Abstract
Background
The contribution of histamine (H1) receptors inhibitory and/or β-adrenoceptors stimulatory mechanisms in the relaxant property of Ferula assa-foetida. (F. asafoetida) was examined in the present study.
Methods
We evaluated the effect of three concentrations of F. asafoetida extract (2.5, 5, and 10 mg/mL), a muscarinic receptors antagonist, and saline on methacholine concentration-response curve in tracheal smooth muscles incubated with β-adrenergic and histamine (H1) (group 1), and only β-adrenergic (group 2) receptors antagonists.
Results
EC50 values in the presence of atropine, extract (5 and 10 mg/mL) and maximum responses to methacholine due to the 10 mg/mL extract in both groups and 5 mg/mL extract in group 1 were higher than saline (P < 0.0001, P = 0.0477, and P = 0.0008 in group 1 and P < 0.0001, P = 0.0438, and P = 0.0107 in group 2 for atropine, 5 and 10 mg/mL extract, respectively). Values of concentration ratio minus one (CR-1), in the presence of extracts were lower than atropine in both groups (P = 0.0339 for high extract concentration in group 1 and P < 0.0001 for other extract concentrations in both groups).
Conclusion
Histamine (H1) receptor blockade affects muscarinic receptors inhibitory property of F. asafoetida in tracheal smooth muscle
Keywords: Ferula extract, muscarinic receptors, muscle relaxation
Introduction
Asafoetida (F. assafoetida) belonging to the family Apiaceae is the main source of asafoetida which is obtained from the exudates of the living underground rhizome or tap roots of the plant. The other local names for gum-resin are “Anghouzeh”, “Khorakoma”, and “Anguzakoma” (1). It has been used in traditional medicine and cuisine in India and Nepal. F. asafoetida is used in folk medicine for the treatment of epilepsy, stomachache, flatulence, intestinal parasites, asthma, and influenza (2–4) and has aphrodisiac, diuretic and sedative effects (5). Several pharmacological effects such as antioxidant (6), anti-viral (6), anti-fungal (7), cancer chemopreventive (8), anti-diabetic (9), anti-spasmodic (10), and hypotensive (10) have been shown for F. asafetida.
F. asafoetida has been traditionally used for the treatment of asthma and angina pectoris (11), bronchitis, whooping cough and pneumonia in children (12–14).
Ferulic acid esters including; resin, gum fraction including glucose, galactose, l-arabinose, rhamnose, and glucuronic acid, volatile oils including sulphur-containing compounds, free ferulic acid, coumarin derivatives (e.g. umbelliferone), and different monoterpenes are different components of the plant (15). The observed relaxant effect of F. asafoetida on tracheal smooth muscle may indicate a bronchodilatory effect (16). The possible mechanisms of the relaxant effect of the plant on tracheal smooth muscle are thought to be mediated through β-adrenoceptors stimulatory (17), muscarinic (18) and/or histamine (H1) receptors inhibitory (19) effects. The effect of F. asafoetida on muscarinic receptors (functional antagonist) in tracheal smooth muscle was also observed (20).
Previously, the functional antagonistic effect of F. asafoetida on muscarinic receptors was suggested. In the present study, we examined if blockade of histamine receptors and/or β-adrenoceptors stimulatory of F. asafoetida has a role in functional antagonistic effect of the plant on muscarinic receptors.
Materials and Methods
Animals
Guinea pigs (both sexes, weight; 600–800 g) were purchased from Razi Institute, Mashhad, Iran. They were kept in a temperature controlled animal room maintained at 22 ± 2 °C with access to food and water ad libitum during the study period. The guidelines of the Institute of Laboratory Animals Resources, Commission on Life Sciences (21) were followed throughout the experiments. The study was approved by Ethical Committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Tissue preparation
Animal tracheal chain was prepared as described previously (22). The tracheal chain was suspended in an organ bath (organ bath 61300, Bio Science, Kent U.K.) containing 10 mL Krebs-Henseliet solution as described previously (22,23). Tissues were suspended under an isometric tension of 1 g and Krebs solution was changed every 15 minutes for 1 hour to equilibrate with organ bath condition. Using an isotonic transducer (MLT0202, AD Instruments, Australia) connected to a power lab system (PowerLab 8/30, ML870, AD Instruments, Australia), contractions of tracheal smooth muscle were measured.
