Abstract
The microfabrication of microfluidic control systems and the development of increasingly sensitive molecular amplificaiton tools has enabled the miniaturization of single cells analytical platforms. Only recently has the throughput of these platforms increased to a level at which populations can be screened at the single cell level. Techniques based upon both active and passive maniuplation are now capable of discriminating between single cell phenotypes for sorting, diagnostic or prognostic applications in a variety of clinical scenarios. The introduction of multiphase microfluidics enables the segmentation of single cells into biochemically discrete picoliter environments. The combination of these techniques are enabling a class of single cell analytical platforms witin great potential for data driven biomedicine, genomics and transcriptomics.
Graphical Abstract

1. Introduction
Over the past few decades, microfabrication has enabled the development of miniaturized analytical platforms that may be integrated to produce portable laboratories, commonly referred to as Lab-on-a-Chip devices. Individual analytical operations, enabled and joined by microfluidic channels, have been reduced to length scales on the order of single cells[1]. Countless microfluidic technologies have been introduced and the pace of innovation accelerated with the introduction of facile fabrication methods, such as poly(dimethylsiloxane)-based soft lithography[2]. Microfabrication and soft lithography have been used not only to develop precise fluid control strategies[3], but also to actively[4,5] or passively[6] manipulate single cells and their environment[7]. As increasingly sensitive molecular biology tools have been introduced, a new class of lab-on-a-chip devices, capable of sorting, segmenting, and individually characterizing large numbers of single cells, has emerged[8].
The increasing throughput of microfluidic single cell analysis technologies has enabled cellular populations to be characterized and parsed for heterogeneity[9], diseased or mutant cells to be isolated[10], and rare cells to be selected from complex cellular backgrounds[8]. Techniques enabling the selection and characterization of single cells have allowed the presence and significance of cellular subpopulations to be identified and analyzed. This has broad implications for biochemical production, clinical diagnostics, therapeutics, and regenerative medicine. The introduction and implementation of multiphase microfluidics as a route to cell encapsulation has provided a method of segregating single cells into discrete picoliter fluid droplets, isolated from other cells and cellular environments. High cell encapsulation rates allow populations to be isolated as single cells for molecular characterization at the genomic or transcriptomic level.
As microfluidic devices for single cell analysis have progressed toward higher throughput capabilities, they have provided new perspectives on how to utilize cellular and molecular information to understand, diagnose, and treat disease. This review summarizes the techniques and technologies that are enabling this nascent shift in biomedical and clinical science.
1. Screening and Sorting of Heterogeneous Cellular Suspensions
The advent of microfluidics promptly introduced techniques for isolating single cells from bulk populations[4] with fine selectivity and sensitivity. Figure 1 provides an overview of microfluidic-enabled technologies and their ability to analyze and isolate increasingly rare single cells from increasingly large populations. Properties of selectively immobilized cells have been directly characterized using static and quasi-static techniques such as MEMS resonant sensing[11], atomic force microscopy (AFM)[12,13] (Figure 1a), and fluorescence microscopy[14]. As microfabrication schemes and fluid control techniques advanced, the selectivity and number of cells that could be simultaneously analyzed increased[1,15]. Cell traps have been used to individually confine large numbers of single cells for long term observation[16], and a variety of methods are used to array cells including passive hydrodynamics[17], dielectrophoresis[18], and optical gradients[19]. The open configuration of cell trap arrays enables the monitoring of cell-cell signaling[14] and has been used to induce and analyze temporal biochemical patterns over time[20].
Figure 1.
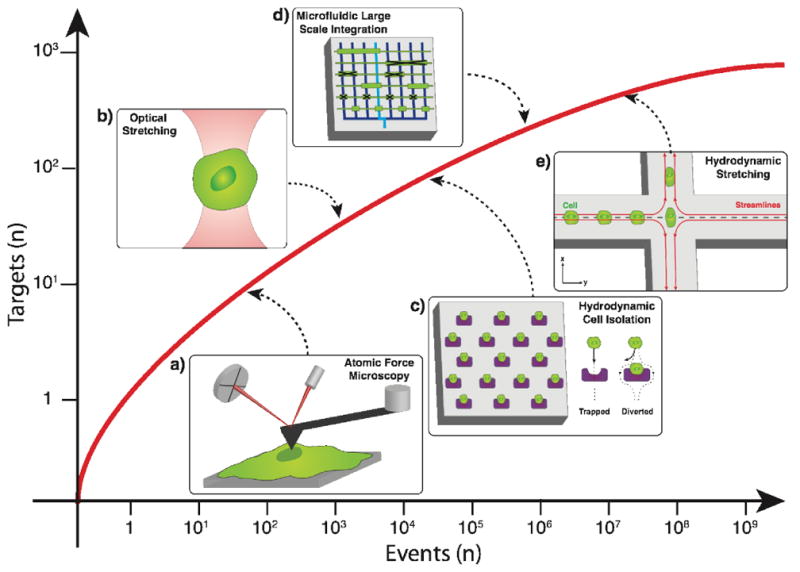
Microfluidic technologies have dramatically increased in throughput capacity while enabling high-purity single cell recognition or separation from bulk samples. Microfluidic techniques that have enabled the evolution of single cell analyses, and their predecessors are illustrated: a) Atomic force microscopy for biophysical marker identification[13] b) Optical stretching for single cell mechanical property measurements[50]. c) Hydrodynamic cell isolation array for single cell capture and analyses[14–15]. d) Microfluidic large scale integration[3]. e) Hydrodynamic stretching for single cell mechanophenotyping[48].
