Abstract
Cardiometabolic disease emerges as a worldwide epidemic and there is urgent need to understand the molecular mechanisms underlying this chronic disease. The chemical environment to which we are exposed has significantly changed in the past few decades and recent research has implicated its contribution to the development of many chronic human diseases. However, the mechanisms of how exposure to chemicals contribute to the development of cardiometabolic disease are poorly understood. Numerous chemicals have been identified as ligands for the pregnane X receptor (PXR), a nuclear receptor functioning as a xenobiotic sensor to coordinately regulate xenobiotic metabolism via transcriptional regulation of xenobiotic-detoxifying enzymes and transporters. In the past decade, the function of PXR in the regulation of xenobiotic metabolism has been extensively studied by many laboratories and the role of PXR as a xenobiotic sensor has been well-established. The identification of PXR as a xenobiotic sensor has provided an important tool for the study of new mechanisms through which xenobiotic exposure impacts human chronic diseases. Recent studies have revealed novel and unexpected roles of PXR in modulating obesity, insulin sensitivity, lipid homeostasis, atherogenesis, and vascular functions. These studies suggest that PXR signaling may contribute significantly to the pathophysiological effects of many known xenobiotics on cardiometabolic disease in humans. The discovery of novel functions of PXR in cardiometabolic disease not only contributes to our understanding of “gene-environment interactions” in predisposing individuals to chronic diseases but also provides strong evidence to inform future risk assessment for relevant chemicals.
Keywords: xenobiotic receptor, cardiovascular disease, obesity, metabolic disorders, lipid homeostasis, endocrine disrupting chemicals
1. Introduction
Cardiometabolic disease, which includes obesity, cardiovascular disease (CVD), hypertension, and type 2 diabetes, is a rapidly growing epidemic representing a serious health threat in an increasing number of countries. There is an urgent need to understand the mechanisms underlying cardiometabolic disease. While considerable progress has been achieved to identify gene variations contributing to cardiometabolic disease, the role played by “gene-environment interactions” in predisposing individuals to cardiometabolic disease remains relatively unexplored. In addition to the obvious contributions of diet and lifestyle on human health, the chemical environment to which we are exposed has significantly changed in the past few decades and has recently been implicated in the etiology of cardiometabolic disease. However, the mechanisms of how exposure to chemicals contribute to the development of chronic human diseases such as cardiometabolic disease are poorly understood.
To sense and respond to environmental chemicals, mammals have evolved a defensive network governed by xenobiotic receptors such as the pregnane X receptor (PXR; also known as steroid and xenobiotic receptor, or SXR; NR1I2 for standard nomenclature) [1–5]. PXR functions as a xenobiotic sensor that induces the expression of genes required for xenobiotic metabolism in the liver and intestine, including cytochrome P450 (CYP) enzymes (e.g. CYP3A4), conjugating enzymes (e.g. glutathione transferase [GST]), and ABC family transporters (e.g. multidrug resistance 1 [MDR1]) [4–6]. Many of PXR-regulated metabolizing enzymes and transporters play a central role in xenobiotic metabolism. For instance, PXR is a key transcriptional factor that regulate the expression of CYP3A4, which is responsible for the metabolism of more than 50% of clinically used drugs in humans [7]. In addition to PXR, another xenobiotic receptor, constitutive androstane receptor (CAR; NR1I3 for standard nomenclature) also has a broad role in xenobiotic metabolism [8]. Unlike PXR, CAR shows relatively high basal activity to activate target genes without ligand. PXR and CAR also can regulate overlapped and distinctive sets of genes involved in xenobiotic metabolism [9–11].
Interestingly, numerous compounds including endogenous hormones, dietary steroids, pharmaceutical agents, and xenobiotic chemicals have been identified to be ligands of PXR [1, 4–6]. The diverse ligand-binding properties of PXR are facilitated by the large volume and smooth shape of its ligand-binding pocket in the ligand-binding domain (LBD). Compared with most other nuclear receptors including CAR, PXR is remarkably divergent across mammalian species with the LBDs sharing only ~60–80% identity compared with the ~90% typically exhibited by orthologous nuclear receptors [5]. Further, PXR also exhibits significant differences in its pharmacology across species (e.g., mouse vs. human) [1, 5, 12, 13]. For example, the antibiotic rifampicin and plastic base chemical bisphenol A (BPA) are potent activators of human and rabbit PXR, but do not affect mouse or rat PXR activity [12, 14]. By contrast, the synthetic steroid pregnenolone-16α-carbonitrile (PCN) is a potent agonist of rat and mouse PXR but does not activate human or rabbit PXR [12, 15, 16]. The unique feature of PXR also explains species-specific differences in xenobiotic induction by CYP3A. Despite its diverse LBD, the DNA-binding domain (DBD) of PXR is well conserved with 95% amino acid sequence homology across various species and PXR target genes appear to be identical in humans and mice [4, 5]. In addition to its species-specific responses, some ligands can also activate PXR and regulate its target genes in a tissue-specific manner [17–19]. For example, rifaximin, a clinically used nonsynthetic antibiotic, has been identified to be an intestine-specific agonist for the human PXR [18]. Tocotrienol forms of vitamin E can selectively regulate the PXR target genes in hepatic and intestinal cell lines, which may be due to different expression levels of nuclear receptor co-repressor in hepatic and intestinal cells [17]. These results indicate that PXR mediate species-and tissue-specific responses to xenobiotic exposure.
Since it was first identified in 1998, the functions of PXR in drug and xenobiotic metabolism have been extensively studied by many laboratories. To date, the role of PXR as a xenobiotic sensor has been well-established and PXR has been considered as a master regulator of xenobiotic metabolism. The identification of PXR as a xenobiotic sensor has provided an important tool for the study of new mechanisms through which xenobiotic exposure impacts diseases. Recent studies have revealed novel functions of PXR beyond xenobiotic metabolism and this review focuses its functions in cardiometabolic disease.
2. Role of PXR in obesity and insulin resistance
The prevalence of obesity has more than doubled over the past 30 years and 60 million people are currently defined as obese in the United States alone. If current trends continue, more than half of the United States population could be obese by 2030 [20]. Obesity is an independent risk factor for insulin resistance, type 2 diabetes, and atherosclerotic CVD, the leading causes of death worldwide [21, 22]. It is generally accepted that environmental factors, most notably consumption of a palatable high-fat diet (HFD), has contributed to the rapidly escalating prevalence of obesity and associated metabolic dysfunctions. Recent findings have implicated exposure to certain chemicals such as endocrine disrupting chemicals (EDCs) in the etiology of obesity and metabolic disorders [23–30]. Mounting evidence demonstrates that many xenobiotics such as EDCs can interfere with complex endocrine signaling mechanisms and result in adverse consequences in humans and wildlife [26, 31–33]. Numerous EDCs, including organochlorine and organophosphate pesticides, alkylphenols, phthalates (e.g. di(2-ethylhexyl)phthalate [DEHP]), polychlorinated biphenyls (PCBs), bisphenol A (BPA) and its analogs (e.g. BPB, BPAF) have been identified to activate PXR [5, 14, 16, 34, 35]. PXR may play a significant role in mediating the pathophysiological effects of those known EDCs and other chemicals in humans and animals. Indeed, recent studies have uncovered novel functions of PXR in obesity and insulin resistance.
