Abstract
Superior canal dehiscence (SCD) is caused by an absence of bony covering of the arcuate eminence or posteromedial aspect of the superior semicircular canal. However, the clinical presentation of SCD syndrome varies considerably, as some SCD patients are asymptomatic and others have auditory and/or vestibular complaints. In order to determine the basis for these observations, we examined the association between SCD length and location with: (1) auditory and vestibular signs and symptoms; (2) air conduction (AC) loss and air-bone gap (ABG) measured by pure-tone audiometric testing, and (3) cervical vestibular-evoked myogenic potential (cVEMP) thresholds. 104 patients (147 ears) underwent SCD length and location measurements using a novel method of measuring bone density along 0.2-mm radial CT sections. We found that patients with auditory symptoms have a larger dehiscence (median length: 4.5 vs. 2.7 mm) with a beginning closer to the ampulla (median location: 4.8 vs. 6.4 mm from ampulla) than patients with no auditory symptoms (only vestibular symptoms). An increase in AC threshold was found as the SCD length increased at 250 Hz (95% CI: 1.7–4.7), 500 Hz (95% CI: 0.7–3.5) and 1,000 Hz (95% CI: 0.0–2.5), and an increase in ABG as the SCD length increased at 250 Hz (95% CI: 2.0–5.3), 500 Hz (95% CI: 1.6–4.6) and 1,000 Hz (95% CI: 1.3–3.3) was also seen. Finally, a larger dehiscence was associated with lowered cVEMP thresholds at 250 Hz (95% CI: −4.4 to −0.3), 500 Hz (95% CI: −4.1 to −1.0), 750 Hz (95% CI: −4.2 to −0.7) and 1,000 Hz (95% CI: −3.6 to −0.5) and a starting location closer to the ampulla at 250 Hz (95% CI: 1.3–5.1), 750 Hz (95% CI: 0.2–3.3) and 1,000 Hz (95% CI: 0.6–3.5). These findings may help to explain the variation of signs and symptoms seen in patients with SCD syndrome.
Keywords: Superior canal dehiscence, size; Superior canal dehiscence, location; Length, auditory; Audiometry; Cervical vestibular-evoked myogenic potential
Introduction
Superior canal dehiscence (SCD) syndrome is caused by an absence of bony covering of the superior semicircular canal (SSC) and was first described by Minor et al. [1998]. Patients with SCD syndrome typically present with auditory and/or vestibular complaints, low-frequency air-bone gap (ABG) and lowered cervical vestibular-evoked myogenic potential (cVEMP) thresholds. The clinical presentation of SCD syndrome varies widely, from auditory symptoms only (hyperacusis, autophony, aural fullness, hearing loss and/or pulsatile tinnitus) to vestibular symptoms only (imbalance, and sound-, pressure- and/or exercise-associated dizziness), to a combination of auditory and vestibular symptoms. It has been theorized that the ‘third window’ created by SCD alters the inner ear fluid flow, causing auditory and vestibular complaints. Vestibular symptoms are thought to be caused by the entrainment of the cupula due to SSC fluid motion elicited by sound [Carey et al., 2004]. Hearing loss may be related to the shunting of acoustic energy away from the cochlea, resulting in a reduction of the stimulus to the hearing mechanism. Rosowski et al. [2004; Songer and Rosowski, 2007] hypothesized that the magnitude of the ABG is influenced by SCD length and location. Our group has recently characterized the effect of SCD length on air conduction (AC) loss in a human temporal bone model [Pisano et al., 2012].
The literature is conflicting regarding the association of SCD length with signs and symptoms of SCD syndrome. A larger defect has been shown to correlate with vestibulocochlear manifestations in one study [Pfammatter et al., 2010], while other studies did not find an association between SCD length and clinical presentation [Martin et al., 2009; Chi et al., 2010; Chien et al., 2012]. The ABG increased with a larger SCD in two studies [Yuen et al., 2009; Chien et al., 2012], while other studies found no correlation between SCD length and degree of hearing loss [Mikulec et al., 2004; Chi et al., 2010]. Relatively small sample sizes and varied methods of measuring SCD length might explain these conflicting results. Research in chinchillas and in a human temporal bone model suggests that the correlation of dehiscence length with hearing sensitivity is more complicated than a monotonic relationship. For example, Pisano et al. [2012] showed that the smallest dehiscence had the largest effects, consistent with more mid-frequency hearing loss, in some ears. Furthermore, the effect of SCD length appears to reach a maximum once the dehiscence has reached a certain length [Songer and Rosowski, 2007; Niesten et al., 2013a]. Recent reports that examined SCD location and clinical presentation were limited by varied methods of measuring the defect and a relatively small sample size [Martin et al., 2009; Pfammatter et al., 2010]. To resolve these contradictory findings, we performed a detailed analysis on a large cohort of SCD patients using a novel method for measuring SCD length and location by high-resolution CT. Specifically, we sought to determine the association of SCD length and location with auditory and vestibular signs and symptoms, magnitude of the ABG and cVEMP thresholds.