Cell traps are ideal for analyzing representative cross sections of cellular populations, but the initial distribution of cells is random, and therefore, the discrimination of subpopulations is difficult. Many strategies have emerged for the identification and collection of subpopulations from bulk suspensions with applications in clinical medicine such as malarial diagnostics[21,22] and cancer prognostics[23]. Immunoaffinity capture surfaces with integrated fluidics were shown to be particularly adept at selectively and quantifiably isolating subpopulations from large volumes of clinical samples[24]. The sensitivity of these platforms has become sufficiently sophisticated to enable the isolation of rare cells from complex media, a capability that has cultivated particular interest in devices suited for the capture of circulating tumor cells (CTCs), cells shed from primary tumors into the bloodstream, from whole blood. The extremely low abundance of CTCs present in peripheral blood poses challenges for clinical quantification, which demands high yield and purity capture. The accurate enumeration and characterization of CTCs from peripheral blood for prognostic, diagnostic, and individualized therapeutic applications therefore requires high throughput microfluidic characterization of CTCs at the single cell level. While affinity-based capture devices have been refined[25,26], recent work has provided high-throughput and label-free alternatives[27–29].
Actuated cell sorting relies upon external electrical, optical, acoustic, or magnetic field-induced stimuli to selectively manipulate particles across fluid streamlines. Passive sorting, alternatively, utilizes adhesion, filtration, and inertial hydrodynamic forces for sample debulking. Label-free sorting mechanisms exploit physical properties of the cell including size, shape, density, elasticity, polarizability, and magnetic susceptibility. Recently high throughput label-free platforms have been reported for CTC isolation[5]. Magnetophoretic-based CTC isolation platforms have also been demonstrated that address throughput and label-based limitations[6], while acoustophoresis has been suggested for high throughput label-free separation[30].
Among many passive particle separation motifs, high throughput hydrodynamic sorting includes size-exclusion filtration[31], cross-flow[32], hydrophoretic[33] and hydrodynamic[34] filtration, and pinched flow fractionation[35]. Inertial focusing is a more recently described cell focusing and sorting technique. Passive and size selective, inertial focusing is governed by inertial lift forces that induce lateral migration of cells or particles across fluid streamlines to predictable equilibrium positions[36]. Precise spatiotemporal focusing positions arise from a combination of lateral focusing by inertial forces and longitudinal ordering arising from hydrodynamic repulsions (Figure 2a). This sheathless alignment is ideal for high throughput optical single cell analyses[37]. A secondary inertial flow, perpendicular to the primary flow direction, is also observable in curved microchannels at finite inertia[36], which can be utilized to further manipulate focusing behavior. Inertial focusing has enabled numerous promising technologies and separation modalities ranging from miniaturized analogs of existing techniques to fundamentally new innovations. Microfiltration of bacteria from dilute blood[38], plasma separation[39], and particle enrichment[40,41] have been demonstrated with inertial focusing devices and exhibit numerous advantages over centrifugation or mechanical filtration, including a compact, portable device footprint, precise and pure size-selective separations, and the elimination of manual sample handling and fouling issues.