It has long been suspected that PXR signaling is involved in the regulation of glucose homeostasis as many clinically relevant PXR-agonistic drugs can affect blood glucose levels [36–39]. For example, rifampicin, phenytoin, and cyclophosphamide which are all PXR ligands have been documented to induce hyperglycemia in patients [36–38]. By contrast, long-term treatment with another PXR ligand, phenobarbital, can reduce plasma glucose levels and improve insulin sensitivity in diabetic patients [40]. By performing mammalian cell-based two-hybrid screening, Kodama et al. [41] revealed an important role of PXR in the regulation of gluconeogenesis by repressing forkhead box protein O1 (FoxO1) activity. FoxO1 is a member of the “forkhead” family of transcription factors that play critical roles in gluconeogenesis in the liver [42]. FoxO1 promotes hepatic gluconeogenesis in liver in the fasted state by activating gluconeogenic genes, including phosphoenolpyruvate carboxykinase 1 (PEPCK1), glucose-6-phosphatase (G-6-P) and insulin-like growth factor-binding protein 1. Kodama et al. [41] identified FoxO1 as a co-activator for PXR. However, PXR acts as a co-repressor of FoxO1 and inhibits FoxO1-mediated transcription by preventing its binding to its response elements in target genes such as PEPCK1 and G-6-P [41]. Further studies revealed that activation of PXR can also repress transcription of PEPCK1 and G-6-P by inhibiting hepatocyte nuclear factor 4α (HNF4α) and cAMP response element-binding protein (CREB) activity, respectively [43, 44]. These studies suggest that PXR can regulate gluconeogenesis through multiple mechanisms. In addition to FoxO1, Nakamura et al. [45] later reported that PXR can also crosstalk with another member of the “forkhead” family, FoxA2 to mediate drug-induced repression of lipid metabolism in fasting mouse livers. FoxA2 regulates ketogenesis and β-oxidation by upregulating transcription of genes including mitochondrial 3-hydroxy-3-methylglutartate-CoA synthase 2 (HMGCS2) and carnitine palmitoyltransferase 1A (CPT1A) during fasting or after prolonged exercise [46]. Similar to the crosstalk with FoxO1, PXR can directly interact with FoxA2 and repress FoxA2-mediated expression of HMGCS2 and CPT1A. Thus, the crosstalk between PXR and FoxO1 and FoxA2 indicates an important role of PXR in mediating hepatic glucose and energy homeostasis.
Although these studies demonstrated a novel role for hepatic PXR signaling in gluconeogenesis, the function of PXR in the regulation of obesity and whole-body insulin sensitivity was not revealed until very recently. In a well-designed study, He et al. [39] revealed a critical role of PXR in obesity and type 2 diabetes. By feeding WT and PXR−/− mice a HFD, they found that PXR−/− mice were resistant to diet-induced obesity, hepatic steatosis, and insulin resistance. While deficiency of PXR did not affect food intake, PXR−/− mice had increased oxygen consumption and mitochondrial beta-oxidation, but decreased hepatic lipogenesis and inflammation. Consistently, deficiency of PXR improved insulin sensitivity in mice. In addition to diet-induced obesity, the authors also found that ablation of PXR in leptin-deficient ob/ob mice prevented genetic obesity by increasing oxygen consumption and energy expenditure [39]. Further, ob/ob mice with PXR deficiency also had improved diabetic phenotype, decreased gluconeogenesis and increased rate of glucose disposal during euglycemic clamp. The metabolic benefits of PXR deficiency were likely due to the inhibited c-Jun NH2-terminal kinase (JNK) activation and downregulation of lipin-1 which is a bona fide PXR target gene [39]. Consistently, treatment with the PXR antagonist ketaconazole improved the diabetic phenotype of HFD-fed mice. By contrast, expression of a constitutively active form of PXR (VP-PXR) in the liver of ob/ob mice exacerbated the diabetic phenotype [39].
While He et al. [39] convincingly demonstrated that PXR signaling promotes obesity and insulin resistance in mice, another study reported that PCN-mediated chronic activation of PXR prevented HFD-induced obesity and insulin resistance in a different strain of mouse model, AKR/J mice [47]. However, the authors also found that PCN treatment decreased hepatic lipid accumulation in HFD-fed AKR/J mice [47], which is not consistent with well-established role of PXR in promoting hepatic steatosis [5, 39, 48–52]. Further, the high concentration of PCN (50 mg/kg), the AKR/J mouse strain, and the lack of control PXR−/− mice in the study also made it difficult to interpret their results.
Consistent with He et al.’s finding [39], Spruiell and colleagues [53] also reported that deficiency of PXR protected male mice from diet-induced obesity [53]. Male PXR−/− mice resisted to HFD-induced repression of peroxisome proliferator-activated receptor (PPAR)α in white adipose tissue (WAT) and induction of CPT1 expression in liver, which could lead to increased energy expenditure [53]. Interestingly, introduction of human PXR gene to male PXR−/− mice also led to resistance to diet-induced obesity [53]. Therefore, the mouse PXR gene promoted obesity but human PXR gene inhibited obesity in male mice. Despite of decreased obesity, both male PXR−/− and PXR-humanized mice had increased fasting glucose levels and severely impaired glucose tolerance which were coincident with impaired induction of glucokinase involved in glucose utilization in liver [53]. The increased insulin resistant phenotype of male PXR−/− mice contradicted to what He et al. demonstrated in their study [39]. Nevertheless, the authors concluded that the impact of PXR on HFD-induced obesity and hyperglycemia is species-dependent in male mice. Spruiell and colleagues then conducted a similar study in pre-menopausal female mice [54]. They found that female PXR-humanized mice also had hyperinsulinemia and impaired glucose tolerance when fed a HFD [54]. Unlike male mice, female PXR-humanized mice were more susceptible to diet-induced obesity [54]. Under basal condition, female PXR-humanized had increased protein levels of hepatic CYP3A11. The key gluconeogenic enzymes including PEPCK1 and G-6-P were constitutively activated in female PXR-humanized mice [54]. Compared with WT mice, female PXR-humanized mice also had reduced ERα but enhanced UCP1 protein levels in WAT when fed a control diet [54]. While HFD induced UCP1 expression in WAT and glucokinase protein expression in liver of WT mice, these enzymes were not affected by HFD in female PXR-humanized mice [54]. Further, serum 17β-estradiol levels and ERα expression in WAT were decreased by HFD in female WT mice but were unaffected by HFD in female PXR-humanized mice [54]. Collectively, these studies demonstrated an important role of PXR in obesity and insulin resistance. However, the functions of PXR in obesity and insulin resistance are complex and the impact of PXR on metabolic dysfunctions is not only species-dependent but also gender-dependent. Future studies are needed to define detailed mechanisms through which PXR modulate obesity, glucose homeostasis, and energy metabolism in various animal models as well as in humans.
