role in insulin-regulated GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. Exp Cell Res. 2007;313:2404–2416. doi: 10.1016/j.yexcr.2007.03.024. http://dx.doi.org/10.1016/j.yexcr.2007.03.024 (S0014-4827(07)00113-9 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sbrissa D, Ikonomov OC, Shisheva A. PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin. J Biol Chem. 1999;274:21589–21597. doi: 10.1074/jbc.274.31.21589. [DOI] [PubMed] [Google Scholar]
- 126.Ikonomov OC, Sbrissa D, Ijuin T, Takenawa T, Shisheva A. Sac3 is an insulin-regulated PtdIns(3,5)P2 phosphatase: gain in insulin responsiveness through Sac3 downregulation in adipocytes. J Biol Chem. 2009 doi: 10.1074/jbc.M109.025361. http://dx.doi.org/10.1074/jbc.M109.025361 (M109.025361 [pii]) [DOI] [PMC free article] [PubMed]
- 127.Ikonomov OC, Sbrissa D, Mlak K, Shisheva A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology. 2002;143:4742–4754. doi: 10.1210/en.2002-220615. [DOI] [PubMed] [Google Scholar]
- 128.Sbrissa D, Ikonomov OC, Strakova J, Shisheva A. Role for a novel signaling intermediate, phosphatidylinositol 5-phosphate, in insulin-regulated F-actin stress fiber breakdown and GLUT4 translocation. Endocrinology. 2004;145:4853–4865. doi: 10.1210/en.2004-0489. http://dx.doi.org/10.1210/en.2004-0489. [DOI] [PubMed] [Google Scholar]
- 129.Ikonomov OC, Sbrissa D, Delvecchio K, Feng H-Z, Cartee GD, Jin J-P, et al. Muscle-specific Pikfyve gene disruption causes glucose intolerance, insulin resistance, adiposity and hyperinsulinemia but not muscle fiber-type switching. Am J Physiol Endocrinol Metab. 2013;305:E119–E131. doi: 10.1152/ajpendo.00030.2013. http://dx.doi.org/10.1152/ajpendo.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Olsen HL, Hoy M, Zhang W, Bertorello AM, Bokvist K, Capito K, et al. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells. Proc Natl Acad Sci U S A. 2003;100:5187–5192. doi: 10.1073/pnas.0931282100. http://dx.doi.org/10.1073/pnas.0931282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Waselle L, Gerona RRL, Vitale N, Martin TFJ, Bader M-F, Regazzi R. Role of phosphoinositide signaling in the control of insulin exocytosis. Mol Endocrinol. 2005;19:3097–3106. doi: 10.1210/me.2004-0530. http://dx.doi.org/10.1210/me.2004-0530. [DOI] [PubMed] [Google Scholar]
- 132.Lawrence JTR, Birnbaum MJ. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2003;100:13320–13325. doi: 10.1073/pnas.2232129100. http://dx.doi.org/10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. http://dx.doi.org/10.1172/JCI200420016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tong Z, Fan Y, Zhang W, Xu J, Cheng J, Ding M, et al. Pancreas-specific Pten deficiency causes partial resistance to diabetes and elevated hepatic AKT signaling. Cell Res. 2009;19:710–719. doi: 10.1038/cr.2009.42. http://dx.doi.org/10.1038/cr.2009.42. [DOI] [PubMed] [Google Scholar]
- 135.Stiles BL, Kuralwalla-Martinez C, Guo W, Gregorian C, Wang Y, Tian J, et al. Selective deletion of Pten in pancreatic $\$beta$\$ cells leads to increased islet mass and resistance to STZ-induced diabetes. Mol Cell Biol. 2006;26:2772. doi: 10.1128/MCB.26.7.2772-2781.2006. http://dx.doi.org/10.1128/MCB.26.7.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dominguez V, Raimondi C, Somanath S, Bugliani M, Loder MK, Edling CE, et al. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. J Biol Chem. 2010:1–22. doi: 10.1074/jbc.M110.200295. http://dx.doi.org/10.1074/jbc.M110.200295. [DOI] [PMC free article] [PubMed]
- 137.Meunier FA, Osborne SL, Hammond GRV, Cooke FT, Parker PJ, Domin J, et al. Phosphatidylinositol 3-kinase C2alpha is essential for ATP-dependent priming of neurosecretory granule exocytosis. Mol Biol Cell. 2005;16:4841–4851. doi: 10.1091/mbc.E05-02-0171. http://dx.doi.org/10.1091/mbc.E05-02-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wen PJ, Osborne SL, Morrow IC, Parton RG, Domin J, Meunier FA. Ca2+-regulated pool of phosphatidylinositol-3-phosphate produced by phosphatidylinositol 3-kinase C2alpha on neurosecretory vesicles. Mol Biol Cell. 2008;19:5593–5603. doi: 10.1091/mbc.E08-06-0595. http://dx.doi.org/10.1091/mbc.E08-06-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012:1–9. doi: 10.1038/nm.2686. http://dx.doi.org/10.1038/nm.2686. [DOI] [PMC free article] [PubMed]
- 140.Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, et al. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. http://dx.doi.org/10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Puigserver P, Rhee J, Donovan J, Kitamura Y, Altomonte J, Dong H. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1 a interaction. :4232003. doi: 10.1038/nature01667. http://dx.doi.org/10.1038/nature01606.1. [DOI] [PubMed]