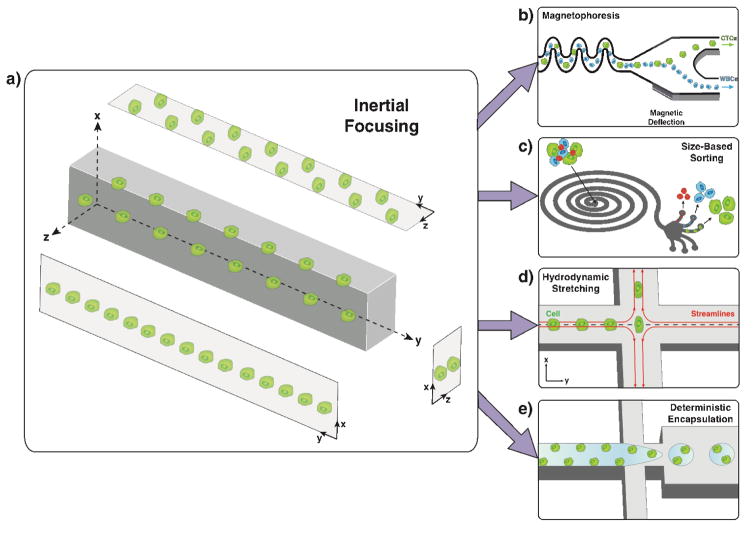
Figure 2.
Overview of inertial focusing and its application in various high throughput biological sample processing for single cell analyses. a) Inertial focusing behavior in microchannels with rectangular cross-section. b) High throughput inertial focusing mediated magnetophoresis[6]. c) Size based cell sorting in spiral microchannels with rectangular cross section. d) Single cell hydrodynamic stretching for mechanical phenotyping[48]. e) Deterministic cell encapsulation for precise modulation of single cell environments[53].
Inertial microfluidics have been applied to CTC characterization and enrichment in a variety of ways[8]. A multistage, integrated, inertial focusing-based device[6] combined deterministic lateral displacement, inertial focusing, and magnetic deflection in series to effectively demonstrate the marginal gain of microfluidic sample handling relative to bulk processing. Capable of both positive or negative selection modes of tumor membrane epitopes, this device is applicable to a broad range of cancer phenotypes (Figure 2b). Inertial focusing within spiral microchannels can exploit size differences for CTC fractionation[42] (Figure 2c). Related work has introduced geometric refinements for improved isolation of CTCs from peripheral blood components[43]. Microvortex-generating microfluidic devices[44] have been demonstrated for label-free CTC separation based upon size distributions of targeted CTC and bulk blood components[45,46].
In the absence of cell capture or enrichment, inertial focusing has been particularly useful as a high throughput tool to characterize the mechanical phenotype - mechanical properties that can be used as a label-free biomarker for predicting disease - of heterogeneous cell suspensions on a single cell basis[47,48] (Figure 1e, 2d). Inertial focusing and high speed image analysis have enhanced the throughput of mechanotyping measurements over static approaches, such as AFM. With applications in rare cell characterization, hydrodynamic mechanotyping is a powerful method to discriminate distinct subpopulations of CTCs or diseased lymphocytes for prognostics or predictive individualized therapies. Optophoresis[49], optical compression[50], and optical stretching[51] (Figure 1b), while lower throughput than inertial focusing mechanotyping, can detect more subtle variations in refractive index, a measure of cell size, density and cytoskeletal properties, with broader mechanical deformation profiles.
1. Single cells within Segmented Environments
In addition to selecting, positioning, and isolating single cells, microfluidic tools are also well suited to create unique, segmented environments in which to isolate individual cells from other cells. Discrete microenvironments were first developed as arrays of individual microchambers connected through micromechanical valves. Consisting of PDMS micro-molded chambers with pressure driven valves, these devices were analogous to integrated circuits, enabling access to individual microchambers[3] and the fine tuning of biochemical environments around sequestered cells (Figure 1d). Though convenient for examining the temporal variation in biochemical expression for a multitude of cells under distinct conditions, their nuanced design limits parallelization and extension to single cell applications[18]. Restrictions on throughput and associated analysis time have motivated new approaches to engineering single cell environments.
Microfluidic emulsification was introduced and quickly adopted as a robust technique for generating precise fluidic aliquots[52], performing custom particle syntheses, and isolating single cells. Combining deterministic encapsulation[53] with precision biochemical microenvironment control and unmatched throughput, droplet-based segmentation has enabled a host of unique assays on the single cell level (Figure 2e).