Plant and extract
From the local market in Mashhad, F. assafoetida was purchased, identified and kept with Herbarium number 293-0606-2, Pharmacognosy Department, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. The aqueous extract of the resin was prepared by dissolving 5 g of the crushed resin in 1 mL dimethyl sulfoxide (DMSO) and 20 mL normal saline. The concentration of F. asafoetida in the stock solution was 250 mg/mL.
Protocol
The effects of the extract of F. assafoetida (2.5, 5 and 10 mg/mL), 0.01 μm atropine (Sigma Chemical Ltd, UK) as positive and saline as negative control on muscarinic receptors were examined as described previously (22) by producing cumulative concentrations of methacholine hydrochloride (Sigma Chemical Ltd UK) and measuring the concentration of methacholine causing 50% of maximum response (EC50). To produce concentration-response curve, the percentage of contraction induced by each concentration in proportion to the maximum contraction obtained in the presence of saline was plotted against log-concentration of methacholine. Slope of methcholine concentration-response curves and its maximum responses to in the presence of three concentrations of the extract and atropine were also measured (24). The concentration-ratio-1 (CR-1) as an indicator of competitive antagonism effect, in experiments with parallel shift in methacholine-response curve was also calculated as previously described (24,25).
The study was done in two experimental groups of incubated tracheal smooth muscle which were incubated with; 1) 1 μM chlorpheniramine (Sigma Chemical Ltd UK), an H1 receptor antagonist and 1 μM propranolol hydrochloride (Sigma Chemical Ltd UK), a β-adrenergic receptor antagonist, (group 1, n = 6) and 2) incubated with only 1 μM propranolol hydrochloride (group 2, n = 7).
Statistical analysis
Data were presented as mean ± SEM. We performed paired t tests to compare the mean of EC50, slope and maximum response values among saline and other treatment groups and the mean of CR-1 between the extract and atropine groups. ANOVA with Tukey Kramer post-hoc test were used to compare the mean of measured parameter among group 1, group 2, and the results of previous study (20) as well as the results of three extract concentrations. InStat version 3.00 (GraphPad Software, San Diego California, USA) was used for statistical analysis. The level of significance was set at P < 0.05.
Results
Comparison of methacholine concentration-response curves between saline, atropine and extract
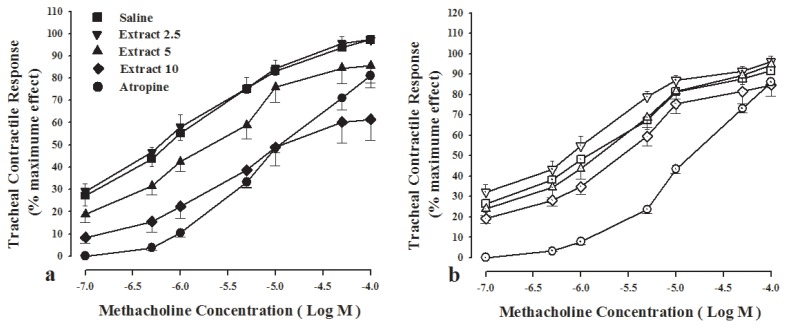
In both groups, methacholine concentration-response curves obtained in the presence of two concentrations of the extract (5 and 10 mg/mL) and atropine showed right-ward shift (Figure 1).
Figure 1.
Concentration-response curves of metacholin in tracheal smooth muscle, in the presence of three concentrations from F. asafoetida extract, saline and 10 nM atropine. (a) Incubated tissues with 1 μM chlorpheniramine and 1 μM propranolol, (group 1, filled, n = 6). The curves showed right ward in the presence of atropine and two higher concentrations of the extract but maximum response to methacholine in the presence of the extract was not achieved in the presence of high extract concentration. (b) Tissues incubated with propranolol, (group 2, open symbols, n = 7). The curves showed right-ward shift in the presence of atropine and two higher concentrations of the extract and maximum response to methacholine in the presence of the extract was achieved.