3. Role of PXR in cholesterol metabolism and lipid homeostasis
Despite enormous research efforts and advances in treatments in the past few decades, atherosclerotic CVD is predicated to remain the leading cause of death worldwide for the next two decades, with annual deaths due to CVD expected to reach 24 million by 2030 [55, 56]. Atherosclerosis is a complex chronic disease involving the interaction of genetic and environmental factors over multiple years. Epidemiological studies have revealed numerous risk factors for atherosclerosis including factors with strong genetic components (e.g., elevated levels of low density lipoproteins [LDL] or very low density lipoproteins [VLDL]) and environmental factors such as HFD [57, 58]. The most prominent risk factor for development of atherosclerosis is hypercholesterolemia which may be due to genetic or environmental factors. Much work has been done in an effort to identify genetic variations contributing to atherosclerosis and many genes with small to modest effects have been identified to affect atherosclerosis. However, the impact of xenobiotic exposure on CVD remains relatively unexplored.
Recent studies have demonstrated that PXR signaling may also contribute to the development of CVD. It is well-known that many clinically relevant PXR ligands can elevate plasma lipid levels in patients and increase their CVD risk [48, 59–63]. For example, treatment with rifampicin, a PXR ligand used in the clinic for the treatment of tuberculosis, can cause hyperlipidemia [59], and short-term treatment increased the ratio of lathosterol to cholesterol, indicator of increased cholesterol synthesis [64]. Treatment with ritonavir, an HIV protease inhibitor and a potent PXR activator [65], caused hyperlipidemia and was also associated with increased risk of CVD in HIV patients [60, 61, 66, 67]. Long-term treatment with the antiepileptic drugs carbamazipine and phenobarbital which are also PXR ligands, increased cholesterol levels in children [62]. Further, a meta-analysis of seven genome-wide association studies found that the common genetic variants in PXR are associated with plasma LDL cholesterol levels in humans [68].
Previous studies have also demonstrated an important role of PXR in cholesterol metabolism. In addition to xenobiotics, various endogenous sterol metabolites have been identifed to activate PXR. For example, the secondary bile acid lithocholic acid and its 3-keto metabolite efficiently activate PXR [69, 70] and the bile acid intermediates, 5-cholestanoic acid-3,7,12-triols and 7α-hydroxy-4-cholesten-3-one and 4-cholesten-3-one are also ligands for PXR [71]. These bile acid precursors have been claimed to be endogenous ligands for murine PXR but they do not affect human PXR activity [71, 72]. Activation of PXR by these sterol compounds provides an important alternative pathway for sterol clearance by stimulating CYP3A expression, which hydroxylates the side chain of sterols and bile acid intermediates [71, 72]. Further, activation of PXR can also repress the expression of CYP7A1, the first and rate limiting step in the metabolism of cholesterol to bile acids [69, 70]. Consistently, deficiency of PXR led to acute hepatorenal failure in mice when fed a diet containing high cholesterol and cholic acid levels [73]. Therefore, PXR plays an important role in the detoxification of cholesterol metabolites in liver. Paradoxically, PXR also transcriptionally regulates many hepatic lipogenic genes including CD36, stearoyl-CoA desaturase-1 (SCD-1), fatty acid elongase (FAE), 7-dehydrocholesterol reductase, S14, lipin-1, and SLC13A5 [5, 39, 48–52, 74, 75]. Activation of PXR can lead to hepatic steatosis in several animal models [48, 51, 76, 77]. To date, hepatic PXR signaling has been well-established to promote lipid accumulation, which may contribute to drug-induced steatosis.
Although these studies suggest that PXR regulates cholesterol and lipid homeostasis at multiple levels, only a few studies have investigated the impact of PXR on whole body lipid homeostasis and plasma lipid levels in animal models. Several early studies showed that PXR activation affected serum high density lipoprotein (HDL) cholesterol and apolipoprotein (Apo)A-I levels [78, 79]. For example, Masson et al. [79] found that the inhibitory effects of bile acids on HDL and ApoA-I levels were more pronounced in PXR-deficient mice whereas these effects were blocked in PXR-humanized mice [79]. Another study claimed that induction of CYP3A by some PXR ligands was positively correlated with induction of ApoA-I mRNA as well as plasma HDL and ApoA-I levels in mice [78]. However, they also found that the human PXR-specific ligand, rifampicin, which lacks the ability to activate the rodent PXR, gave positive results in mice [78], suggesting the involvement of a non-PXR dependent mechanism. Moreover, de Hann et al. [80] reported that activation of PXR by PCN treatment increased plasma total cholesterol and VLDL levels in ApoE*3-Leiden mice which exhibit a human-like lipoprotein distribution on a cholesterol-rich diet. Contrary to Bachmann et al.’s findings [78], PCN-mediated PXR activation decreased HDL cholesterol levels in ApoE*3-Leiden cholesteryl ester transfer protein (CETP) transgenic mice [80]. Although several hepatic genes involved in HDL metabolism, including ATP-binding cassette transporter (ABCA)1 and ApoA1 were affected by treatment of PCN at relatively high concentration (0.1% in diet), the detailed mechanisms through which PXR regulates HDL metabolism remain elusive.
The systemic impact of chronic PXR activation on plasma lipid levels were further investigated by several independent groups. Activation of PXR by feeding PCN to WT mice was found to significantly increase plasma total cholesterol levels and VLDL and LDL cholesterol levels in one study [63]. By contrast, PCN had no effect on plasma lipid level in PXR knockout mice (PXR−/−) mice [63], suggesting that the PCN-mediated effects were through PXR signaling. Consistent with de Hann et al.’s report [80], chronic PXR activation in ApoE−/− mice was also found to decrease plasma HDL levels [63]. PXR activation significantly regulated genes in the liver involved in lipoprotein transportation and cholesterol metabolism, including CD36, ApoA-IV and CYP39A1, in both WT and ApoE−/− mice [63]. Another study demonstrated that short-term activation PXR by intraperitoneal injection of relatively high concentration of PCN at 80 mg/kg/day for 3 days increased plasma triglyceride levels but decreased plasma LDL levels in LDL receptor knockout (LDLR−/−) mice [51]. Similar treatment also caused increased plasma triglyceride levels in ApoE−/− mice but the plasma cholesterol and lipoprotein levels were not reported [51]. While the detailed mechanisms through which PXR signaling regulates plasma lipid and lipoprotein levels remain to be determined, all of the evidence suggests that modulation of PXR can affect lipid metabolism and plasma lipid levels in different animal models.