- 142.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. http://dx.doi.org/10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 143.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. http://dx.doi.org/10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. http://dx.doi.org/10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 145.Le Marchand-Brustel Y, Gautier N, Cormont M, Van Obberghen E. Wortmannin inhibits the action of insulin but not that of okadaic acid in skeletal muscle: comparison with fat cells. Endocrinology. 1995;136:3564–3570. doi: 10.1210/endo.136.8.7628394. [DOI] [PubMed] [Google Scholar]
- 146.Shepherd PR, Navé BT, Siddle K. Insulin stimulation of glycogen synthesis and glycogen synthase activity is blocked by wortmannin and rapamycin in 3T3-L1 adipocytes: evidence for the involvement of phosphoinositide 3-kinase and p70 ribosomal protein-S6 kinase. Biochem J. 1995;305(Pt 1):25–28. doi: 10.1042/bj3050025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. http://dx.doi.org/10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peterson TR, Sengupta S, Harris TEE, Carmack AEE, Kang SAA, Balderas E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. http://dx.doi.org/10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huffman TA, Mothe-Satney I, Lawrence JJC. Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Natl Acad Sci U S A. 2002;99:1047–1052. doi: 10.1073/pnas.022634399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Laplante M, Sabatini DM. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3281–3282. doi: 10.1073/pnas.1000323107. http://dx.doi.org/10.1073/pnas.1000323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu B, Bridges D, Yang Y, Fisher K, Cheng A, Chang L, et al. Metabolic crosstalk: molecular links between glycogen and lipid metabolism in obesity. Diabetes. 2014:1–49. doi: 10.2337/db13-1531. http://dx.doi.org/10.2337/db13-1531. [DOI] [PMC free article] [PubMed]
- 152.Cross DaE, Watt PW, Shaw M, van der Kaay J, Downes CP, Holder JC, et al. Insulin activates protein kinaseB, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997;406:211–215. doi: 10.1016/s0014-5793(97)00240-8. http://dx.doi.org/10.1016/S0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 153.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. http://dx.doi.org/10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 154.Bouskila M, Hirshman MF, Jensen J, Goodyear LJ, Sakamoto K. Insulin promotes glycogen synthesis in the absence of GSK3 phosphorylation in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E28–E35. doi: 10.1152/ajpendo.00481.2007. http://dx.doi.org/10.1152/ajpendo.00481.2007. [DOI] [PubMed] [Google Scholar]
- 155.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. http://dx.doi.org/10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wan M, Leavens KF, Hunter RW, Koren S, von Wilamowitz-Moellendorff A, Lu M, et al. A noncanonical, GSK3-independent pathway controls postprandial hepatic glycogen deposition. Cell Metab. 2013;18:99–105. doi: 10.1016/j.cmet.2013.06.001. http://dx.doi.org/10.1016/j.cmet.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ruiz R, Jideonwo V, Ahn M, Surendran S, Tagliabracci VS, Hou Y, et al. Gene regulation: sterol regulatory element binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J Biol Chem. 2014;289(9):5510–5517. doi: 10.1074/jbc.M113.541110. http://dx.doi.org/10.1074/jbc.M113.541110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harris TE, Huffman TA, Chi A, Shabanowitz J, Hunt DF, Kumar A, et al. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J Biol Chem. 2007;282:277–286. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- 159.Owen JL, Zhang Y, Bae S-H, Farooqi MS, Liang G, Hammer RE, et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci U S A. 2012:1–6. doi: 10.1073/pnas.1213343109. http://dx.doi.org/10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed]
- 160.Quinn WJ, Birnbaum MJ. Distinct mTORC1 pathways for transcription and cleavage of SREBP-1c. Proc Natl Acad Sci U S A. 2012:1–2. doi: 10.1073/pnas.1214113109. http://dx.doi.org/10.1073/pnas.1214113109. [DOI] [PMC free article] [PubMed]
- 161.Bi L, Okabe I, Bernard DJ, Wynshaw-boris A, Nussbaum RL. Proliferative defect and embryonic lethality inmice homozygous for a deletion in the p110α Subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 162.Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110 catalytic subunit of PI 3-kinase, New York. Mamm Genome. 2002;172:169–172. doi: 10.1007/BF02684023. http://dx.doi.org/10.1007/s00335-001-2123-x. [DOI] [PubMed] [Google Scholar]
