Abstract
Background
To investigate factors influencing disconnection hyperprolactinemia, including tumour volume, degree of pituitary stalk displacement and extent of tumour growth based on a modified Wilson-Hardy classification in a non-functioning pituitary macroadenoma and to confirm reductions in serum prolactin levels after endoscopic transphenoidal surgery.
Methods
This prospective, descriptive study was conducted in the Department of Neurosurgery, General Hospital Kuala Lumpur from Jan 1, 2011 to Jan 1, 2013. Forty patients fulfilling the inclusion criteria were enrolled. All patients underwent endoscopic transphenoidal resection of non-functioning pituitary macroadenoma. Pituitary stalk angle, tumour volume and extent of tumour growth were measured from Magnetic Resonance Imaging (MRI) pre- and post-operatively. These variables were compared to serum prolactin levels measured pre and post operatively. SPSS 21 was used to perform statistical analyses.
Results
In 40 patients, the mean tumour volumes were 10.58 cm3 (SD 7.81) pre-operatively and 3.1 cm3 (SD 3.45) post-operatively. There was a 70% reduction in tumour volume post-operatively (P < 0.01). The mean serum prolactin was 457 mIU/L (SD 66.93) pre-operatively and 297 mIU/L (SD 6.73) post-operatively. There was a 65% reduction in prolactin serum levels after surgery (P < 0.01). The mean pituitary stalk angles were 93.45 ± 3.89 degrees pre-operatively and 51.45 ± 1.46 degrees post-operatively (P = 0.01). The mean pituitary stalk angle in the control group was 50.4 ± 8.80 degrees. Hence, there was a 98% reduction in pituitary stalk angle after surgery (P < 0.01). This study showed a linear correlation between the pre-operative and post-operative tumour volumes and serum prolactin levels (P = 0.01 pre-and post-operative) and between serum prolactin levels and pituitary stalk angle (P = 0.20 pre-operative; P = 0.01 post-operative).
Conclusion
Tumour volume and pituitary stalk angle displacement have positive predictive values for disconnection hyperprolactinemia in non-functioning pituitary macroadenoma. However, a larger sample size and further objective studies are needed to confirm these findings.
Keywords: pituitary macroadenoma, stalk effect, disconnection hyperprolactinemia
Introduction
Pituitary adenoma accounts for 10% of all cases of intracranial neoplasm. Among these cases, 80% are non-functioning pituitary adenomas. A pituitary tumour presenting with a high serum prolactin level due to hormone secretion (prolactinoma) is called functioning adenoma. Non-secretory pituitary macroadenoma causing hyperprolactinemia is attributed to the stalk effect. In the stalk effect, a compressed pituitary stalk causes a decrease in dopamine release that subsequently leads to unopposed prolactin secretion. Little is known regarding the upper level of serum prolactin levels caused by the stalk effect or the factors influencing the stalk effect, although a few hypotheses have been established. Hence, the objective of this study was to establish clinical correlations for the extent of stalk displacement in a non-functioning pituitary macroadenoma between serum prolactin levels and pituitary tumour volume. Another objective of this study was to reduce serum prolactin levels to near normal ranges after surgical resection.
Methods
This prospective, descriptive study was conducted in the Department of Neurosurgery, General Hospital Kuala Lumpur from Jan 1, 2011 to Jan 1, 2013. Forty patients fulfilling the inclusion criteria were enrolled. This study was designed to evaluate the factors influencing disconnection hyperprolactinemia in a non-functioning pituitary macroadenoma. The serum prolactin level, pituitary adenoma volume, degree of pituitary stalk angle displacement and extent of tumour growth based on a modified Wilson-Hardy classification were evaluated pre- and post-operatively. To reduce the surgical bias, only patients undergoing endoscopic transphenoidal pituitary surgery were included. All patients were screened for other secondary causes of hyperprolactinemia and were confirmed negative.
Blood sampling for serum prolactin was performed in all patients pre-operatively and three months post-operatively. Magnetic Resonance Imaging (MRI) examinations were performed pre- and post-operatively using a SIEMENS AVANTO (1.5 Tesla) MRI scanner unit with 0.1 mg/kg of gadolinium diethylenetriaminepentaacetic acid (DTPA). The pituitary tumour volume was calculated using Cavalieri’s principle after measuring the tumour diameter in three orthogonal planes and using the following formula: tumour volume = 4/3π (a/2.b/2.c/2) (where a, b and c represent the diameters measured in the three dimensions).