4. Role of PXR in mediating intestinal lipid uptake and transport
The discovery of the role of PXR in lipid homeostasis has provided a novel mechanism for drug or xenobiotic-induced dyslipidemia and prompted more research to investigate the contribution of PXR to adverse effects of clinically relevant drugs on lipid homeostasis. For example, CVD has become a major comorbidity for individuals being treated for HIV with anti-retroviral (ARV) therapy and large-scale clinical studies have concluded that ARV drugs are associated with dyslipidemia and increased risk of CVD in HIV-infected patients [81–85]. Interestingly, several widely-used ARV drugs such as ritonavir have been previously demonstrated to activate PXR [65] and recent studies have identified more ARV drugs including amprenavir and nelfinavir as PXR ligands [86]. These PXR agonistic ARV drugs have been associated with dyslipidemia and increased CVD risk in HIV-infected patients [82, 87, 88]. A recent study showed that short-term exposure to amprenavir by oral delivery can significantly increase plasma total cholesterol and LDL cholesterol levels in WT mice [86]. By contrast, amprenavir did not affect plasma cholesterol levels in PXR−/− mice [86], demonstrating a potential role of PXR in mediating adverse effects of ARV drugs. PXR is expressed at high levels in the liver and intestine, two organs that play a central role in whole body lipid homeostasis. Interestingly, amprenavir regulated PXR target genes in the intestine but not in the liver, which was likely due to the low dose of amprenavir (10 mg/kg body weight/day) and short-term treatment (1 week) used in this study [86]. In addition to prototypic PXR target genes involved in xenobiotic metabolism such as CYP3A11 and MDR1a, amprenavir stimulated expression of several key genes involved in intestinal lipid homeostasis including CD36, diacylglycerol acyltransferase 1 and 2 (DGAT1 and 2).
Several other studies also suggested that PXR plays an important role in the regulation of intestine lipid homeostasis. Ricketts et al. [89] reported that cafestol, presented in unfiltered brewed coffee and the most potent cholesterol-elevating compound known in the human diet, is an agonist of both PXR and farnesoid X receptor (FXR). Cafestol induced intestinal CYP27A1 and ABCA1 expression and promoted cholesterol efflux to the liver via PXR activation [89], which was consistent with a previous report demonstrating similar effects in intestinal cells in vitro [90]. Cheng et al. [50] also demonstrated that chronic exposure to rifaximin, a nonsystemic antibiotic that activates human PXR only in the gut [91], stimulated the expression of lipid transportation genes including CD36, diglyceride acyltransferase (DGAT)1 and DGAT2 in the intestine of PXR-humanized mice, leading to increased triglyceride secretion and hepatic steatosis [50]. However, altered expression of these genes cannot fully explain the elevated plasma cholesterol levels elicited by either amprenavir [86] or rifaximin [50].
The link between intestinal PXR signaling and xenobiotic-induced hyperlipidemia was further confirmed by a more recent study. Tributyl citrate (TBC), one of a large group of FDA-approved pharmaceutical plasticizers, has been identified as a potent and selective PXR agonist [92, 93]. Similar to rifaximin, TBC activated intestinal PXR but does not affect hepatic PXR activity [92]. Nevertheless, short-term TBC exposure increased plasma total cholesterol and atherogenic LDL cholesterol levels in WT mice, but not in PXR−/− mice [92]. In addition to CD36, TBC-mediated PXR activation stimulated the expression of the intestinal transporter Niemann-Pick C1-Like 1 (NPC1L1), an essential transporter in mediating intestinal cholesterol uptake [94–96]. NPC1L1 takes up free cholesterol into cells via vesicular endocytosis and is required for intestinal cholesterol absorption [94, 95, 97]. Indeed, TBC promoted cholesterol uptake by both murine and human intestinal cells in a PXR-dependent manner [92]. Inactivating mutations in NPC1L1 has recently been associated with reduced plasma LDL cholesterol levels and a reduced risk of CVD in a large scale human study [98]. NPC1L1 is also the molecular target of the clinically used drug ezetimibe, a potent cholesterol absorption inhibitor widely used to treat hypercholesterolemia [95]. Interestingly, ezetimibe can effectively reduce LDL cholesterol levels in HIV-infected patients taking ARV drugs [99, 100]. Despite the established function of NPC1L1 in intestinal cholesterol absorption, the transcriptional regulation of NPC1L1 was not fully understood. A PXR-binding site in the human NPC1L1 promoter was then identified, indicating NPC1L1 is a bona fide PXR target gene [92]. Thus, PXR-mediated NPC1L1 upregulation may contribute to TBC and other PXR ligand-induced hypercholesterolemia.
While NPC1L1 plays an essential role in intestinal cholesterol absorption, another PXR-regulated transporter, CD36 mediates enterocyte uptake of fatty acids, which are then converted to triglycerides for transport into chylomicrons [97, 101]. Several studies have also indicated that CD36 mediates cholesterol uptake in the intestine [101, 102] and cholesterol uptake was significantly decreased in the enterocytes isolated from CD36-deficient (CD36−/−) mice [102]. In a lipid infusion study, CD36−/− mice exhibited accumulation of dietary cholesterol in the intestinal lumen and reduction of cholesterol transport into the lymph [101]. It is plausible that the PXR-mediated CD36 upregulation also contributes to xenobiotic-stimulated elevation of cholesterol levels. Further, DHR96, a Drosophila PXR ortholog, has been demonstrated to regulate the intestine lipase Magro (CG5932) which mediates cholesterol and triglyceride homeostasis in Drosophila [103]. Magro protein is most similar to mammalian gastric lipase (LipF) (56% similarity) and lysosomal lipase (LipA) (50% similarity) [86, 103]. LipA plays an important role in the hydrolysis of cholesterol esters and triglycerides within lipoprotein particles internalized by receptor-mediated endocytosis [104, 105] and LipF contributes to lipid catabolism by hydrolysis of dietary triglycerides in the stomach and intestine sequentially producing free fatty acids and diacylglycerol [106, 107]. Interestingly, activation of PXR can induce both LipA and LipF expression in mouse intestine [86] but it is currently unclear whether LipA and LipF are direct transcriptional targets of PXR.