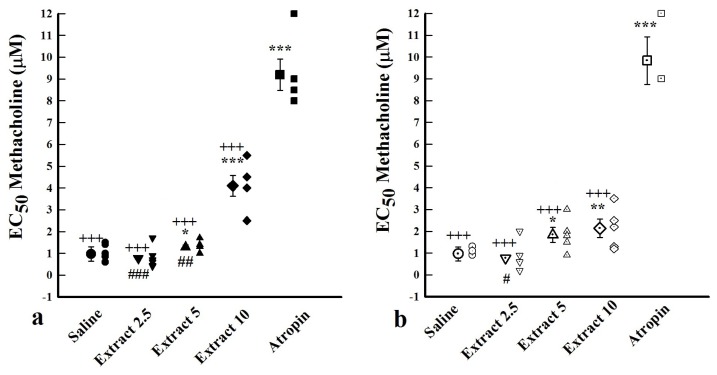
Comparison of EC50 values between saline, atropine and extract
In both groups, EC50 values for methacholine obtained in the presence of atropine and two extract concentrations (5 and 10 mg/mL) were significantly higher compared to saline (P < 0.0001, P = 0.0477, and P = 0.0008 in group 1 and P < 0.0001, P = 0.0438, and P = 0.0107 in group 2 for atropine 5 and 10 mg/mL extract respectively). In group1, EC50 for methacholine in the presence of lower extract concentrations (2.5 and 5 mg/mL) were less than that of the high concentration (10 mg/ml), (P = 0.0008 and P = 0.0029 for low and medium concentrations respectively). The low concentration of the extract (2.5 mg/mL) caused significantly lower EC50 than its high concentration in group 2 (P = 0.0178) (Figure 2).
Figure 2.
Methacholine EC50 values in the presence of three concentrations from F. asafoetida extract, saline and 10 nM atropine. (a) Tissues incubated with 1 μM chlorpheniramine and 1 μM propranolol (group 1, filled symbols, n = 6). (b) Tissues incubated with propranolol (group 2, open symbols, n = 7).
* P < 0.05, *** P < 0.001 compared to saline. +++ P < 0.001 compared to atropine. # P < 0.05, ## P < 0.01, ### P < 0.001 compared to high extract concentration (10 mg/mL).
EC50 values increased in the presence of high extract concentrations and atropine in both groups.
Comparison of maximum response to methacholine among saline, atropine and extract
The P value of 0.001 came from comparing maximum contractile responses to methacholine obtained in the presence of high extract concentration (10 mg/mL) in group 1 and p value 0f 0.01 in group 2 compared to that of saline. We obtained P value of 0.049 when comparing maximum contractile responses to methacholine in the presence of medium extract concentration (5 mg/mL) in group 1 compared to that of saline (Table 1).
Table 1.
Comparisons of maximum response to metacholine in the presence of F. asafoetida extract concentrations (2.5, 5, and 10 mg/mL) and 10 nM atropine with the results of saline in group 1 and 2
| Solutions | Concentration | Group 1 (n=6) | P value vs saline | Group 2 (n = 7) | P value vs saline |
|---|---|---|---|---|---|
| Saline | 100.00 ± 0.00 | 100.00 ± 0.00 | |||
| 2.5 mg/mL | 98.37 ± 1.45 | 0.398 | 98.71 ± 1.28 | 0.519 | |
| Ferula | 5 mg/mL | 85.50 ± 6.98 | 0.049 | 95.57 ± 4.42 | 0.414 |
| 10 mg/mL | 61.45 ± 5.75 | 0.001 | 84.80 ± 5.04 | 0.010 | |
| Atropine | 94.60 ± 2.71 | 0. 356 | 98.80 ± 0.58 | 0.210 |
Mean ± SEM of data in group 1 (tissues incubated with propranolol and chlorpheniramine) and group 2 (tissues incubated with propranolol). The data were compared between saline and other solutions using paired t test.