Methods
Selection of Patients, Patient Characteristics, and Signs and Symptoms
This study was approved by the Human Studies Committee of the Massachusetts Eye and Ear Infirmary (Protocol No. 09-08-088; principal investigator: D.J.L.). We identified 147 ears from 104 patients with SCD syndrome that underwent high-resolution temporal bone CT (HR-CT) at the Massachusetts Eye and Ear Infirmary between 2000 and 2011. The presence of an anatomical SCD was based on HR-CT of the temporal bone without contrast. The diagnosis of SCD syndrome was based on clinical signs and symptoms with complementary audiometric and vestibular testing. A chart review was performed to collect demographic patient data and to assess various clinical signs and symptoms, including auditory signs and symptoms (hyperacusis, autophony, aural fullness, report of hearing loss and/or tinnitus) and vestibular signs and symptoms (imbalance; sound-, pressure- and exercise-associated dizziness; Tullio’s phenomenon and/or Hennebert’s sign). It is important to note that ‘auditory signs and symptoms’ are subjective findings reported by the patient and are not always associated with abnormalities on audiometric measurements.
Audiometric Data
Audiometric testing was done at the Massachusetts Eye and Ear Infirmary as part of the patients’ clinical evaluation as described in Niesten et al. [2013b]. If multiple audiograms were performed, the test closest in time to the HR-CT was used for analysis. AC and bone conduction (BC) thresholds were measured at 250, 500, 1,000, 2,000, 4,000 and 8,000 Hz (for BC up to 4,000 Hz). The ABG was calculated as a difference between the AC threshold and the BC threshold from 250 to 4,000 Hz.
cVEMP Data
cVEMP testing is a standard procedure at our institution to aid in diagnoses of SCD syndrome. cVEMP thresholds were determined at 250, 500, 750 and 1,000 Hz, and converted from normal hearing level to peak sound pressure as described by Rauch et al. [2004].
Radiology
CT imaging was performed in a multidetector row CT scanner (Somatom Sensation 40; Siemens Healthcare, Erlangen, Germany) by using a standardized temporal bone CT protocol. Radiologic assessment of the superior canal was performed by HR-CT, available on our picture archiving and communication system (Synapse). The settings were as follows: 120 kVp tube voltage, 320 mAs effective tube current and a helical scanning mode with a pitch factor of 0.55. Axial, Pöschl and Stenvers views were reconstructed at 0.5-mm intervals separately for the left and right ears by using a 0.6-mm image thickness, 10-cm reconstruction field-of-view and an ultra-high-resolution kernel (U70u).
Curved Reconstruction of the Superior Canal
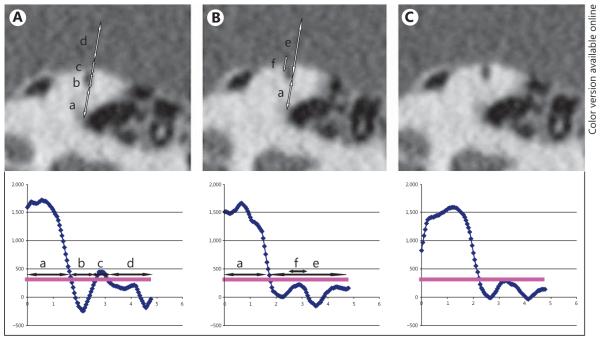
Voxar 3D (Toshiba) was used to view the oblique multiplanar reformatted images while making a curved reconstruction of the SSC, which was divided into approximately 80 radial sections of 0.2 mm in width (fig. 1). Measurements of bone density around the SSC were used to identify a dehiscence on radial sections. Specifically, the density profile along the radial dimension of each section (based on a straight line beginning at the temporal bone, through the center of the canal, passing through the SSC and ending in the brain) was plotted in Hounsfield units (HU) using ImageJ software (National Institutes of Health) [Schneider et al., 2012]. To determine the bone density in intact superior canals, we made measurements in 8 patients (16 ears) who (1) did not have a bony dehiscence or (2) had an intact but thin bone covering the SSC. We measured the thinnest part of the bony covering of the intact superior canal (mean = 1,130 HU; SD = 267 HU). To minimize the risk of diagnosing intact bone as a dehiscence, an HU value below 300 (less than the mean value of the bone minus 3 SD) was selected as a threshold below which the HU values were assumed to indicate an absence of the bony covering of the SSC (fig. 2).