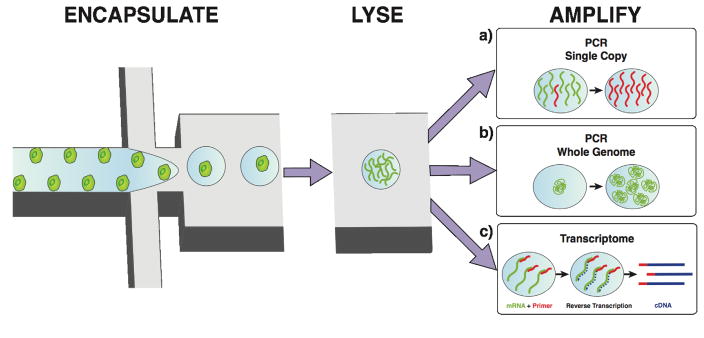
Improved throughput is particularly critical for single cell genomic sequencing[1] due, in part, to the scarcity of microbial species amenable to isolation using standard culturing methods, with even fewer (less than 0.01%) of known viruses readily isolatable[54]. Bench scale DNA sequencing methods are time consuming and expensive, and require serial dilutions or flow cytometry. In the last decade the field of single cell genomics has experienced a paradigm shift as integrated microfluidic techniques have been adopted, beginning with the polymerase chain reaction (PCR) to isolate nucleic acids from single cells, followed by digital PCR (dPCR) sequencing. More recently, high throughput methods have emerged to detect copy number variation-related disease and infer clonal evolution of a breast tumor by measuring single cell copy number using droplet dPCR[55] (Figure 3a). Purification and genome sequencing of single viral species directly from environmental samples via droplet-based dPCR has also been demonstrated, precluding the need for culturing entirely[56] (Figure 3b). These tools have been integrated into systems to understand mutation rates in gametogenesis[57].
Figure 3.
Overview of microfluidic single cell encapsulation and droplet microenvironment manipulations for downstream molecular analyses. a) Droplet single copy PCR amplification[55]. b) Droplet whole genome PCR amplification[56]. c) Droplet barcoding for single cell transcriptomics[63].
Epigenetics, variations in the local cellular environment, and signaling cues are all additional sources of phenotypic heterogeneity. Microfluidic real time reverse transcription PCR (RT-qPCR) capable of detecting single RNA copies was first demonstrated several years ago, but suffered from a lack of throughput[58]. Subsequently, an integrated microfluidics-based quantitative RT-qPCR platform introduced single cell specific gene expression, surpassing previous throughput by a factor of one hundred[59]. Recently, droplet platforms were developed to prepare cDNA from single cells for high-throughput whole-transcriptome sequencing in droplets[60], as well as to conduct a comparison of single-cell RNA sequencing (Figure 3c). This illustrates that current RNA-sequencing techniques had become quantitatively comparable to commercial bench-scale qPCR but with less sample bias[61]. Most recently, two groups independently described the development of Drop-seq[62] and In-drop[63], which are cost-effective, integrated, highly parallel microfluidic platforms that rely on genetic code barcoding to remember the cell-of-origin, enabling the simultaneous preparation of thousands of single-cell libraries for transcriptome-sequencing. A present challenge is the measurement of data from single cells in situ to preserve their spatial context while collecting combinations of DNA and RNA data in parallel from the same cell[64].
1. Conclusions and Outlook
The technologies described in this review have enabled the high throughput sorting, capture, and manipulation of single cells. High throughput droplet microfluidics enables the large scale encapsulation of therapeutic-level quantities of cells while simultaneously enabling a host of screening and sorting applications. Techniques such as fluorescent droplet analysis and encapsulation of therapeutic cells[65] exploit the high throughput capacity of this technology. Emerging applications will build upon the techniques described here to develop technologies for in vitro cell culture within microfluidically prepared hydrogels. Potential applications include controlled cell growth in 3D biomaterial niches[66], stem cell trophic factor therapeutics[67], tissue engineering[68], and in vitro screening[69]. Three dimensional tissue bioprinting[70] and related techniques adapted from inkjet printing may incorporate these capabilities for cell and biomaterial patterning[71].
Microfluidics, coupled with increasingly sensitive molecular biology tools has changed the landscape of single cell analysis in fundamental ways. The past few years has witnessed the introduction of high throughput encapsulation, sorting, and analytical techniques that have allowed cellular populations to be characterized at the level of the single cell genotype or phenotype. These tools are only just beginning to have an impact upon clinical medicine and global health. Increasingly, microfluidic platforms will be capable of monitoring cellular dynamics in response to environmental changes, signaling events, and mechanical stimuli. Using single cell analytical platforms to inform the design of biomaterials and multicellular systems will prove transformative to other disciplines, including tissue engineering and regenerative medicine.