Comparison of the slope of methacholine concentration-response curves among saline, atropine and extract
There was not significant difference in the slopes of methacholine concentration-response curves among different studied solutions and different groups (Table 2).
Table 2.
Comparisons of slope of metacholine response curves in the presence of F. asafetida extract concentrations (2.5, 5, and 10 mg/mL), 10 nM atropine with the results of saline in group 1and 2 as well as between two groups
| Solutions | Concentration | Group 1 (n = 6) | Group 2 (n = 7) |
|---|---|---|---|
| Saline | 0.92.23 ± 0.076 | 0.97.72 ± 0.01 | |
| 2.5 mg/mL | 0.86.17 ± 0.068 | 0.88.77 ± 0.03 | |
| Ferula | 5 mg/mL | 0.95.19 ± 0.01 | 0.95.63 ± 0.02 |
| 10 mg/mL | 0.95.60 ± 0.005 | 0.97.71 ± 0.01 | |
| Atropine | 0.99.59 ± 0.01 | 0.93.88 ± 0.06 |
Mean ± SEM of data in group 1 (tissues incubated with propranolol and chlorpheniramine) and group 2 (tissues incubated with propranolol). No significance difference was observed among the data of different solution using one-way analysis of variance (ANOVA) with post-hoc test. There was also no significant difference in slope of the curve between two groups as assessed by independent t test.
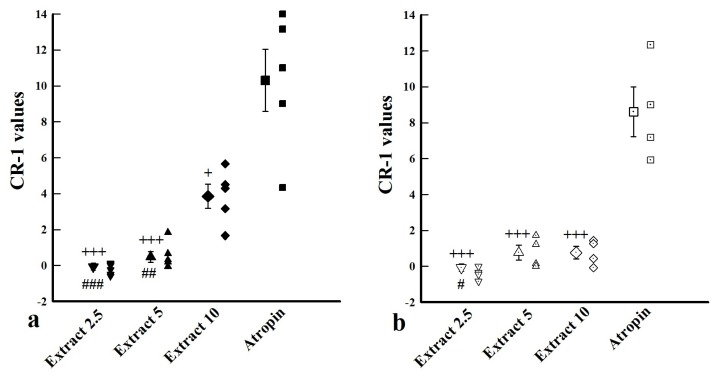
Comparison of CR-1 values among saline, atropine and extract
The (CR-1) values in the presence of all concentrations of the extract in both groups were lower than those of atropine (p value was 0.0339 for high extract concentration in group 1 and for other extract concentrations in both groups were <0.0001, Figure 3). The (CR-1) values in the presence of low extract concentration in both groups (P = 0.0023 and P = 0.0468 for group 1 and 2 respectively) and the medium concentration in groups 1 (P = 0.007) were also lower than the high concentration (Figure 3).
Figure 3.
The values of (CR-1) in the presence of three concentrations from F. asafoetida extract, saline and 10 nM atropine. (a) Tissues incubated with 1 μM chlorpheniramine and 1 μM propranolol (group 1, filled symbols, n=6). (b) Tissues incubated with propranolol (group 2, open symbols, n = 7).
+ P < 0.05; +++ P < 0.001, compared to atropine. ## P < 0.01, ### P < 0.001, compared to high extract concentration (10 mg/mL).
Comparison of EC50, maximum response, slope and (CR-1) among groups 1, 2 and those of previous study
The values of EC50 obtained in the presence of high extract concentration in group 1 were significantly higher than those of group 2 and previous study (P = 0.034 and P = 0.047 respectively; Tables 3 and 4). Maximum response obtained in the presence of high extract concentration in group 1 was lower than that of the group 2 and previous study (P = 0.015 and P = 0.032 respectively, Table 3 and 4). The (CR-1) value in the presence of high extract concentration in group 1 was also higher than those of group 2 and previous study (P = 0.048 and P = 0.014, respectively; Tables 3 and 4).