Fig. 1.
Reconstruction of an oblique multiplanar reformatted CT image in the plane axial and parallel through the semicircular canal (A, B) of the left ear of a patient. Images C and D show the lines drawn through the semicircular canal, starting at the side of the ampulla and ending toward the common crus. Images E and F show the reconstruction divided into radial sections with a thickness of 0.2 mm, perpendicular to the bony covering of the superior canal, on which density measurements were performed.
Fig. 2.
Density measurements made in three 0.2-mm-thick radial CT images through the SSC taken at different positions in a single patient. The radiodensity of the SSC was measured by drawing a line through the semicircular canal (a–d). Density measurements below 300 HU (horizontal line in the plot) indicate an absence of bone overlying the semicircular canal. Y-axis: HU; x-axis: length of the line that is drawn, in millimeters. The length and location of each dehiscence can be determined by counting the number of sections showing a dehiscence and the number of sections between the ampulla and the start of the SCD, respectively. In the figure, lines are drawn slightly off centre to show the dehiscence or thin bony covering in this figure. A Example of a thin bony covering of the SSC. Arrow a: temporal bone; arrow b: lumen of the canal; arrow c: bony covering of the SSC; arrow d: brain. B Example of an SCD. Arrow e: lumen of the SSC and the brain; arrow f: lack of bony covering (the density measurement stays just below 300 HU, confirming a dehiscence). C Similar to B, but the dehiscence is less obvious; however, the density measurements show thresholds below 300 HU, indicating a dehiscence.
The length of a dehiscence was calculated by multiplying the number of radial sections in which the dehiscence was found with the thickness of the radial section (0.2 mm). If multiple dehiscences were present (with bone covering the SSC between two dehiscent sections), the total SCD length was the sum of the thickness of multiple sections. SCD location was determined by counting the number of radial sections from the ampulla to the start and end of the SCD, and by multiplying this by the thickness of the radial sections. In our later descriptions of SCD location, we use the start of the SCD in millimeters from the ampulla, because we did not find any statistically significant correlation between any of our variables and the end of the SCD. Independent measurements of dehiscence length and location were performed by two of the authors; one author measured them in the first 46 patients, and another author in the remaining 58 patients. To determine consistency between these two authors, 5 patients were measured by both authors, and the mean difference in SCD length was 0.24 mm and the mean difference in SCD starting location was 0.2 mm, i.e. the differences between authors were at the limit of the imaging resolution.
Data Analysis
Statistical software (SPSS version 15.0) was used to analyze the data. We assessed the strength of the relationship between SCD length and location and patient characteristics (as well as the presence of a sign or symptom) by calculating the ‘effect size’ (<0.3 indicates a small effect). We analyzed whether differences in SCD length and location existed by: gender; patient age (patients younger or older than the median age of 45 years); duration of complaints until CT scanning (shorter or longer than the median period of 18 months); or the presence or absence of a second event preceding the onset of symptoms. A ‘first event’ is considered to be congenital thinning of the bone overlying the SSC. In some patients, the onset of SCD symptoms is associated with a ‘second event’, an activity that dramatically affects inner ear pressure (such as head trauma, excessive straining, coughing or child birth [Watters et al., 2006]). We also assessed the strength of the relationship between the presence of a sign or symptom (for each sign separately) and SCD length and location, by using effect size. In the case of a bilateral dehiscence, the more symptomatic ear in each patient was used for analysis.
We divided the SCD patients into three groups: (1) those with only auditory signs and symptoms, such as hyperacusis, autophony, aural fullness, hearing loss and/or tinnitus; (2) those with only vestibular signs and symptoms, such as imbalance, sound-, pressure- and exercise-associated dizziness, Tullio’s phenomenon and/ or Hennebert’s sign, and (3) those with both auditory and vestibular signs and symptoms. Because the distribution of SCD location data in these patients barely met the criteria for a normal distribution, we used nonparametric statistics [that do not assume a particular distribution, e.g. median, interquartile ranges (IQRs) and Mann-Whitney U tests] to compare for differences in SCD length and location among the three different groups. In each case we compared the difference between one group and the two other groups combined, to determine whether a difference exists between these patients and the remainder of the group.