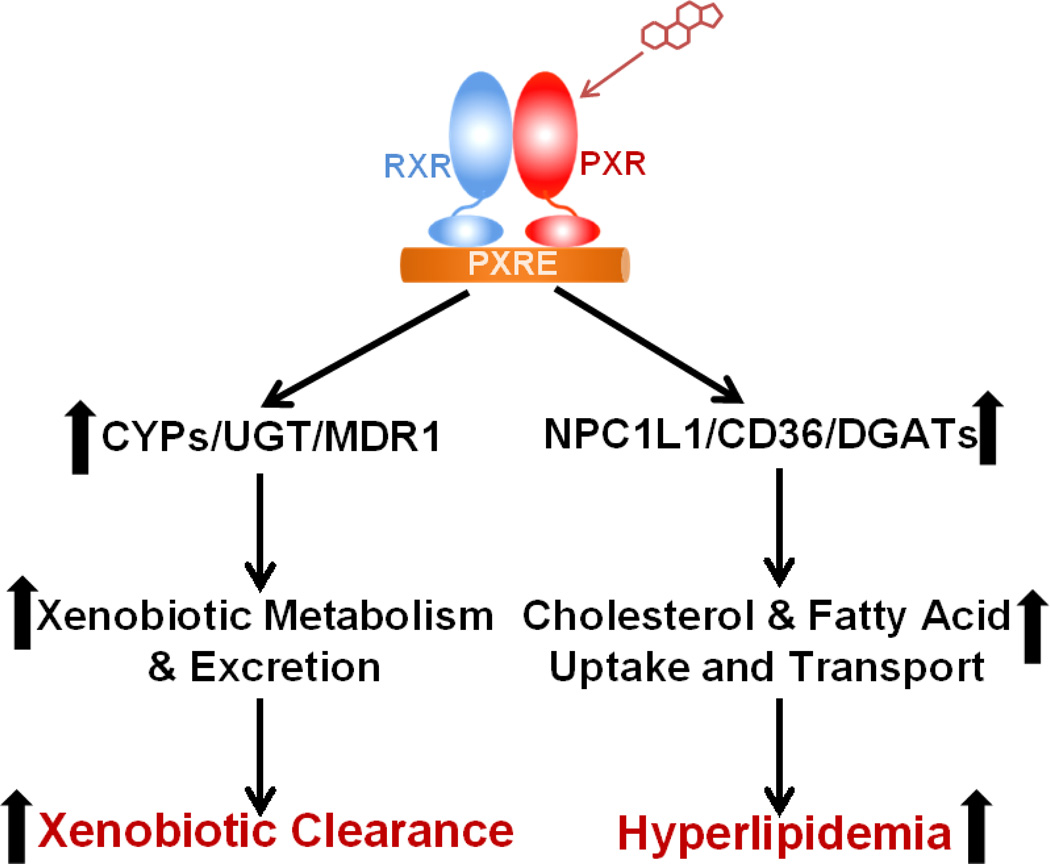
Collectively, these studies demonstrated that intestinal PXR plays a dual role in xenobiotic metabolism and lipid homeostasis (Fig. 1). In addition to promoting xenobiotic metabolism and excretion through regulation of xenobiotic metabolizing enzymes and transporters, PXR signaling also regulates key genes involved in intestinal lipid uptake and transportation including NPC1L1, CD36, and DGATs to modulate lipid homeostasis. Future studies are needed to define the precise mechanisms through which intestinal PXR transcriptionally regulates potential target genes such as LipA and LipF and modulates lipid homeostasis in animal models as well as in humans.
Figure 1. Dual role of intestinal PXR in xenobiotic metabolism and lipid homeostasis.
Activation of PXR stimulates expression of xenobiotic metabolizing enzymes and transporters to promote xenobiotic metabolism and excretion. PXR also regulates key genes mediating intestinal lipid uptake and transport to induce hyperlipidemia. PXRE, PXR response element.
5. Role of PXR in regulating atherosclerosis development and vascular functions
In addition to liver and intestine, PXR is also expressed in immune cells including T cells, B cells, and macrophages [63, 108–113]. Many of those cells directly contribute to atherosclerosis initiation and development. For example, macrophages play a critical role in atherogenesis and accumulation of lipid-loaded macrophages is a hallmark or atherosclerosis [57, 58]. Several studies have indicated that PXR may directly regulate atherosclerosis development independent of dyslipidemia. CD36 [48], a direct transcriptional target of PXR, is a key molecule that mediates macrophages lipid uptake and foam cell formation [114–118]. Activation of PXR was found to increase CD36 expression and lipid accumulation in macrophages of ApoE−/− mice and chronic PXR activation significantly increased atherosclerotic lesions in ApoE−/− mice [63]. By contrast, PXR loss-of-function decreased atherosclerosis in ApoE−/− mice without altering plasma lipid levels, in part due to decreased CD36 expression and CD36-mediated lipid uptake in macrophages [119].
These studies provided novel mechanistic links explaining how exposure to certain xenobiotics causes atherogenic effects without affecting plasma lipid levels. For example, numerous studies implicate that exposure to BPA, a ubiquitous environmental chemical, may cause adverse health effects in humans [120–122]. Recent large and well-controlled cross-sectional and longitudinal studies have found that higher BPA exposure is consistently associated with an increased risk of CVD or atherosclerosis development [123–127]. Further, these associations are independent of traditional CVD risk factors including body mass index, blood pressure, lipid concentrations, and levels of physical activity [123, 125]. However, the underlying mechanisms responsible for these associations remain elusive, which continues to hamper rational assessment of the health risks of BPA exposure [120, 128]. BPA and several its analogs have been identified as ligands of PXR [14], suggesting that BPA-mediated PXR activation could potentially accelerate atherosclerosis development and increase CVD risk in humans. Interestingly, BPA is a potent agonist for human PXR but not for mouse or rat PXR [14]. To investigate the effects of BPA exposure on atherosclerosis development, a PXR-humanized ApoE deficient mouse model was generated [129]. Feeding study concluded that BPA increased atherosclerosis in ApoE−/− mice in a human PXR-dependent manner [129]. BPA-mediated human PXR activation also increased CD36 expression, lipid accumulation and foam cell formation in macrophages of PXR-humanized ApoE−/− deficient mice [129]. These findings identified a potential molecular mechanism that links BPA exposure to increased risk of CVD in exposed individuals and provided evidence to inform future risk assessment for BPA as well as other relevant chemicals.
Atherosclerosis has also been considered a chronic inflammatory disease [130, 131]. Many inflammatory pathways that contribute to the initiation and progression of atherosclerosis are regulated by the transcription factor NF-κB, a master regulator of the innate and adaptive immune responses [132–134]. Interestingly, it has been demonstrated that PXR can regulate inflammation via cross-talk with NF-κB signaling pathway [134–136]. NF-κB signaling activation has been implicated in pathogenesis of atherosclerosis [131, 133] and studies have demonstrated the complex functions of NF-κB signaling in atherosclerosis [137–140]. However, the crosstalk between PXR and NF-κB signaling has not been investigated in the concept of atherosclerosis and future studies are needed to determine whether PXR-NF-κB crosstalk can regulate atherosclerosis development when exposure to PXR-relevant xenobiotics in appropriate animal models.