Table 3.
Comparisons of EC50, Max (maximum response to methacholine), and (CR-1) values in the presence of F. asafoetida extract concentrations (2.5, 5, and 10 mg/mL) between tracheal smooth muscle incubated with propranolol and chlorpheniramine (group 1) and tissues incubated with propranolol (group 2)
| Parameter | Extract concentration (mg/ml) | Group 1 (n = 6) | Group 2 (n = 7) | Group 2 vs group 1 |
|---|---|---|---|---|
| EC50 | 2.5 | 0.68 ± 0.18 | 0.56 ± 0.08 | |
| 5 | 1.18 ± 0.12 | 1.35 ± 0.32 | ||
| 10 | 3.38 ± 0.57 | 1.73 ± 0.29 | 0.034 | |
| Max | 2.5 | 98.37 ± 1.45 | 98.71 ± 1.28 | |
| 5 | 85.50 ± 6.98 | 95.57 ± 4.42 | ||
| 10 | 61.45 ± 5.75 | 84.80 ± 5.04 | 0.015 | |
| CR-1 | 2.5 | −0.08 ± 0.36 | −0.17 ± 0.12 | |
| 5 | 1.17 ± 0.25 | 1.64 ± 0.90 | ||
| 10 | 6.64 ± 1.49 | 2.50 ± 1.13 | 0.048 |
Data were presented as Mean ± SEM. The data of three groups (groups 1, 2) were compared using one-way analysis of variance (ANOVA) with post-hoc test.
Table 4.
Comparisons of EC50, Max (maximum response to methacholine), and (CR-1) values in the presence of F. asafoetida extract concentrations (2.5, 5, and 10 mg/mL) between incubated tracheal smooth muscle with propranolol & chlorpheniramine (group 1) and the results of non incubated tissues (previous study) (16)
| Parameter | Extract concentration (mg/ml) | Previous study (n = 6) | Group 1 (n = 6) | Group 1 vs previous study |
|---|---|---|---|---|
| EC50 | 2.5 | 0.54 ± 0.14 | 0.68 ± 0.18 | |
| 5 | 1.20 ± 0.41 | 1.18 ± 0.12 | ||
| 10 | 1.88 ± 0.37 | 3.38 ± 0.57 | 0.047 | |
| Max | 2.5 | 97.00 ± 1.43 | 98.37 ± 1.45 | |
| 5 | 93.20 ± 2.26 | 85.50 ± 6.98 | ||
| 10 | 80.20 ± 4.13 | 61.45 ± 5.75 | 0.032 | |
| CR-1 | 2.5 | −0.21 ± 0.17 | −0.08 ± 0.36- | |
| 5 | 0.65 ± 0.18 | 1.17 ± 0.25 | ||
| 10 | 1.87 ± 0.29 | 6.64 ± 1.49 | 0.014 |
Data were presented as Mean ± SEM. The data of three groups (group 1, 2 and previous study) were compared using unpaired one-way analysis of variance (ANOVA) with post-hoc test. There was no significant difference between the data of group 2 and previous study.
Correlation between extract concentrations and EC50 values
Significant positive correlations were observed between concentrations of the extract and EC50 in groups 1 (r = 0.79, P < 0.001) and group 2 (r = 0.58, P < 0.01).
Discussion
In previous studies, the relaxant effect of F. assafoetida on smooth muscle in the tracheal chain was shown. Different mechanism(s) responsible for the relaxant effect of F. assafoetida on smooth muscle (16,26) have been suggested including inhibitory effect on muscarinic and histamine (H1) receptors and/or stimulatory effect on β-adrenergic receptors (17–19). Therefore, in the present study, the contribution of (H1) receptors inhibitory and/or β-adrenoceptors stimulatory effects to non-competitive muscarinic receptors seen for F. asafoetida was examined by plotting concentration-response curves in the presence of saline, extract and atropine in incubated tissues with both chlorpheniramine and propranolol to block (H1) receptors and β-adrenergic receptors (group 1) and only propranolol, to inhibit β-adrenergic receptors (group 2).