Microfluidics complements high sensitivity molecular tools, allowing single cell analysis
Cells can be driectly manipulated by a variety of fields, with forces equivalent microfluidic flows
Passive, high throughput microfluidic systems enable single cell subpopulation classification
Microfluidic emulsificaiton is a high throughput route to single cell genomic classification
Acknowledgments
This work was supported by the NIH-funded Wyoming IDeA Networks of Biomedical Research Excellence program (P20GM103432), the National Science Foundation Faculty Early Career Development (CAREER) Program (BBBE 1254608) and the Department of Defense (Congressionally Directed Medical Research Program, Prostate Cancer Research Program) under Award no. W81XWH-13-1-0273.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins DJ, Neild A, deMello A, Liu A-Q, Ai Y. The Poisson distribution and beyond: methods for microfluidic droplet production and single cell encapsulation. Lab Chip. 2015;15:3439–3459. doi: 10.1039/c5lc00614g. [DOI] [PubMed] [Google Scholar]
- 2.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 3.Thorsen T, Maerkl SJ, Quake SR. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- 4.Oakey J, Allely J, Marr D. Laminar-flow-based separations at the microscale. Biotechnol Prog. 2002;18:1439–1442. doi: 10.1021/bp0256216. [DOI] [PubMed] [Google Scholar]
- 5.Huang S-B, Wu MH, Lin Y-H, Hsieh C-H, Yang C-L, Lin H-C, Tseng C-P, Lee G-B. High-purity and label-free isolation of circulating tumor cells (CTCs) in a microfluidic platform by using optically-induced-dielectrophoretic (ODEP) force. Lab Chip. 2013;13:1371–1383. doi: 10.1039/c3lc41256c. [DOI] [PubMed] [Google Scholar]
- 6**.Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, Yu M, Chen PI, Morgan B, Trautwein J, et al. Supplemental Material for Inertial Focusing for Tumor Antigen-Dependent and -Independent Sorting of Rare Circulating Tumor Cells. Science Translational Medicine. 2013;5:179ra47–179ra47. doi: 10.1126/scitranslmed.3005616. A high throughput multistage microfluidic device capable of both positive or negative selection modes of tumor membrane epitopes for CTC isolation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger R, Kurzbuch D, Gorkin R, Kijanka G, Glynn M, McDonagh C, Ducrée J. An integrated centrifugo-opto-microfluidic platform for arraying, analysis, identification and manipulation of individual cells. Lab Chip. 2014;15:378–381. doi: 10.1039/c4lc01002g. [DOI] [PubMed] [Google Scholar]
- 8.Shields CW, IV, Reyes CD, López GP. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip. 2015;15:1230–1249. doi: 10.1039/c4lc01246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson RA, Jimenez R. Microfluidic cytometer for high-throughput measurement of photosynthetic characteristics and lipid accumulation in individual algal cells. Lab Chip. 2013;13:2893–2901. doi: 10.1039/c3lc41429a. [DOI] [PubMed] [Google Scholar]
- 10.Dean KM, Davis LM, Lubbeck JL, Manna P, Friis P, Palmer AE, Jimenez R. High-Speed Multiparameter Photophysical Analyses of Fluorophore Libraries. Anal Chem. 2015;87:5026–5030. doi: 10.1021/acs.analchem.5b00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbin EA, Dorvel BR, Millet LJ, King WP, Bashir R. Micro-patterning of mammalian cells on suspended MEMS resonant sensors for long-term growth measurements. Lab Chip. 2014;14:1401–1405. doi: 10.1039/c3lc51217g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Oorschot R, Garza H, Derks R, Staufer U, Ghatkesar MK. A microfluidic AFM cantilever based dispensing and aspiration platform. EPJ Techniques and Instrumentation. 2015;2:1–11. [Google Scholar]
- 13.Lee WC, Bhagat A, Huang S, Van Vliet KJ, Han J. High-throughput cell cycle synchronization using inertial forces in spiral microchannels. Lab Chip. 2011;11:1359–1367. doi: 10.1039/c0lc00579g. [DOI] [PubMed] [Google Scholar]
- 14.He L, Kniss A, San-Miguel A, Rouse T, Kemp ML, Lu H. An automated programmable platform enabling multiplex dynamic stimuli delivery and cellular response monitoring for high-throughput suspension single-cell signaling studies. Lab Chip. 2015;15:1497–1507. doi: 10.1039/c4lc01070a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Carlo D, Wu LY, Lee LP. Dynamic single cell culture array. Lab Chip. 2006;6:1445–1449. doi: 10.1039/b605937f. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Sun J, Wolvetang E, Cooper-White J. High-throughput, deterministic single cell trapping and long-term clonal cell culture in microfluidic devices. Lab Chip. 2015;15:1072–1083. doi: 10.1039/c4lc01176g. [DOI] [PubMed] [Google Scholar]