In addition to macrophages, PXR is also expressed in vascular tissue [141] and vascular cells including smooth muscle cells (SMCs) and endothelial cells (ECs) [142–144]. Hagedorn et al. [141] first reported that PXR regulates vascular tone and contributes to the development of vascular adaptations to pregnancy. They found that treatment with progesterone metabolite, 5β-dihydroprogesterone, led to PXR-dependent increases in vasorelaxation in both nonpregnant and pregnant mice, which was likely due to activation of cytochrome p450 epoxygenases [141]. Swales et al. [142] confirmed that PXR is expressed in primary human and rat aortic SMCs as well as human and rat aorta. Activation of PXR increased xenobiotic metabolism by stimulating expression of Phase 1 and II drug-metabolisms and transporters including CYP3A23, GSTM1, multidrug resistance-associated protein 1 (MRP1) in vascular cells, leading to decreased oxidative stress in those cells [142]. PXR therefore may protect the vasculature from oxidative stress elicited by endogenous and exogenous insults. More recently, Wang et al. [143] showed that the atheroprotective flow, laminar shear stress, activated PXR and induced expression of PXR-regulated genes encoding phase I and II metabolizing enzyme and transporters. By contrast, the atheroprone flow, oscillatory shear stress, suppressed PXR. Laminar shear stress-mediated PXR activation protected ECs from apoptosis triggered by doxorubicin via the induction of detoxification genes including CYP1B1, GSTM4, and MDR1. Consistent with previous reports [134, 135], activation of PXR also suppressed the NF-κB activity and inhibit TNFα or LPS-induced expression of proinflammatory adhesion molecules such as vascular cell adhesion molecule-1 and E-selectin in ECs and in rat carotid arteries [143]. These results suggest that PXR signaling may protect SMCs, ECs and vasculature against potential harmful effects induced by endobiotics or environmental xenobiotics. Interestingly, Wang et al. [144] also found that PXR can regulate innate immunity by activating NLRP3 inflammasome in cultured ECs in another study. PXR can transcriptionally regulate NLRP3 expression and activation of PXR triggered the activation of NLRP3 inflammasome, leading to the cleavage and maturation of caspase-1 and IL-1β in ECs [144]. Therefore, PXR signaling can potentially have both pro-atherogenic and anti-atherogenic effects in different vascular cells. The precise mechanisms through which PXR modulates vascular functions and atherosclerosis, in animal models and in humans remain to be determined.
6. Conclusion
Influences of the chemical environment on human health have recently become the subject of intense interest. PXR was originally identified as a xenobiotic sensor that regulates the metabolism and excretion of a large variety of endobiotic, dietary, and xenobiotic chemicals. The extraordinary chemical diversity of PXR ligands and the marked species-specific differences in the pharmacological activation profiles of PXR have led many laboratories to study the impact of numerous xenobiotics, including clinically used drugs and known and suspected EDCs, on activation of PXR [5]. The discovery of novel and unsuspected roles for PXR in obesity, insulin resistance, lipid homeostasis, atherogenesis, and vascular functions, suggests that PXR signaling may contribute significantly to the pathophysiological effects of many known xenobiotics on cardiometabolic disease in humans. However, the functions of PXR in cardiometabolic disease are complex and future studies are needed to define the cell/tissue-specific role of PXR in cardiometabolic disease in a clinically relevant environment. In addition to cardiometabolic disease, PXR has a number of other important functions in the body including inflammation, bone homeostasis, and tumorigenesis that remain to be fully explored. Further, recent studies also revealed that PXR can be synergistically activated by a mixture of xenobiotics including pharmaceutical and environmental chemicals [14, 145, 146]. For examples, Sui et al. reported that BPA and analogs can synergistically activate human PXR and computational docking study indicated that BPA and its analog may bind to PXR LBD simultaneously [14]. Venkatesh et al. demonstrated that indole 3-propionic acid (IPA), an indole metabolite produced in the gut, is a weak human PXR agonist but , IPA can robustly activate PXR in the presence of indole [146]. More recently, it has been shown that pesticide trans-nonachlor and pharmaceutical chemical 17α-ethinylestradiol can also produce synergistic effects on PXR activity [145]. A fundamental question about exposure to xenobiotic chemicals including EDCs is whether low-dose exposure to those chemicals can influence various signaling pathways and induce adverse effects in humans. The synergism between different PXR-agonistic chemicals support the need to include mixtures for future in vitro and in vivo studies, which may have important implications for toxicology, endocrine disruption study, and chemical risk assessment. Combinations of PXR-agonistic chemicals including pharmaceutical drugs and environmental chemicals may produce significant effects on PXR activity and cardiometabolic disease in humans, even when each chemical is present at low doses that individually do not induce observable effects. There are now more than 100,000 man-made chemicals on the market and only a relatively small subset of chemicals have been identified to have potential adverse effects such as endocrine disrupting activities [147]. Considering PXR’s extraordinary ligand-binding properties including the ability to be activated by mixtures, it is important to identify more xenobiotics as PXR ligand and to further characterize its role in cardiometabolic disease. Such studies will not only significantly contribute to our understanding of “gene-environment interactions” in predisposing individuals to chronic diseases but also provide a novel therapeutic target to combat these diseases.
Table 1.
Summary of genes directly or indirectly regulated by PXR and their functions in cardiometabolic disease.
| Organ/Cell Type | Function | Gene | Reference |
|---|---|---|---|
| Liver | Cholesterol and Lipid Metabolism |
CD36 | [48, 76, 77] |
| FAS | [48, 76] | ||
| SCD-1 | [48, 74, 76] | ||
| ACC-1 | [48] | ||
| PPARγ | [48, 77] | ||
| SREBP1a | [148] | ||
| S14 | [52] | ||
| Lipin-1 | [39] | ||
| SLC13A5 | [75] | ||
| AKR1B10 | [148] | ||
| Insig-1 | [149] | ||
| HMGCS2 | [45] | ||
| CPT1a | [45] | ||
| CYP7A1 | [69, 70] | ||
| DHCR24 | [150] | ||
| Lipoprotein Metabolism |
ApoA-I | [78–80] | |
| ApoA-IV | [63] | ||
| ABCA-1 | [79, 80, 151] | ||
| HL | [51, 80] | ||
| SR-B1 | [51, 80, 151] | ||
| Glucose Metabolism |
PEPCK1 | [41, 152] | |
| G-6-P | [41, 44, 152] | ||
| Glucokinase | [53, 54] | ||
| Intestine | Cholesterol and Lipid Metabolism |
CD36 | [50, 86, 92] |
| NPC1L1 | [50, 92] | ||
| DGAT1 | [50, 86] | ||
| DGAT2 | [50, 86] | ||
| FABP2 | [50] | ||
| LipA | [86] | ||
| LipF | [86] | ||
| CYP27A1 | [89, 90] | ||
| ABCA1 | [89] | ||
| Immune cells (T cells, B cells, and macrophages) |
Inflammation and Atherosclerosis |
CD36 | [63, 119, 129] |
| CD25 | [108] | ||
| IFN-γ | [108] | ||
| IL-10 | [108] | ||
| TNFα | [113] | ||
| COX2 | [113] | ||
| Vasculature and vascular cells (ECs and SMCs) |
Inflammation and Atherosclerosis |
TNFα | [143] |
| VCAM-1 | [143] | ||
| E-selectin | [143] | ||
| NLRP3 | [144] | ||
| IL-1β | [144] | ||
| Detoxification and Oxidative Stress Protection |
CYP3A23 | [142] | |
| GSTM1 | [142] | ||
| GSTM4 | [144] | ||
| MDR1 | [144] | ||
| MRP2 | [142] |
Highlights.