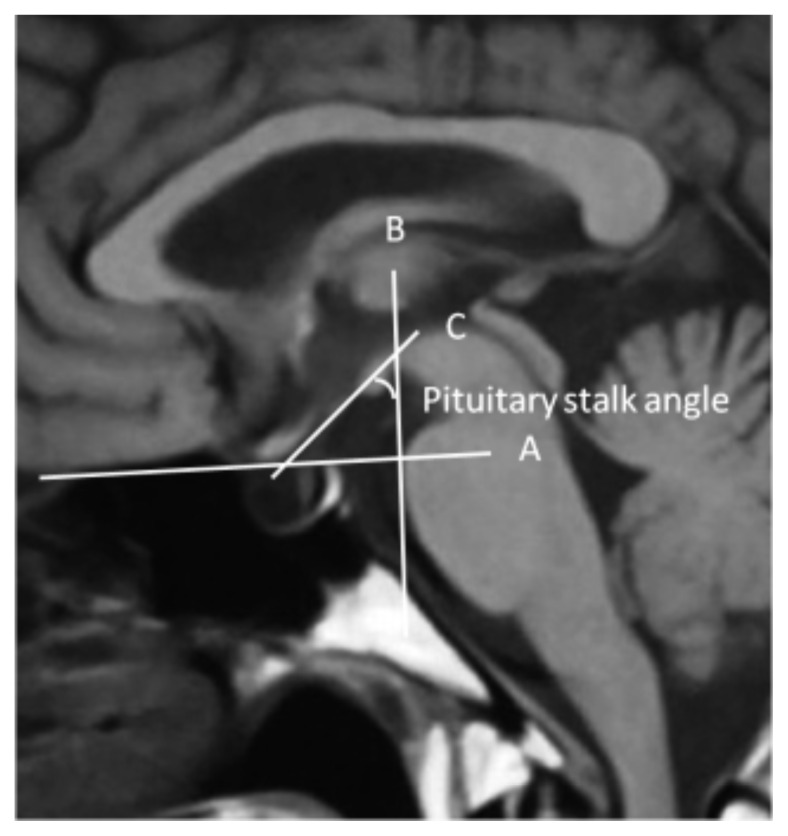
Pituitary stalk angle was calculated from saggital T1 gadolinium-enhanced MRI, as is shown in figure 1. In the figure, line A is drawn horizontally from the frontal base across the sellar incorporating a point in the roof of the sphenoid sinus and dorsum sella and is extrapolated horizontally. Line B is drawn perpendicularly from line A to the origin of the pituitary stalk. Line C is drawn along the pituitary stalk, and it intersects line B. The angle formed by the intersection of these 2 lines was taken as the pituitary stalk angle (calculated in degrees).
Figure 1.
MRI image of Pituitary Gland (Saggital View), shows method of Pituitay Stalk Angle measuremen.
This study also enrolled healthy individuals with normal serum prolactin levels as controls. The pituitary stalk angle was also calculated and analysed in the controls.
Data were statistically analysed using SPSS version 21.0. A descriptive analysis was performed for demographic, biochemical and radiological data. The results are presented as means for quantitative variables and percentages (%) for qualitative variables.
Results
A total of 40 patients (n = 40) were included in our study. The mean age was 48.5 years (SD 12.3), and there was a slight male preponderance with male to female ratio of 3:2. The tumour volumes ranged from 3–33 cm3, with a mean pre-operative value of 10.58 cm3 (SD 7.81). As patients had a non-functioning adenoma, almost 80% of the patients presented with a visual field defect. Others had hypopituitarism and headache as a presenting complaint. Post-operatively, the average tumour volume was 3.1 cm3 (SD 3.45), which, on average, was a 7.48 cm3 (SD 3.46) gross and 70% reduction in tumour volume (P < 0.01).
Increased serum prolactin levels were observed between 100 and 2300 mIU/L. The average serum prolactin level was 457 66. mIU/L (SD 93) pre-operatively and 160 mIU/L (SD 16.93) post-operatively. Therefore, serum prolactin levels decreased by an average of 297 mIU/L (SD 50.00), which amounted to a 65% reduction (P < 0.01). We also identified 5 patients with markedly high serum prolactin levels > 1000 mIU/L. These 5 patients had a small intratumoural haemorrhage without panhypopituitarism.