We compared AC and BC thresholds and the ABG with SCD length and location (due to a significant correlation between SCD length and location) by using linear regression, reported as the 95% CI around the slope. In patients with bilateral dehiscence, both ears were used for audiometric testing as the pure-tone average could be measured for each ear individually. Similarly, cVEMP thresholds were analyzed by linear regression analysis for both SCD length and location, reported as the 95% CI around the slope. Regression analyses were done on ears without a history of ear disease or ear surgery, because these conditions could affect the pure-tone average and cVEMP threshold data independently of the SCD status.
Results
Selection of Patients
From our database of 146 patients diagnosed with SCD between 2000 and 2011, 104 patients (147 ears) with SCD syndrome underwent temporal bone HR-CT imaging at our institution. Out of the 104 patients, 34 (33%) had left SCD, 27 (26%) had right SCD, and 43 (41%) had bilateral SCD. The median SCD length was 4.4 mm (IQR: 2.8–5.4 mm) and the median SCD starting location was 5.0 mm (IQR: 4.2–6.0 mm) from the ampulla. Patient characteristics and signs and symptoms were analyzed for 104 ears (only the more symptomatic ear of the 104 patients). Audiometric data were available for 146 ears, and cVEMP data for 76 ears. After exclusion of ears with a history of ear disease or surgery, we analyzed the audiometric data on 118 ears and cVEMP data on 66 ears. See figure 3 for the flowchart showing patient selection.
Fig. 3.
Patient selection flowchart. From our database of 146 patients with SCD, 128 had symptomatic SCD. Of these 128 patients, 104 underwent CT scanning at the Massachusetts Eye and Ear Infirmary (MEEI). Signs and symptoms were analyzed in all 104 patients. Audiometric and cVEMP testing was performed on ears without a history of ear surgery.
Patient Characteristics and Signs and Symptoms
The mean age of the patients was 47 years (range: 15– 85 years), and 58 of the 104 patients (56%) were female. The presence of a second event prior to the onset of SCD symptoms was noted in 25 out of the 50 patients that were specifically asked this question based on their medical record. We did not find a strong correlation of gender, age, duration of complaints and the presence or absence of a preceding second event with SCD length or location (effect size: <0.15).
Signs and symptoms were correlated separately with SCD length and location, and included hyperacusis, autophony, aural fullness, tinnitus, imbalance and sound- and pressure-associated dizziness. These variables individually did not show a significant correlation with SCD length or location.
We then divided the SCD patients into three groups based on signs and symptoms, as described in the methods section: (1) auditory signs and symptoms only; (2) vestibular signs and symptoms only, and (3) both auditory and vestibular signs and symptoms. We compared each group with the other two groups combined. Our data indicate that a larger dehiscence starting closer to the ampulla was found in patients with auditory symptoms (with or without vestibular symptoms) as compared with the group that had no auditory symptoms (vestibular symptoms only; Mann-Whitney U test for SCD length = 0.03, and for SCD location = 0.004; fig. 4; table 1).
Fig. 4.
Box-and-whisker plots showing the association of signs and symptoms with SCD length (A) and SCD location (B) in millimeters from the ampulla. Box: median and IQR; whiskers: range; auditory: audiometric complaints (hyperacusis, autophony, aural fullness, report of hearing loss and/or tinnitus) with or without vestibular complaints; no auditory: vestibular complaints only (imbalance, sound-, pressure- or exercise-induced dizziness, Tullio’s phenomenon and/or Hennebert’s sign). The group with auditory complaints had 98 patients (94%), the group with no auditory (only vestibular) symptoms had 6 patients (6%). Significant differences between the auditory group and the ‘no auditory’ (vestibular only) group were found for both length (Mann-Whitney p = 0.03) and location (Mann-Whitney p = 0.004).
Table 1.
Relationship of SCD size and location with signs and symptoms
| Group | Median | Comparison group | Median | MW | |
|---|---|---|---|---|---|
| Size | auditory only | 4.4 (3.4 – 5.4) | vestibular only + auditory and vestibular | 4.4 (2.9 – 5.9) | 0.821 |
| no auditory (vestibular only) | 2.7 (1.4 – 4.2) | auditory only + auditory and vestibular | 4.5 (3.4 – 5.9) | 0.030 | |
| auditory and vestibular | 4.6 (3.2 – 6.2) | auditory only + vestibular only | 4.2 (3.0 – 5.1) | 0.382 | |
|
| |||||
| Location | auditory only | 4.8 (4.0 – 6.0) | vestibular only + auditory and vestibular | 5.0 (4.1 – 5.7) | 0.838 |
| no auditory (vestibular only) | 6.4 (5.7 – 10.7) | auditory only + auditory and vestibular | 4.8 (4.0 – 5.6) | 0.004 | |
| auditory and vestibular | 5.0 (4.0 – 5.6) | auditory only + vestibular only | 5.0 (4.1 – 6.3) | 0.209 | |
Values in parentheses denote IQR. Auditory only = auditory signs and symptoms only; no auditory (vestibular only) = vestibular signs and symptoms only; auditory and vestibular = auditory and vestibular signs and symptoms; MW = Mann-Whitney test.