PXR functions as a xenobiotic sensor that regulates xenobiotic metabolism.
Numerous chemicals have been identified as ligands for PXR.
Recent studies have revealed novel functions of PXR in cardiometabolic disease.
PXR may play a key role in linking xenobiotic exposure and cardiometabolic disease.
PXR should be taken into consideration for future risk assessment of chemicals.
Acknowledgments
We apologize to all the authors whose work could not be cited because of space restrictions. This work was supported by NIH grants (R01ES023470, R01HL123358, and R21ES022745) to C.Z.
Abbreviations
- ABCA
ATP-binding cassette transporter
- Apo
apolipoprotein
- ARV
anti-retroviral
- BPA
bisphenol A
- CAR
constitutive androstane receptor
- CETP
cholesteryl ester transfer protein
- CPT1
carnitine palmitoyltransferase 1
- CREB
cAMP response element-binding protein
- CVD
cardiovascular disease
- CYP
cytochrome P450
- DBD
DNA-binding domain
- DEHP
di(2-ethylhexyl)phthalate
- DGAT
diglyceride acyltransferase
- EC
endothelia cell
- EDC
endocrine disrupting chemicals
- FAC
fatty acid elongase
- FoxO1
forkhead box protein O1
- FoxA2
forkhead box protein A2
- FXR
farnesoid X receptor
- G-6-P
glucose-6-phosphatase
- GST
glutathione transferase
- HFD
high-fat diet
- HDL
high density lipoproteins
- HMGCS2
3-hydroxy-3-methylglutartate-CoA synthase 2
- HNF4α
hepatocyte nuclear factor 4α
- JNK
c-Jun NH2-terminal kinase
- LBD
ligand-binding domain
- LDL
low-density lipoprotein
- LDLR
LDL receptor
- LipA
lysosomal lipase
- LipF
Gastric lipase
- MDR1
multidrug resistance 1
- MRP1
Multidrug resistance-associated protein 1
- NPC1L1
Niemann-Pick C1-Like 1
- PCB
polychlorinated biphenyl
- PEPCK1
phosphoenolpyruvate carboxykinase 1
- PCN
pregnenolone 16α-carbonitrile
- PPAR
peroxisome proliferator-activated receptor
- PXR
pregnane X receptor
- PXRE
PXR response element
- RIF
rifampicin
- RXR
retinoid X receptor
- SCD-1
stearoyl-CoA desaturase-1
- SFN
sulforaphane
- SMC
smooth muscle cell
- SXR
steroid and xenobiotic receptor
- TBC
tributyl citrate
- VLDL
very low density lipoproteins
- WAT
white adipose tissue
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author, Changcheng Zhou, declares no conflict of interest.
References
- 1.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 3.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Verma S, Blumberg B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal. 2009;7:e001. doi: 10.1621/nrs.07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg B, Evans RM. Orphan nuclear receptors--new ligands and new possibilities. Genes Dev. 1998;12:3149–3155. doi: 10.1101/gad.12.20.3149. [DOI] [PubMed] [Google Scholar]
- 7.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 10.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 11.Xie W, Evans RM. Orphan nuclear receptors: the exotics of xenobiotics. J Biol Chem. 2001;276:37739–37742. doi: 10.1074/jbc.R100033200. [DOI] [PubMed] [Google Scholar]
- 12.Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, Kliewer SA, Moore JT. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 13.LeCluyse EL. Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–289. doi: 10.1016/s0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 14.Sui Y, Ai N, Park SH, Rios-Pilier J, Perkins JT, Welsh WJ, Zhou C. Bisphenol a and its analogues activate human pregnane x receptor. Environ Health Perspect. 2012;120:399–405. doi: 10.1289/ehp.1104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuyama H, Hiramatsu Y, Kunitomi M, Kudo T, MacDonald PN. Endocrine disrupting chemicals, phthalic acid and nonylphenol, activate Pregnane X receptor-mediated transcription. Mol Endocrinol. 2000;14:421–428. doi: 10.1210/mend.14.3.0424. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita A, Koibuchi N, Oka J, Taguchi M, Shishiba Y, Ozawa Y. Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur J Endocrinol. 2001;145:513–517. doi: 10.1530/eje.0.1450513. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Tabb MM, Sadatrafiei A, Grun F, Blumberg B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab Dispos. 2004;32:1075–1082. doi: 10.1124/dmd.104.000299. [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, Krausz KW, Gonzalez FJ. Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther. 2010;335:32–41. doi: 10.1124/jpet.110.170225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley DP, Dai X, He YD, Carlini EJ, Wang B, Huskey SE, Ulrich RG, Rushmore TH, Evers R, Evans DC. Activators of the rat pregnane X receptor differentially modulate hepatic and intestinal gene expression. Mol Pharmacol. 2004;65:1159–1171. doi: 10.1124/mol.65.5.1159. [DOI] [PubMed] [Google Scholar]
- 20.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 21.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 22.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 23.Vom Saal FS, Myers JP. Bisphenol a and risk of metabolic disorders. JAMA. 2008;300:1353–1355. doi: 10.1001/jama.300.11.1353. [DOI] [PubMed] [Google Scholar]
- 24.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 25.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environmental research. 2011;111:825–830. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol. 2007;23:290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 29.Grun F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8:161–171. doi: 10.1007/s11154-007-9049-x. [DOI] [PubMed] [Google Scholar]
- 30.Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996;104(Suppl 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colborn T, Dumanoski D, Meyers JP. Our Stolen Future. 1996 [Google Scholar]
- 33.Gross L. The Toxic Origins of Disease. PLoS Biol. 2007;5:e193. doi: 10.1371/journal.pbio.0050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabb MM, Kholodovych V, Grun F, Zhou C, Welsh WJ, Blumberg B. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR) Environ Health Perspect. 2004;112:163–169. doi: 10.1289/ehp.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeKeyser JG, Laurenzana EM, Peterson EC, Chen T, Omiecinski CJ. Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol Sci. 2011;120:381–391. doi: 10.1093/toxsci/kfq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takasu N, Yamada T, Miura H, Sakamoto S, Korenaga M, Nakajima K, Kanayama M. Rifampicin-induced early phase hyperglycemia in humans. Am Rev Respir Dis. 1982;125:23–27. doi: 10.1164/arrd.1982.125.1.23. [DOI] [PubMed] [Google Scholar]
- 37.Peters BH, Samaan NA. Hyperglycemia with relative hypoinsulinemia in diphenylhydantoin toxicity. N Engl J Med. 1969;281:91–92. doi: 10.1056/NEJM196907102810208. [DOI] [PubMed] [Google Scholar]
- 38.Suvannasankha A, Fausel C, Juliar BE, Yiannoutsos CT, Fisher WB, Ansari RH, Wood LL, Smith GG, Cripe LD, Abonour R. Final report of toxicity and efficacy of a phase II study of oral cyclophosphamide, thalidomide, and prednisone for patients with relapsed or refractory multiple myeloma: A Hoosier Oncology Group Trial, HEM01-21. Oncologist. 2007;12:99–106. doi: 10.1634/theoncologist.12-1-99. [DOI] [PubMed] [Google Scholar]