- 17.Kukhtevich IV, Belousov KI, Bukatin AS, Dubina MV, Evstrapov AA. A microfluidic chip with hydrodynamic traps for in vitro microscopic investigations of single cells. Tech Phys Lett. 2015;41:255–258. [Google Scholar]
- 18.Kim HS, Devarenne TP, Han A. A high-throughput microfluidic single-cell screening platform capable of selective cell extraction. Lab Chip. 2015;15:2467–2475. doi: 10.1039/c4lc01316f. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Liu KK. Optical tweezers for single cells. Journal of The Royal Society Interface. 2008;5:671–690. doi: 10.1098/rsif.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chingozha L, Zhan M, Zhu C, Lu H. A Generalizable, Tunable Microfluidic Platform for Delivering Fast Temporally Varying Chemical Signals to Probe Single-Cell Response Dynamics. Anal Chem. 2014;86:10138–10147. doi: 10.1021/ac5019843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Birch CM, Hou HW, Han J, Niles JC. Identification of malaria parasite-infected red blood cell surface aptamers by inertial microfluidic SELEX (I-SELEX) Sci Rep. 2015;5:11347–11363. doi: 10.1038/srep11347. A high throughput spiral microchannel separation device used to recognize distinct epitopes present on parasite-infected RBCs for malarial diagnostics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Warkiani ME, Tay AKP, Khoo BL, Xiaofeng X, Han J, Lim CT. Malaria detection using inertial microfluidics. Lab Chip. 2015;15:1101–1109. doi: 10.1039/c4lc01058b. A high throughput multi-orifice microfluidic device demonstrating efficacy for sensitive detection of parasites in diseased blood for malarial studies. [DOI] [PubMed] [Google Scholar]
- 23.Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, et al. Isolation and Characterization of Circulating Tumor Cells from Patients with Localized and Metastatic Prostate Cancer. Science Translational Medicine. 2010;2:25ra23–25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toner M, Irimia D. Blood-on-a-chip. Annu Rev Biomed Eng. 2005;7:77–103. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AM, Yu M, Nakamura Z, Ciciliano J, Ulman M, Kotz K, Stott SL, Maheswaran S, Haber DA, Toner M. Biopolymer System for Cell Recovery from Microfluidic Cell Capture Devices. Anal Chem. 2012;84:3682–3688. doi: 10.1021/ac300190j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murlidhar V, Zeinali M, Grabauskiene S, Ghannad-Rezaie M, Wicha MS, Simeone DM, Ramnath N, Reddy RM, Nagrath S. A Radial Flow Microfluidic Device for Ultra-High-Throughput Affinity-Based Isolation of Circulating Tumor Cells. Small. 2014;10:4895–4904. doi: 10.1002/smll.201400719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S-W, Hyun K-A, Kim S-I, Kang JY, Jung H-I. Continuous enrichment of circulating tumor cells using a microfluidic lateral flow filtration chip. Journal of Chromatography A. 2015;1377:100–105. doi: 10.1016/j.chroma.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 29**.Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto DT, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Meth. 2015;12:685–691. doi: 10.1038/nmeth.3404. Microfluidic platform for the capture and enumeration of CTC clusters using specialized low-shear bifurcation traps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antfolk M, Magnusson C, Augustsson P, Lilja H, Laurell T. Acoustofluidic, Label-Free Separation and Simultaneous Concentration of Rare Tumor Cells from White Blood Cells. Anal Chem. 2015;87:9322–9328. doi: 10.1021/acs.analchem.5b02023. [DOI] [PubMed] [Google Scholar]
- 31.McFaul SM, Lin BK, Ma H. Cell separation based on size and deformability using microfluidic funnel ratchets. Lab Chip. 2012;12:2369–2376. doi: 10.1039/c2lc21045b. [DOI] [PubMed] [Google Scholar]
- 32.Earhart CM, Hughes CE, Gaster RS, Ooi CC, Wilson RJ, Zhou LY, Humke EW, Xu L, Wong DJ, Willingham SB, et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab Chip. 2014;14:78–88. doi: 10.1039/c3lc50580d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi S, Song S, Choi C, Park J-K. Continuous blood cell separation by hydrophoretic filtration. Lab Chip. 2007;7:1532. doi: 10.1039/b705203k. [DOI] [PubMed] [Google Scholar]