Data Analysis: Audiometric Testing
We analyzed AC and BC thresholds and the ABG in 118 ears with SCD. Twenty-eight out of 147 ears were excluded due to a history of ear disease or ear surgery (severe sensorineural hearing loss, prolapse of the dura abutting the ossicular chain, mastoidectomy, atticotomy, stapes surgery, glomus tympanicum, acoustic neuroma removal and middle fossa craniotomy). Audiometric data were not available for 1 patient. By linear regression analysis, we found that a larger dehiscence showed significantly more AC loss at 250 Hz (95% CI: 1.7–4.7), 500 Hz (95% CI: 0.7–3.5) and 1,000 Hz (95% CI: 0.0–2.5) and a significantly larger ABG at 250 Hz (95% CI: 2.0–5.3), 500 Hz (95% CI: 1.6–4.6) and 1,000 Hz (95% CI: 1.3–3.3). No correlation with SCD length was found for AC loss or the ABG for frequencies above 1,000 Hz, as well as for BC loss.
Finally, linear regression analyses showed a statistically significant relationship of SCD length and location with ABG. After adjusting for this correlation, we did not observe a statistically significant relationship between SCD location and AC loss, BC loss or ABG. Figure 5 shows a scatter plot of the association of ABG at 250 Hz with SCD length and location.
Fig. 5.
Scatter plots correlating ABG with SCD length (A) and location (B) in patients with data on the ABG (excluding 28 patients with a history of ear disease; n = 118). Each graph shows the linear regression line. When both length and location are used in two-factor linear regression (R2 = 0.19, F = 12.6), SCD length shows the 95% CI around the slope of 2.0–5.3 (p < 0.001), and SCD location shows the 95% CI around the slope of −1.7 to 1.8 (p = 0.954). A Intercept = 4.9; slope = 3.6; R2 = 0.19; F = 25.4 (p < 0.001). B Intercept = 31.1; slope = −2.0; R2 = 0.05; F = 5.8 (p = 0.018).
Data Analysis: cVEMP Testing
We analyzed cVEMP testing in 66 ears with SCD. On 80 ears, cVEMP testing results were not available, and 10 patients were excluded due to a history of ear disease or surgery. Linear regression analyses showed a statistically significant relationship of SCD length and location with cVEMP thresholds. After adjusting for this relationship, lowered cVEMP thresholds were seen with a larger dehiscence at 250 Hz (95% CI: −4.4 to −0.3), 500 Hz (95% CI: −4.1 to −1.0), 750 Hz (95% CI: −4.2 to −0.7) and 1,000 Hz (95% CI: −3.6 to −0.5) and a starting location closer to the ampulla at 250 Hz (95% CI: 1.3–5.1), 750 Hz (95% CI: 0.2–3.3) and 1,000 Hz (95% CI: 0.6–3.5). These data can be found in figure 6.
Fig. 6.
Scatter plots correlating cVEMP with SCD length (A) and location (B) in patients with cVEMP testing (excluding 10 patients with a history of ear disease; n = 66). Each graph shows the linear regression line. When both length and location are used in two-factor linear regression (R2 = 0.36, F = 17.4), SCD length shows the 95% CI around the slope of −4.4 to −0.3 (p = 0.024), and SCD location shows the 95% CI around the slope of 1.3–5.1 (p = 0.001). pSP = peak sound pressure. A Intercept = 125.0; slope = −4.2; R2 = 0.23; F = 19.9 (p < 0.001). B Intercept = 83.9; slope = 4.4; R2 = 0.30; F = 27.6 (p < 0.001).
Exclusive of SCD length, the location of the dehiscence did not significantly correlate with cVEMP thresholds at 500 Hz (95% CI: −0.4 to 2.5).
Discussion
Our radiologic study of 104 patients with SCD syndrome showed that (1) a larger dehiscence located closer to the ampulla was associated with auditory symptoms, with or without vestibular symptoms; (2) a larger ABG was associated with a larger dehiscence and not associated with SCD location, and (3) a lower cVEMP threshold was associated with a larger dehiscence located closer to the ampulla. Differences in length and location of the bony defect may help to explain the varied clinical presentation of SCD syndrome.