- 163.Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. http://dx.doi.org/10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. http://dx.doi.org/10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 165.Brachmann SM, Ueki K, Engelman JA, Kahn CR, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Society. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. http://dx.doi.org/10.1128/MCB.25.5.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sopasakis VR, Liu P, Suzuki R, Kondo T, Winnay J, Tran TT, et al. Specific roles of the p110a isoform of phosphatidylinositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 2010;11:220–230. doi: 10.1016/j.cmet.2010.02.002. http://dx.doi.org/10.1016/j.cmet.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chattopadhyay M, Selinger ES, Ballou LM, Lin RZ. Ablation of PI3K p110-a prevents high-fat diet-induced liver steatosis. Liver. 2011;60 doi: 10.2337/db10-0869. http://dx.doi.org/10.2337/db10-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson Ma, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 169.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI (3) K–p110b in cell growth, metabolism and tumorigenesis. Nature. 2008;454 doi: 10.1038/nature07091. http://dx.doi.org/10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ribel-Madsen R, Poulsen P, Holmkvist J, Mortensen B, Grarup N, Friedrichsen M, et al. Impact of rs361072 in the phosphoinositide 3-kinase p110beta gene on whole-body glucose metabolism and subunit protein expression in skeletal muscle. Diabetes. 2010;59:1108–1112. doi: 10.2337/db09-1359. http://dx.doi.org/10.2337/db09-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suzuki a, de la Pompa JL, Stambolic V, Elia aJ, Sasaki T, del Barco Barrantes I, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 173.Wong JT, Kim PTW, Peacock JW, Yau TY, Mui aL-F, Chung SW, et al. Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity. Diabetologia. 2007;50:395–403. doi: 10.1007/s00125-006-0531-x. http://dx.doi.org/10.1007/s00125-006-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Butler M, McKay Ra, Popoff IJ, Gaarde Wa, Witchell D, Murray SF, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 175.Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene. 2008;27:5398–5415. doi: 10.1038/onc.2008.238. http://dx.doi.org/10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- 176.Wijesekara N, Konrad D, Eweida M, Jefferies C, Liadis N, Giacca A, et al. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Society. 2005;25:1135–1145. doi: 10.1128/MCB.25.3.1135-1145.2005. http://dx.doi.org/10.1128/MCB.25.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113 doi: 10.1172/JCI20513. http://dx.doi.org/10.1172/JCI200420513.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim Y-J, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci U S A. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. http://dx.doi.org/10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25:2498–2510. doi: 10.1128/MCB.25.6.2498-2510.2005. http://dx.doi.org/10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- 181.Nguyen K-TT, Tajmir P, Lin CH, Liadis N, Zhu X-D, Eweida M, et al. Essential role of Pten in body size determination and pancreatic beta-cell homeostasis in vivo. Mol Cell Biol. 2006;26:4511–4518. doi: 10.1128/MCB.00238-06. http://dx.doi.org/10.1128/MCB.00238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rosivatz E, Matthews JG, McDonald NQ, Mulet X, Ho KK, Lossi N, et al. A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN) ACS Chem Biol. 2006;1:780–790. doi: 10.1021/cb600352f. http://dx.doi.org/10.1021/cb600352f. [DOI] [PubMed] [Google Scholar]
- 183.Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. http://dx.doi.org/10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 184.Fisher JS, Nolte La, Kawanaka K, Han D-H, Jones TE, Holloszy JO. Glucose transport rate and glycogen synthase activity both limit skeletal muscle glycogen accumulation. Am J Physiol Endocrinol Metab. 2002;282:E1214–E1221. doi: 10.1152/ajpendo.00254.2001. http://dx.doi.org/10.1152/ajpendo.00254.2001. [DOI] [PubMed] [Google Scholar]
- 185.Nolte La, Han D-H, Hansen Pa, Hucker Ka, Holloszy JO. A peroxovanadium compound stimulates muscle glucose transport as powerfully as insulin and contractions combined. Diabetes. 2003;52:1918–1925. doi: 10.2337/diabetes.52.8.1918. [DOI] [PubMed] [Google Scholar]
- 186.Yu ZW, Jansson Pa, Posner BI, Smith U, Eriksson JW. Peroxovanadate and insulin action in adipocytes from NIDDM patients. evidence against a primary defect in tyrosine phosphorylation. Diabetologia. 1997;40:1197–1203. doi: 10.1007/s001250050807. http://dx.doi.org/10.1007/s001250050807. [DOI] [PubMed] [Google Scholar]