- 34.Preira P, Grandné V, Forel JM, Gabriele S, Camara M, Theodoly O. Passive circulating cell sorting by deformability using a microfluidic gradual filter. Lab Chip. 2013;13:161–170. doi: 10.1039/c2lc40847c. [DOI] [PubMed] [Google Scholar]
- 35.Lu X, Xuan X. Continuous Microfluidic Particle Separation via Elasto-Inertial Pinched Flow Fractionation. Anal Chem. 2015;87:6389–6396. doi: 10.1021/acs.analchem.5b01432. [DOI] [PubMed] [Google Scholar]
- 36.Martel JM, Toner M. Inertial Focusing in Microfluidics. Annu Rev Biomed Eng. 2014;16:371–396. doi: 10.1146/annurev-bioeng-121813-120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakey J, Applegate R, Jr, Arellano E, Carlo D, Graves SW, Toner M. Particle Focusing in Staged Inertial Microfluidic Devices for Flow Cytometry. Anal Chem. 2010;82:3862–3867. doi: 10.1021/ac100387b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mach AJ, Di Carlo D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol Bioeng. 2010;107:302–311. doi: 10.1002/bit.22833. [DOI] [PubMed] [Google Scholar]
- 39.Kersaudy-Kerhoas M, Dhariwal R, Desmulliez MPY, Jouvet L. Hydrodynamic blood plasma separation in microfluidic channels. Microfluid Nanofluid. 2009;8:105–114. [Google Scholar]
- 40.Reece AE, Kaastrup K, Sikes HD, Oakey J. Staged inertial microfluidic focusing for complex fluid enrichment. RSC Advances. 2015;5:53857–53864. doi: 10.1039/c5ra10634f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martel JM, Smith KC, Dlamini M, Pletcher K, Yang J, Karabacak M, Haber DA, Kapur R, Toner M. Continuous Flow Microfluidic Bioparticle Concentrator. Sci Rep. 2015;5:11300–11312. doi: 10.1038/srep11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martel JM, Toner M. Inertial focusing dynamics in spiral microchannels. Phys Fluids. 2012;24:032001. doi: 10.1063/1.3681228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS-W, Lim W-T, Han J, Bhagat AAS, Lim CT. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep. 2013;3:1259–1267. doi: 10.1038/srep01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hur SC, Mach AJ, Di Carlo D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics. 2011;5:022206-1–11. doi: 10.1063/1.3576780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Sollier E, Go DE, Che J, Gossett DR, O’Byrne S, Weaver WM, Kummer N, Rettig M, Goldman J, Nickols N, et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip. 2014;14:63–77. doi: 10.1039/c3lc50689d. [DOI] [PubMed] [Google Scholar]
- 46.Deng Y, Zhang Y, Sun S, Wang Z, Wang M, Yu B, Czajkowsky DM, Liu B, Li Y, Wei W, et al. An Integrated Microfluidic Chip System for Single-Cell Secretion Profiling of Rare Circulating Tumor Cells. Sci Rep. 2014;4:7499–8. doi: 10.1038/srep07499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Dudani JS, Gossett DR, Tse HTK, Di Carlo D. Pinched-flow hydrodynamic stretching of single-cells. Lab Chip. 2013;13:3728–7. doi: 10.1039/c3lc50649e. [DOI] [PubMed] [Google Scholar]
- 48.Gossett DR, Henry TK, Lee SA, Ying Y, Lindgren AG, Yang OO, Rao J, Clark AT, Di Carlo D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci USA. 2012;109:7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nerenberg M, Kariv I, McNeeley P, Marchand P, Sur S, Diver J, Riccitelli S, Nieva J, Saven A. Use of optophoresis as an in vitro predictor of cell response to chemotherapy for chronic lymphocytic leukemia. Leukemia & Lymphoma. 2006;47:2194–2202. doi: 10.1080/10428190600799532. [DOI] [PubMed] [Google Scholar]
- 50*.Roth KB, Eggleton CD, Neeves KB, Marr DWM. Measuring cell mechanics by optical alignment compression cytometry. Lab Chip. 2013;13:1571–1577. doi: 10.1039/c3lc41253a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sraj I, Szatmary AC, Desai SA, Marr DWM, Eggleton CD. Erythrocyte deformation in high-throughput optical stretchers. Phys Rev E. 2012;85:041923–9. doi: 10.1103/PhysRevE.85.041923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Link DR, Anna S, Weitz DA, Stone H. Geometrically Mediated Breakup of Drops in Microfluidic Devices. Physical Review Letters. 2004;92:054503-1–4. doi: 10.1103/PhysRevLett.92.054503. [DOI] [PubMed] [Google Scholar]