Signs and Symptoms in SCD
Patients without auditory symptoms (only vestibular symptoms, such as imbalance, sound-, pressure- or exercise-associated dizziness, Tullio’s phenomenon and/or Hennebert’s sign) demonstrated less spread in the length and location of their SCD than patients with auditory symptoms (hyperacusis, autophony, aural fullness, report of hearing loss, tinnitus). The smaller sample size of the ‘vestibular only’ group might contribute to these findings. When auditory signs and symptoms were present, the dehiscence was usually located near the ampulla of the SSC (and thus closer to the cochlea), whereas when auditory signs and symptoms were not present (vestibular symptoms only), the dehiscence was located further from the ampulla. Auditory symptoms were usually associated with a larger bony defect, whereas no auditory symptoms (vestibular symptoms only) were associated with a small dehiscence.
Differences in measurement techniques and patient selection may help to explain why our data are not in agreement with other recent reports. One study correlated intraoperative SCD length with clinical findings, audiometric testing and cVEMP testing [Chien et al., 2012], but because only patients who underwent surgical repair were included, patients with mild symptoms or with only auditory symptoms may not have been adequately represented. We included all symptomatic SCD patients (whether or not they underwent surgery) that had imaging available for detailed analysis, allowing us to study a wide range of SCD lengths and locations to correlate with signs and symptoms. Although we observed a range of symptoms across a number of patients who had a variety of defect sizes and locations, larger dehiscences closer to the ampulla were rather associated with auditory (with or without vestibular) symptoms than with vestibular symptoms alone. Such information is clinically valuable and helps to explain why, for example, a patient with a small bony defect located distant from the ampulla may not have auditory symptoms.
Audiometric Testing in SCD
Rosowski et al. [2004] suggested that the impedance through a superior canal defect will vary according to length and location; thus a larger dehiscence would theoretically lead to lower impedances and more low-frequency conductive loss. Pisano et al. [2012] examined the effect of SCD length on intracochlear pressure measurements in fresh human cadaveric temporal bones and computed the differential pressure across the cochlear partition. This differential pressure measurement is related to sound input to the cochlea, and provides an estimate of hearing in a human temporal bone model. They showed that acoustic input to the cochlea was reduced monotonically with an increase in length of the SCD at low frequencies (below 1 kHz). Surprisingly, at higher frequencies (above 1 kHz), sometimes the smallest SCD (<0.5 mm diameter, generally below the resolution of CT) showed more reduction in cochlear input than the larger SCD [Pisano et al., 2012]. Our clinical data are in accordance with these low-frequency findings in the temporal bone model: patients with a larger SCD have higher AC thresholds (and larger ABG) across three frequencies. SCD location, however, does not significantly contribute to AC thresholds or the magnitude of the ABG. This final observation will need to be validated in our temporal bone model.
cVEMP in SCD
The cVEMP measures the function of the saccule and the inferior vestibular nerve, and low thresholds and large amplitudes are often seen in symptomatic SCD ears [Brantberg et al., 1999; Streubel et al., 2001; Belden et al., 2003; Curtin, 2003; Roditi et al., 2009]. The bony defect is felt to create a ‘third window’ or low-impedance fluid pathway, resulting in enhanced vestibular sensitivity. One study reported lower cVEMP thresholds in patients with larger canal defects [Pfammatter et al., 2010], but another study showed no association with intraoperative SCD length and VEMP thresholds [Chien et al., 2012]. The influence of SCD location on cVEMP thresholds, however, has not been described before. We theorize that an SCD located closer to the saccule (and thus closer to the ampulla of the SSC) creates a lower fluid impedance pathway shunting energy through this third window, leading to lower cVEMP thresholds (as shown by our data). In future studies we will examine the SSC fluid flow in temporal bones and in computational models to better assess these anatomic relationships. However, cVEMP thresholds can be affected by multiple variables (e.g. sternocleidomastoid muscle mass, otosclerosis or middle ear disease) and are found to be lowered in other third-window conditions such as large vestibular aqueduct syndrome [Merchant et al., 2007].