In order to examine the involvement of beta-adrenergic stimulatory effect and/or histamine (H1) inhibitory effect in functional antagonism effect of the plant at muscarinic receptors (20), in group 1, tissues were incubated with chlorpheniramine and propranolol. The results of group 1 experiments showed a more marked parallel rightward shift in methacholine-response curves in the presence of high and medium concentrations compared to the shift seen in previous study (20). The shift obtained in the presence of high plant concentration in group 1 was comparable with that of atropine. In group 1, EC50 methacholine values due to two higher extract concentrations were greater than the effect of saline. However, (CR-1) values in the presence of these two concentrations of the extract were smaller than that in the presence of atropine. The maximum contraction effect to methacholine with two higher concentrations of the extract was lower than that of saline. The greater EC50 and (CR-1) values obtained in this group compared to those of previous study (20), indicate the contribution of β-adrenergic stimulatory and/or histamine (H1) inhibitory effect to the functional antagonism of the plant observed in previous study (27). However, the lower maximum response to methacholie seen in experiments with high concentration of the plant extract in group 1 suggests non-competitive antagonistic effect of the extract on muscarinic receptors in group 1 (27).
In order to examine the influence of stimulatory effect of β-adrenergic or blocking effect of histamine (H1) on functional antagonism seen for the extract at muscarinic receptors in previous study and group 1, the inhibitory effect of the plant on muscarinic receptors was re-examined in group 2 in tissues incubated only with propranolol. The results of this group were more similar to those of the previous study as compared to data from group 1 of the present study. Although the maximum contractile response to methcholine in the presence of concentration of the plant extract was higher than group 1 but it was not fully achieved in this group. Similar results obtained in group 2 with those of previous study, suggest a histamine (H1) inhibitory effect for the plant rather than a β-adrenergic stimulatory effect. However, stimulatory effect of beta-adrenergic and histamine (H1) inhibitory effect of the extract should be evaluate in future studies by performing concentration-response curves to a β and histamine (H1) receptors agonists with plant extract and evaluate the shift in concentration-response curves to respected agonist. In addition, the relaxant effect of the plant on tissues incubated with chlorpheniramine was reduced which suggest a histamine (H1) inhibitory effect for the plant (16) and support the finding of the present study.
In both groups, the effect of F. assafoetida was concentration-dependent and there were significant correlations between the values of EC50 and plant concentrations which indicated a concentration-dependent effect for the plant.
The relaxant effect of the extract may be due to its constituents ambelliprenin and carvacrol because the relaxant effects of these two constituents on tracheal smooth muscle have been shown previously (28,29). The previous study showed that carvacrol has inhibitory effect on muscarinic receptors of tracheal smooth muscle (30). Therefore, carvacrol could contribute to observed relaxant effect of F. assafoetida gum extract.
Conclusion
Results of the present study showed parallel right-ward shift in the concentration-response curve of methacholine and achievement of maximum response in the presence of F. assafoetida which support the competitive antagonistic effect of F. assafoetida at muscarinic receptors. The absence of maximum response to methacholine in group 1, also suggest an inhibitory effect for the plant on histamine (H1) receptors of tracheal smooth muscles.
Acknowledgment
The authors would like to thanks the Research Department of Mashhad University of Medical Sciences for its materials and financial support of this research project.
Footnotes
Funds
Mashhad University of Medical sciences.
Conflict of Interest
There is not any conflict of interest in this study.