- 53.Lagus TP, Edd JF. High Throughput Single-cell and Multiple-cell Micro-encapsulation. JoVE. 2012 doi: 10.3791/4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anthony SJ, Epstein JH, Murray KA, Navarrete-Macias I, Zambrana-Torrelio CM, Solovyov A, Ojeda-Flores R, Arrigo NC, Islam A, Ali Khan S, et al. A Strategy To Estimate Unknown Viral Diversity in Mammals. mBio. 2013;4:e00598-13–1–15. doi: 10.1128/mBio.00598-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumaresan P, Yang CJ, Cronier SA, Blazej RG, Mathies RA. High-Throughput Single Copy DNA Amplification and Cell Analysis in Engineered Nanoliter Droplets. Anal Chem. 2008;80:3522–3529. doi: 10.1021/ac800327d. [DOI] [PubMed] [Google Scholar]
- 56**.Han H-S, Cantalupo PG, Rotem A, Cockrell SK, Carbonnaux M, Pipas JM, Weitz DA. Whole-Genome Sequencing of a Single Viral Species from a Highly Heterogeneous Sample. Angew Chem. 2015;127:14191–14194. doi: 10.1002/anie.201507047. Isolated a specific virus from complex samples followed by complete genome sequencing, which holds great potential for virus discovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Fan HC, Behr B, Quake SR. Genome-wide Single-Cell Analysis of Recombination Activity and De Novo Mutation Rates in Human Sperm. Cell. 2012;150:402–412. doi: 10.1016/j.cell.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beer NR, Wheeler EK, Lee-Houghton L, Watkins N, Nasarabadi S, Hebert N, Leung P, Arnold DW, Bailey CG, Colston BW. On-Chip Single-Copy Real-Time Reverse-Transcription PCR in Isolated Picoliter Droplets. Anal Chem. 2008;80:1854–1858. doi: 10.1021/ac800048k. [DOI] [PubMed] [Google Scholar]
- 59.White AK, VanInsberghe M, Petriv I. High-throughput microfluidic single-cell RT-qPCR. 2011:13999–14004. doi: 10.1073/pnas.1019446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Streets AM, Zhang X, Cao C, Pang Y, Wu X, Xiong L, Yang L, Fu Y, Zhao L, Tang F, et al. Microfluidic single-cell whole-transcriptome sequencing. Proc Natl Acad Sci USA. 2014;111:7048–7053. doi: 10.1073/pnas.1402030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu AR, Neff NF, Kalisky T, Dalerba P, Treutlein B, Rothenberg ME, Mburu FM, Mantalas GL, Sim S, Clarke MF, et al. Quantitative assessment of single-cell RNA-sequencing methods. Nat Meth. 2013;11:41–46. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. Describes a novel Drop-seq approach enabled by genetic code barcoding and capable of profiling thousands of cells individually while remembering all transcripts’ cell-of-origin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63**.Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW. Droplet Barcoding for Single-Cell Transcriptomics Applied to Embryonic Stem Cells. Cell. 2015;161:1187–1201. doi: 10.1016/j.cell.2015.04.044. Describes an alternative high throughput droplet-based approach, In-drop, for mRNA sequencing of thousands of cells at single cell resolution with RNA barcoding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Navin NE. Advances and Applications of Single-Cell Sequencing Technologies. Molecular Cell. 2015;58:598–609. doi: 10.1016/j.molcel.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang A, Park J, Ju J, Jeong GS, Lee S-H. Cell encapsulation via microtechnologies. Biomaterials. 2014;35:2651–2663. doi: 10.1016/j.biomaterials.2013.12.073. [DOI] [PubMed] [Google Scholar]
- 66.Allazetta S, Lutolf MP. ScienceDirectStem cell niche engineering through droplet microfluidics. Current Opinion in Biotechnology. 2015;35:86–93. doi: 10.1016/j.copbio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Chen AK-L, Reuveny S, Oh SKW. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnology Advances. 2013;31:1032–1046. doi: 10.1016/j.biotechadv.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Hazeltine LB, Selekman JA, Palecek SP. Engineering the human pluripotent stem cell microenvironment to direct cell fate. Biotechnology Advances. 2013;31:1002–1019. doi: 10.1016/j.biotechadv.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sjostrom SL, Bai Y, Huang M, Liu Z, Nielsen J, Joensson HN, Andersson Svahn H. High-throughput screening for industrial enzyme production hosts by droplet microfluidics. Lab Chip. 2014;14:806–813. doi: 10.1039/c3lc51202a. [DOI] [PubMed] [Google Scholar]
- 70.Nelson B. 3-dimensional bioprinting makes its mark: New tissue and organ printing methods are yielding critical new tools for the laboratory and clinic. Cancer Cytopathology. 2015;123:203–204. doi: 10.1002/cncy.21543. [DOI] [PubMed] [Google Scholar]
- 71.Leibacher I, Schoendube J, Dual J, Zengerle R, Koltay P. Enhanced single-cell printing by acoustophoretic cell focusing. Biomicrofluidics. 2015;9:024109-1–11. doi: 10.1063/1.4916780. [DOI] [PMC free article] [PubMed] [Google Scholar]