As for the control group, the average pituitary stalk angle was 50.4 ± 8.80 degrees. In patients with pituitary macroadenoma, the average pituitary stalk angle was 93.45 ± 3.89 degrees pre-operatively and 51.45 ± 1.46 degrees post-operatively. On average, the pituitary stalk angle decreased post-operatively by 42 ± 2.43 degrees (P = 0.01). This post-operative pituitary stalk angle was near that measured in the control group. Summary of results are illustrated in table 1 and figure 2.
Table 1.
Mean with standard deviation of pre-operative and post-operative serum prolactin, pituitary stalk angle and pituitary volume
| Subject | Pre-operative | Post-operative |
|---|---|---|
|
|
||
| Mean value (SD) | ||
| Serum prolactin (mIU/L) | 457.11 (66.93) | 160.90 (16.73) |
| Pituitary stalk angle (degree) | 93.45 (3.89) | 51.48 (1.46) |
| Pituitary tumour volume (cm3) | 10.58 (1.23) | 3.13 (0.54) |
SD: Standard Deviation.
Figure 2.
The graph shows clinical correlation between the serum prolactin, pituitary stalk angle and pituitary tumour volume.
Discussion
The pituitary is a small neuro-endocrine organ that is attached to the hypothalamus by the pituitary stalk and a portal system. The pituitary is composed of two components that are morphologically and functionally distinct, which are the anterior (adenohypophysis) and posterior lobes (neurohypophysis). The adenohypophysis contains five endocrine cell types that produce and secrete pituitary hormones. These five cell types were identified by antibodies against pituitary hormones and include the somatotrophic, lactotrophic, corticotrophic, thyrotrophic, and gonadotrophic cells. The neurohypophysis produces the hormones oxytocin and arginine vasopressin.
The prevalence of pituitary tumours ranges between 2 and 27%, with an average prevalence of 11% (1). In patients who were treated surgically for pituitary tumours, pituitary adenoma accounted for more than 90% of the tumours (2). In patients with macroadenomas, non-functioning adenomas were more common than functioning adenomas, as non-functioning adenomas accounted for over 80% of all pituitary tumours (2).
The initial presentation of non-functioning pituitary macroadenoma depends largely on the size and growth pattern of the tumour. The main presenting symptoms of non-functioning pituitary macroadenoma are headache, visual field defects and hypopituitarism due to mass effect of the tumour. In addition to pituitary deficiencies, non-functioning macroadenomas can be accompanied by hyperprolactinemia. The secretion and release of prolactin is inhibited by hypothalamic dopamine release. A pituitary tumour may disrupt dopamine release by compressing the pituitary stalk and may therefore be accompanied by modest hyperprolactinemia. This clinical syndrome is called stalk effect, pituitary stalk compression syndrome or disconnection hyperprolactinemia (3).
It is sometimes clinically difficult to differentiate a prolactin-secreting tumour from disconnection hyperprolactinemia because some cases demonstrate high serum prolactin levels. Biochemical measurement of serum prolactin level > 6000 – 8000 mIU/L indicates macroprolactinoma, whereas levels < 2000 – 3000 mIU/L indicate a non-functioning pituitary tumour (4).
A positive correlation with increased intrasellar pressure causing hyperprolactinemia was reported by in The Journal of Clinical Endocrinology and Metabolism in 2000. An alternative hypothesis in which the suprasellar tumour secretes a specific pars tuberalis factor that stimulates prolactin secretion, and the candidates for the hypothesised factor were the preprotachykinin A derived tachykinins, substance P and neurokinin A. These tachykinins have been shown to stimulate prolactin release (5,6).
The objective of this study was to examine the factors causing disconnection hyperprolactinemia. First, there were no statistically significant correlations between the pre- or post- operative pituitary volumes and increased serum prolactin levels. However, the correlation coefficients revealed an inverse relationship between these two variables. This might suggest that a larger tumour causes compressive ischaemic necrosis resulting in gland hypofunction. Others observed no significant correlations between serum prolactin levels and pituitary tumour size in their study (7,8).