Authors’ Contributions
Conception and design, drafting of the article, critical revision of the article for important intellectual content, provision of study materials or patients: MHB
Analysis and interpretation of the data: MRK
Collection and assembly of data: MK
References
- 1.Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-A review. J Ethnopharmacol. 2011;134(1):1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 2.Takeoka G. Volatile Constituents of Asafoetida, Aroma Active Compounds in Foods. Am Chemic Soc. 2001;794:33–44. doi: 10.1021/bk-2001-0794.ch004. [DOI] [Google Scholar]
- 3.Lee CL, Chiang LC, Cheng LH, Liaw CC, Abd El-Razek MH, Chang FR, Wu YC. Influenza A (H(1)N(1)) Antiviral and Cytotoxic Agents from Ferula assa-foetida. J Nat Prod. 2009;72(9):1568–1572. doi: 10.1021/np900158f. [DOI] [PubMed] [Google Scholar]
- 4.Evans WC. Trease and Evans Pharmacognosy. London (LDN): WB Saunders; 2002. p. 286. [DOI] [Google Scholar]
- 5.Zargari A. Medicinal Plants. Sixth ed. Tehran (TEH): Tehran University Tehran Publications; 1996. [Google Scholar]
- 6.Dehpour AA, Ebrahimzadeh MA, Fazel NS, Mohammad NS. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas y Aceites. 2009;60(4):405–412. doi: 10.3989/gya.010109. [DOI] [Google Scholar]
- 7.Angelini P, Pagiotti R, Venanzoni R, Granetti B. Antifungal and allelopathic effects of Asafoetida against Trichodermaharzianum and Pleurotus spp. Allelopat J. 2009;23(1):357–368. [Google Scholar]
- 8.Saleem M, Alam A, Sultana S. Asafoetida inhibits early events of carcinogenesis: a chemopreventive study. Life Sci. 2001;68(16):1913–1921. doi: 10.1016/S0024-3205(01)00977-8. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Zaiton AS. Anti-diabetic activity of Ferula assafoetida extract in normal and alloxan-induced diabetic rats. Pak J Biol Sci. 2010;15(13):97–100. doi: 10.3923/pjbs.2010.97.100. [DOI] [PubMed] [Google Scholar]
- 10.Fatehi M, Farifteh F, Fatehi-Hassanabad Z. Antispasmodic and hypotensive effects of Ferula asafoetida gum extract. J Ethnopharmacol. 2004;91(2–3):321–324. doi: 10.1016/S0378-8741(04)00028-5. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan K. Spices as influencers of body metabolism: an overview of three decades of research. Food Res Int. 2005;38(1):77–86. doi: 10.1016/j.foodres.2004.09.001. [DOI] [Google Scholar]
- 12.Kapoor LD. Handbook of Ayurvedic Medicinal Plants. first ed. CRC Press: Washington D.C.; 2001. [DOI] [Google Scholar]
- 13.Ross IA. Medicinal plants of the world: chemical constituents, traditional, and modern medicinal uses. Humana Press; Totowa, N.J.: 2005. [DOI] [Google Scholar]
- 14.Appendino G, Maxia L, Bascope M, Houghton PJ, Sanchez-Duffhues G, Munoz E, Sterner O. A meroterpenoid NF-kappaB inhibitor and drimanesesquiterpenoids from Asafetida. J Nat Prod. 2006;69(7):1101–1104. doi: 10.1021/np0600954. [DOI] [PubMed] [Google Scholar]
- 15.Kajimoto T, Yahiro K, Nohara T. Sesquiterpenoid and disulphide derivatives from ferula assa-foetida. Phytochemistry. 1989;28(6):1761–1763. doi: 10.1016/S0031-9422(00)97841-5. [DOI] [Google Scholar]
- 16.Gholamnezhad Z, Byrami G, Boskabady MH, Iranshahi M. Possible mechanism(s) of the relaxant effect of asafoetida (Ferula assa-foetida) oleo-gum-resin extract on guinea-pig tracheal smooth muscle. Avicenna J Phytomed. 2012;2(1):10–16. [Google Scholar]