Limitations of This Study
Most studies that have assessed SCD length used linear CT scanning measurements of the curved superior canal. We developed a method of assessing SCD length taking the curvature of the superior canal into account by making an oblique reconstruction of the SSC. However, recent studies have described the risk of overestimation of the SCD length by using CT scans [Sequeira et al., 2011; Tavassolie et al., 2012]. Risk of overestimation and false-positive diagnoses of SCD have especially been described for patients with a small dehiscence (<3 mm) [Sequeira et al., 2011]. To minimize the risk of (1) overestimating SCD length or (2) missing a very thin layer of bone due to the partial volume averaging effect, we determined a conservative HU cutoff point (less than the mean HU value for bone minus 3 SD) for the bone to be defined as absent. In addition, SCD size on CT scanning measurements can be correlated with the intraoperative measurements. However, precise measurements of the dehiscence are difficult to obtain intraoperatively due to (1) angulation of the defect relative to the measuring instrument; (2) blood and occasionally cerebrospinal fluid in the field; (3) the need to continuously immerse the field with irrigation fluid to reduce injury to the labyrinth, and (4) the desire to repair the dehiscence in a timely manner to minimize prolonged exposure of the membranous labyrinth. Finally, we used radial measurements in this study, and the measurement during surgery is linear. A more precise and efficient way to measure these defects intraoperatively needs to be developed, perhaps incorporated into the microscope or endoscope (which provides a superior and high-magnification view of the defect; data in press (accepted by the Laryngoscope, November 2013) as a superimposed heads-up display scale bar that could be rotated to conform to the defect.
While we describe significant correlations of SCD length and location with the magnitude of the ABG and cVEMP thresholds, these relationships only explain about 20–30% (R2 of 0.19 and 0.36, respectively; fig. 5, 6) of the variability in ABG and cVEMP thresholds. Other factors that might influence these outcomes need to be further studied, such as the effect of natural plugging or ‘autoplugging’ of the dura and/or brain on SCD signs and symptoms, as recently described [Brandolini and Modugno, 2012].
Clinical Implications
When a patient presents with signs and symptoms suspicious for SCD syndrome, high-resolution CT imaging with Stenvers and Pöschl reformats are used to confirm the presence of a bony defect of the superior canal. Audiometric and VEMP testing are used to support the diagnosis and determine the ‘worse ear’, but not all patients present with classical test results (e.g., low-frequency ABG, lower cVEMP thresholds). Our data help explain why normal cVEMP thresholds or no ABG are seen in some patients with positive SCD symptoms and CT findings and provide important information in the surgical counseling of patients with SCD.
Conclusions
We found that: (1) patients with auditory symptoms (with or without vestibular symptoms) have a larger dehiscence located closer to the ampulla; (2) a larger ABG is associated with a larger SCD, while dehiscence location did not influence the AC threshold or ABG, and (3) lower cVEMP thresholds are found in patients with a larger dehiscence located closer to the ampulla. We believe that both SCD length and location are important features to assess in the evaluation of temporal bone CT imaging in patients with SCD syndrome. Our findings help explain why symptomatic patients with a smaller SCD located further away from the ampulla may not present with an ABG or with lowered cVEMP thresholds.
Acknowledgments
We appreciate the help of Saumil Merchant, MD, and John Rosowski, PhD, with the initial design of this study and for reviewing previous versions of the manuscript. This article is dedicated to the memory of Saumil Merchant (1960–2012).
References
- Belden CJ, Weg N, Minor LB, Zinreich SJ. CT evaluation of bone dehiscence of the superior semicircular canal as a cause of sound- and/or pressure-induced vertigo. Radiology. 2003;226:337–343. doi: 10.1148/radiol.2262010897. [DOI] [PubMed] [Google Scholar]
- Brandolini C, Modugno GC. Do signs of natural plugging of superior semicircular canal dehiscence exist? Am J Otolaryngol. 2012;33:268–271. doi: 10.1016/j.amjoto.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Brantberg K, Bergenius J, Tribukait A. Vestibular-evoked myogenic potentials in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol. 1999;119:633–640. doi: 10.1080/00016489950180559. [DOI] [PubMed] [Google Scholar]
- Carey JP, Hirvonen TP, Hullar TE, Minor LB. Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol Neurotol. 2004;25:345–352. doi: 10.1097/00129492-200405000-00024. [DOI] [PubMed] [Google Scholar]