Next to review is the relationship between pituitary tumour volumes and pituitary stalk angle deviation. This study showed a linear correlation between pituitary tumour volume and pituitary stalk angle (P = 0.01). This correlation was also observed in suprasellar extension of pituitary tumours (P < 0.01). All patients showed a post-operative regression in stalk angle with a decrease in pituitary volume. Such a correlation was not observed between pituitary stalk angle deviation and increased serum prolactin, which we expected to see. However, the correlation coefficient showed a linear but clinically insignificant correlation (P = 0.20).
Based on our study involving 40 patients with confirmed non-functioning pituitary macroadenoma, we found a positive correlation between pituitary tumour volumes and serum prolactin levels and between serum prolactin levels and pituitary stalk angles. In addition, we observed post operatively a 70% reduction in tumour volume, 60% reduction in serum prolactin level and 98% reduction in displaced pituitary stalk angle. Hence, some of these findings are clinically significant in confirming our hypothesis of a positive correlation among pituitary volumes, increased prolactin levels and displaced pituitary stalk angles, and the return of serum prolactin levels to near normal levels with a reduction in the displaced pituitary stalk angle.
Acknowledgement
A special thanks to the entire specialist from Department of Neurosurgery, Radiology, Pathology and Endocrinology, Hospital Kuala Lumpur. I would like to also extend my gratitude to Profesor Jafri Malim from Department of Neuroscience, University Sains Malaysia (USM) and Dr Azmi Alias from Department of Neurosurgery, Hospital Kuala Lumpur.
Footnotes
Conflicts of Interest
None.
Funds
None.
Authors’ Contributions
Conception and design, final approval of the articl: AA, SH
Analysis and interpretation of the data: SH
Drafting of the article, collection and assembly of data: TK
Critical revision of the article for important intellectual content: TK, AA, FRV, JMA
Statistical expertise: FRV
References
- 1.Adams CBT, Burke CW. Current modes of treatment of pituitary tumours. British J Neurosurg. 1993;7(2):123–127. doi: 10.3109/02688699309103468. [DOI] [PubMed] [Google Scholar]
- 2.Arafah BM, Kailani SH, Nekl KE, Gold RS, Selman WR. Immediate recovery of pituitary function following transsphenoidal resection of pituitary macroadenomas. J Clin Endocrinol Metab. 1994;79(2):348–354. doi: 10.1210/jcem.79.2.8045946. doi: http://dx.doi.org/10.1210/jcem.79.2.8045946. [DOI] [PubMed] [Google Scholar]
- 3.Arafah BM, Nekl KE, Gold RS, Selman WR. Dynamics of prolactin secretion in patients with hypopituitarism and pituitary macroadenomas. J Clin Endocrinol Metab. 1995;80(12):3507–3512. doi: 10.1210/jcem.80.12.8530591. doi: http://dx.doi.org/10.1210/jcem.80.12.8530591. [DOI] [PubMed] [Google Scholar]
- 4.Albuquerque FC, Hinton DR, Weiss MH. Excessively high prolactin level in a patient with a nonprolactin-secreting adenoma. J Neurosurg. 1998;89(6):1043–1046. doi: 10.3171/jns.1998.89.6.1043. [DOI] [PubMed] [Google Scholar]
- 5.Lees PD, Pickard JD. Hyperprolactinemia, intra-sellar pituitary tissue pressure, and the pituitary stalk compression syndrome. J Neurosurg. 1987;67(2):192–196. doi: 10.3171/jns.1987.67.2.0192. [DOI] [PubMed] [Google Scholar]
- 6.Lees PD, Falhbusch R, Zrinzo A, Pickard JD. Intra-sellar pituitary tissue pressure, tumor size, and endocrine status-an international comparison in 107 patients. Br J Neurosurg. 1994;8(3):313–318. doi: 10.3109/02688699409029619. [DOI] [PubMed] [Google Scholar]
- 7.Anders K, Jens A, George C, Hans H, Hansen Pressure and blood flow in pituitary adenomas measured during transsphenoidal surgery. Br J Neurosurg. 1992;6(4):333–341. doi: 10.3109/02688699209023792. [DOI] [PubMed] [Google Scholar]
- 8.Arafah BM. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 1986;62(6):1173–1179. doi: 10.1210/jcem-62-6-1173. [DOI] [PubMed] [Google Scholar]