- 17.Loenders B, Rampart M, Herman AG. Selective M3 muscarinic receptor inhibits smooth muscle contraction in rabbit trachea without increasing the release of acetylcholine. J Pharmacol Exp Ther. 1992;263(2):773–977. [PubMed] [Google Scholar]
- 18.Popa VT, Somani P, Simon P, Simon V. The effect of inhaled verapamil on resting bronchial tone and airway constriction by histamine and acetylcholine in normal and asthmatic subjects. Am Rev Respir Dis. 1984;130(6):106–113. doi: 10.1164/arrd.1984.130.6.1006. doi: 10.1002ptr.1796. M. H[1] [DOI] [PubMed] [Google Scholar]
- 19.Linden A, Bergendal A, Ullman A, Skoogh BE, Lofdahl CG. Salmetrol, formetrol, and salbutamol in the isolated guinea-pig trachea: differences in maximum relaxant effect and potency but not in functional antagonism. Thorax. 1993;48(5):547–553. doi: 10.1136/thx.48.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khazdair MR, Boskabady MH, Kiyanmehr M, Hashemzehi M, Iranshahi M. The effects of Ferula assafoetida on muscarinic receptors of guinea-pig tracheal smooth muscle. Jundishapur J Nat Pharm Prod. 2015;10(3):e20008. doi: 10.17795/jjnpp-20008. [DOI] [Google Scholar]
- 21.Clark J, Baldwin R, Bayne K, et al. Nat Res Council. Washington, DC: Institute of Laboratory Animal Resources; 1996. Guide for the care and use of laboratory animals. [DOI] [Google Scholar]
- 22.Boskabady MH, Kiani S, Aslani MR. Tracheal responsiveness to both isoprenaline and beta2-adrenoreceptor blockade by propranolol in cigarette smoke exposed and sensitized guinea pigs. Respirology. 2006;11(5):572–578. doi: 10.1111/j.1440-1843.2005.00893. [DOI] [PubMed] [Google Scholar]
- 23.Boskabady MH, Ziaei T. Effect of ascorbic acid on airway responsiveness in ovalbumin sensitized guinea pigs. Respirology. 2003;8(4):473–478. doi: 10.1046/j.1440-1843.2003.00511. [DOI] [PubMed] [Google Scholar]
- 24.Jafari Z, Boskabady MH, Pouraboli I, Babazade B. Zataria multiflora Boiss Inhibited Muscarinic Receptors of Incubated Tracheal Smooth Muscle with Propranolol. Avicenna J Phytomed. 2011;1(1):7–13. doi: 10.1155/2014/802189. [DOI] [Google Scholar]
- 25.Boskabady MH, Ghasemzadeh Rahbardar M, Nemati H, Esmaeilzadeh M. Inhibitory effect of Crocus sativus (saffron) on histamine (H1) receptors of guinea pig tracheal chains. Pharmazie. 2010;65(4):300–305. doi: 10.1691/ph.2010.9760. [DOI] [PubMed] [Google Scholar]
- 26.Bayrami G, Boskabady MH, Gholamnezhad Z, Iranshahi M. Dose asafoetida (Ferula aassafoetida oleo-gum-resin) extract and its constituent umbelliprenin have relaxant effects on guinea-pig tracheal smooth muscle. Chin J Integ Med. 2013 doi: 10.1007/s11655-013-1550-3. Epub Ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Arunlakshana O, Schild HO. Some quantitive use of drug antagonist. Br J Pharmacol. 1959;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandyopadhyay D, Basak B, Chatterjee A, Lai TK, Banerji A, Banerji J, Neuman A, Prangé T. Saradaferin, a new sesquiterpenoid coumarin from Ferula asafoetida. Nat Prod Res. 2006;20(10):961–965. doi: 10.1080/14786410600823431. [DOI] [PubMed] [Google Scholar]
- 29.Boskabady MH, Jandaghi P. Relaxant effects of carvacrol on guinea pig tracheal chains and its possible mechanisms. Pharmazie. 2003;58(9):661–663. [PubMed] [Google Scholar]
- 30.Boskabady MH, Jafari Z, Pouraboli I. The effect of carvacrol on muscarinic receptors of guinea pig tracheal chains. Phytother Res. 2011;25(4):530–535. doi: 10.1002/ptr.3290. [DOI] [PubMed] [Google Scholar]