- Chi FL, Ren DD, Dai CF. Variety of audiologic manifestations in patients with superior semicircular canal dehiscence. Otol Neurotol. 2010;31:2–10. doi: 10.1097/mao.0b013e3181bc35ce. [DOI] [PubMed] [Google Scholar]
- Chien WW, Janky K, Minor LB, Carey JP. Superior canal dehiscence size: multivariate assessment of clinical impact. Otol Neurotol. 2012;33:810–815. doi: 10.1097/MAO.0b013e318248eac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin HD. Superior semicircular canal dehiscence syndrome and multi-detector row CT. Radiology. 2003;226:312–314. doi: 10.1148/radiol.2262021327. [DOI] [PubMed] [Google Scholar]
- Martin C, Chahine P, Veyret C, Richard C, Prades JM, Pouget JF. Prospective radiological study concerning a series of patients suffering from conductive or mixed hearing loss due to superior semicircular canal dehiscence. Eur Arch Otorhinolaryngol. 2009;266:1175–1181. doi: 10.1007/s00405-008-0862-y. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Nakajima HH, Halpin C, Nadol JB, Jr, Lee DJ, Innis WP, Curtin H, Rosowski JJ. Clinical investigation and mechanism of airbone gaps in large vestibular aqueduct syndrome. Ann Otol Rhinol Laryngol. 2007;116:532–541. doi: 10.1177/000348940711600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulec AA, McKenna MJ, Ramsey MJ, Rosowski JJ, Herrmann BS, Rauch SD, Curtin HD, Merchant SN. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol. 2004;25:121–129. doi: 10.1097/00129492-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–258. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- Niesten MEF, Lee DJ, Stieger C, Rosowski JJ, Grolman W, Nakajima HH. Assessment of superior canal dehiscence location and size on intracochlear sound pressures; Association for Research in Otolaryngology, 36th Midwinter Meeting; Baltimore. 2013a. [Google Scholar]
- Niesten MEF, McKenna MJ, Herrmann BS, Grolman W, Lee DJ. Utility of cVEMPs in bilateral superior canal dehiscence syndrome. Laryngoscope. 2013b;123:226–232. doi: 10.1002/lary.23550. [DOI] [PubMed] [Google Scholar]
- Pfammatter A, Darrouzet V, Gärtner M, Somers T, van Dinther J, Trabalzini F, Ayache D, Linder T. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol. 2010;31:447–454. doi: 10.1097/MAO.0b013e3181d27740. [DOI] [PubMed] [Google Scholar]
- Pisano DV, Niesten ME, Merchant SN, Nakajima HH. The effect of superior semicircular canal dehiscence on intracochlear sound pressures. Audiol Neurotol. 2012;17:338–348. doi: 10.1159/000339653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SD, Zhou G, Kujawa SG, Guinan JJ, Herrmann BS. Vestibular evoked myogenic potentials show altered tuning in patients with Ménière’s disease. Otol Neurotol. 2004;25:333–338. doi: 10.1097/00129492-200405000-00022. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Eppsteiner RW, Sauter TB, Lee DJ. Cervical vestibular evoked myogenic potentials (cVEMPs) in patients with superior canal dehiscence syndrome (SCDS) Otolaryngol Head Neck Surg. 2009;141:24–28. doi: 10.1016/j.otohns.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Songer JE, Nakajima HH, Brinsko KM, Merchant SN. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004;25:323–332. doi: 10.1097/00129492-200405000-00021. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira SM, Whiting BR, Shimony JS, Vo KD, Hullar TE. Accuracy of computed tomography detection of superior canal dehiscence. Otol Neurotol. 2011;32:1500–1505. doi: 10.1097/MAO.0b013e318238280c. [DOI] [PubMed] [Google Scholar]
- Songer JE, Rosowski JJ. A mechano-acoustic model of the effect of superior canal dehiscence on hearing in chinchilla. J Acoust Soc Am. 2007;122:943–951. doi: 10.1121/1.2747158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel SO, Cremer PD, Carey JP, Weg N, Minor LB. Vestibular-evoked myogenic potentials in the diagnosis of superior canal dehiscence syndrome. Acta Otolaryngol Suppl. 2001;545:41–49. doi: 10.1080/000164801750388090. [DOI] [PubMed] [Google Scholar]
- Tavassolie TS, Penninger RT, Zuniga MG, Minor LB, Carey JP. Multislice computed tomography in the diagnosis of superior canal dehiscence: how much error, and how to minimize it? Otol Neurotol. 2012;33:215–222. doi: 10.1097/MAO.0b013e318241c23b. [DOI] [PubMed] [Google Scholar]
- Watters KF, Rosowski JJ, Sauter T, Lee DJ. Superior semicircular canal dehiscence presenting as postpartum vertigo. Otol Neurotol. 2006;27:756–768. doi: 10.1097/01.mao.0000227894.27291.9f. [DOI] [PubMed] [Google Scholar]
- Yuen HW, Boeddinghaus R, Eikelboom RH, Atlas MD. The relationship between the airbone gap and the size of superior semicircular canal dehiscence. Otolaryngol Head Neck Surg. 2009;141:689–694. doi: 10.1016/j.otohns.2009.08.029. [DOI] [PubMed] [Google Scholar]