- 187.Clark aS, Fagan JM, Mitch WE. Selectivity of the insulin-like actions of vanadate on glucose and protein metabolism in skeletal muscle. Biochem J. 1985;232:273–276. doi: 10.1042/bj2320273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Duckworth WC, Solomon SS, Liepnieks J, Hamel FG, Hand S, Peavy DE. Insulin-like effects of vanadate in isolated rat adipocytes. Endocrinology. 1988;122:2285–2289. doi: 10.1210/endo-122-5-2285. [DOI] [PubMed] [Google Scholar]
- 189.Westergaard N, Brand CL, Lewinsky RH, Andersen HS, Carr RD, Burchell a, et al. Peroxyvanadium compounds inhibit glucose-6-phosphatase activity and glucagon-stimulated hepatic glucose output in the rat in vivo. Arch Biochem Biophys. 1999;366:55–60. doi: 10.1006/abbi.1999.1181. http://dx.doi.org/10.1006/abbi.1999.1181. [DOI] [PubMed] [Google Scholar]
- 190.Brand RM, Hamel FG. Transdermally delivered peroxovanadium can lower blood glucose levels in diabetic rats. Int J Pharm. 1999;183:117–123. doi: 10.1016/s0378-5173(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 191.Dubois E, Jacoby M, Blockmans M, Pernot E, Schiffmann SN, Foukas LC, et al. Developmental defects and rescue from glucose intolerance of a catalytically-inactive novel Ship2 mutant mouse. Cell Signal. 2012;24:1971–1980. doi: 10.1016/j.cellsig.2012.06.012. http://dx.doi.org/10.1016/j.cellsig.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 192.Suwa A, Yamamoto T, Sawada A, Minoura K, Hosogai N, Tahara A, et al. Discovery and functional characterization of a novel small molecule inhibitor of the intracellular phosphatase, SHIP2. Br J Pharmacol. 2009;158:879–887. doi: 10.1111/j.1476-5381.2009.00358.x. http://dx.doi.org/10.1111/j.1476-5381.2009.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xiong Q, Deng C-Y, Chai J, Jiang S-W, Xiong Y-Z, Li F-E, et al. Knockdown of endogenous SKIP gene enhanced insulin-induced glycogen synthesis signaling in differentiating C2C12 myoblasts. BMB Rep. 2009;42:119–124. doi: 10.5483/bmbrep.2009.42.2.119. [DOI] [PubMed] [Google Scholar]
- 194.Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in IDDM. Diabetes. 1993;42:1700–1707. doi: 10.2337/diab.42.12.1700. http://dx.doi.org/10.2337/diab.42.12.1700. [DOI] [PubMed] [Google Scholar]
- 195.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. http://dx.doi.org/10.1016/S0140-6736(98)07019-6. [PubMed] [Google Scholar]
- 196.The DCCT Research Group. Weight gain associated with intensive therapy in the diabetes control and complications trial. The DCCT Research Group. Diabetes Care. 1988;11:567–573. doi: 10.2337/diacare.11.7.567. http://dx.doi.org/10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- 197.Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with Type II diabetes mellitus. Diabetologia. 1999:406–412. doi: 10.1007/s001250051172. [DOI] [PubMed] [Google Scholar]
- 198.Kelly IE, Han TS, Walsh K, Lean ME. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care. 1999;22:288–293. doi: 10.2337/diacare.22.2.288. [DOI] [PubMed] [Google Scholar]
- 199.Mori Y, Murakawa Y, Okada K, Horikoshi H, Yokoyama J, Tajima N, et al. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care. 1999;22:908–912. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]
- 200.Carey DG, Cowin GJ, Galloway GJ, Jones NP, Richards JC, Biswas N, et al. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected] Obes Res. 2002;10:1008–1015. doi: 10.1038/oby.2002.137. http://dx.doi.org/10.1038/oby.2002.137. [DOI] [PubMed] [Google Scholar]
- 201.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. http://dx.doi.org/10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 202.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan WQ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mowers J, Uhm M, Reilly SM, Simon J, Leto D, Chiang S-H, et al. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKK{varepsilon} and TBK1. Elife. 2013;2:e01119. doi: 10.7554/eLife.01119. http://dx.doi.org/10.7554/eLife.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. http://dx.doi.org/10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fisher FM, Chui PC, Antonellis PJ, Bina Ha, Kharitonenkov A, Flier JS, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. http://dx.doi.org/10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Manganiello V, Vaughan M. Selective loss of adipose cell responsiveness to glucagon with growth in the rat. J Lipid Res. 1972;13:12–16. [PubMed] [Google Scholar]
- 207.Livingston JN, Cuatrecasas P, Lockwood DH. Studies of glucagon resistance in large rat adipocytes: 125I-labeled glucagon binding and lipolytic capacity. J Lipid Res. 1974;15:26–32. [PubMed] [Google Scholar]