- 39.He J, Gao J, Xu M, Ren S, Stefanovic-Racic M, O’Doherty RM, Xie W. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes. 2013;62:1876–1887. doi: 10.2337/db12-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahtela JT, Arranto AJ, Sotaniemi EA. Enzyme inducers improve insulin sensitivity in non-insulin-dependent diabetic subjects. Diabetes. 1985;34:911–916. doi: 10.2337/diab.34.9.911. [DOI] [PubMed] [Google Scholar]
- 41.Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montminy M, Koo SH. Diabetes: outfoxing insulin resistance? Nature. 2004;432:958–959. doi: 10.1038/432958a. [DOI] [PubMed] [Google Scholar]
- 43.Bhalla S, Ozalp C, Fang S, Xiang L, Kemper JK. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem. 2004;279:45139–45147. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- 44.Kodama S, Moore R, Yamamoto Y, Negishi M. Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J. 2007;407:373–381. doi: 10.1042/BJ20070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura K, Moore R, Negishi M, Sueyoshi T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem. 2007;282:9768–9776. doi: 10.1074/jbc.M610072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Liu D. Activation of pregnane X receptor by pregnenolone 16 alpha-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS One. 2012;7:e38734. doi: 10.1371/journal.pone.0038734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281:15013–15020. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Haan W, de Vries-van der Weij J, Mol IM, Hoekstra M, J AR, Jukema JW, Havekes LM, Princen HM, Rensen PC. PXR agonism decreases plasma HDL levels in ApoE3-Leiden.CETP mice. Biochim Biophys Acta. 2009;1791:191–197. doi: 10.1016/j.bbalip.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Cheng J, Krausz KW, Tanaka N, Gonzalez FJ. Chronic exposure to rifaximin causes hepatic steatosis in pregnane x receptor-humanized mice. Toxicol Sci. 2012;129:456–468. doi: 10.1093/toxsci/kfs211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoekstra M, Lammers B, Out R, Li Z, Van Eck M, Van Berkel TJ. Activation of the nuclear receptor PXR decreases plasma LDL-cholesterol levels and induces hepatic steatosis in LDL receptor knockout mice. Molecular pharmaceutics. 2009;6:182–189. doi: 10.1021/mp800131d. [DOI] [PubMed] [Google Scholar]
- 52.Moreau A, Teruel C, Beylot M, Albalea V, Tamasi V, Umbdenstock T, Parmentier Y, Sa-Cunha A, Suc B, Fabre JM, Navarro F, Ramos J, Meyer U, Maurel P, Vilarem MJ, Pascussi JM. A novel pregnane X receptor and S14-mediated lipogenic pathway in human hepatocyte. Hepatology. 2009;49:2068–2079. doi: 10.1002/hep.22907. [DOI] [PubMed] [Google Scholar]
- 53.Spruiell K, Richardson RM, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA. Role of pregnane X receptor in obesity and glucose homeostasis in male mice. J Biol Chem. 2014;289:3244–3261. doi: 10.1074/jbc.M113.494575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spruiell K, Jones DZ, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA. Role of human pregnane X receptor in high fat diet-induced obesity in pre-menopausal female mice. Biochem Pharmacol. 2014;89:399–412. doi: 10.1016/j.bcp.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 56.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 57.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 59.Khogali AM, Chazan BI, Metcalf VJ, Ramsay JH. Hyperlipidaemia as a complication of rifampicin treatment. Tubercle. 1974;55:231–233. doi: 10.1016/0041-3879(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 60.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 61.Shafran SD, Mashinter LD, Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 2005;6:421–425. doi: 10.1111/j.1468-1293.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 62.Eiris JM, Lojo S, Del Rio MC, Novo I, Bravo M, Pavon P, Castro-Gago M. Effects of long-term treatment with antiepileptic drugs on serum lipid levels in children with epilepsy. Neurology. 1995;45:1155–1157. doi: 10.1212/wnl.45.6.1155. [DOI] [PubMed] [Google Scholar]
- 63.Zhou C, King N, Chen KY, Breslow JL. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J Lipid Res. 2009;50:2004–2013. doi: 10.1194/jlr.M800608-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lutjohann D, Hahn C, Prange W, Sudhop T, Axelson M, Sauerbruch T, von Bergmann K, Reichel C. Influence of rifampin on serum markers of cholesterol and bile acid synthesis in men. Int J Clin Pharmacol Ther. 2004;42:307–313. doi: 10.5414/cpp42307. [DOI] [PubMed] [Google Scholar]
- 65.Dussault I, Lin M, Hollister K, Wang EH, Synold TW, Forman BM. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J Biol Chem. 2001;276:33309–33312. doi: 10.1074/jbc.C100375200. [DOI] [PubMed] [Google Scholar]
- 66.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. Aids. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr HIV Res. 2006;4:79–85. doi: 10.2174/157016206775197664. [DOI] [PubMed] [Google Scholar]
- 68.Lu Y, Feskens EJ, Boer JM, Muller M. The potential influence of genetic variants in genes along bile acid and bile metabolic pathway on blood cholesterol levels in the population. Atherosclerosis. 2010;210:14–27. doi: 10.1016/j.atherosclerosis.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 69.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dussault I, Yoo HD, Lin M, Wang E, Fan M, Batta AK, Salen G, Erickson SK, Forman BM. Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance. Proc Natl Acad Sci U S A. 2003;100:833–838. doi: 10.1073/pnas.0336235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodwin B, Gauthier KC, Umetani M, Watson MA, Lochansky MI, Collins JL, Leitersdorf E, Mangelsdorf DJ, Kliewer SA, Repa JJ. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc Natl Acad Sci U S A. 2003;100:223–228. doi: 10.1073/pnas.0237082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sonoda J, Chong LW, Downes M, Barish GD, Coulter S, Liddle C, Lee CH, Evans RM. Pregnane X receptor prevents hepatorenal toxicity from cholesterol metabolites. Proc Natl Acad Sci U S A. 2005;102:2198–2203. doi: 10.1073/pnas.0409481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Wei Y, Hu B, Huang M, Xie W, Zhai Y. Activation of human stearoyl-coenzyme A desaturase 1 contributes to the lipogenic effect of PXR in HepG2 cells. PLoS One. 2013;8:e67959. doi: 10.1371/journal.pone.0067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li L, Li H, Garzel B, Yang H, Sueyoshi T, Li Q, Shu Y, Zhang J, Hu B, Heyward S, Moeller T, Xie W, Negishi M, Wang H. SLC13A5 is a novel transcriptional target of the pregnane X receptor and sensitizes drug-induced steatosis in human liver. Mol Pharmacol. 2015;87:674–682. doi: 10.1124/mol.114.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitro N, Vargas L, Romeo R, Koder A, Saez E. T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett. 2007;581:1721–1726. doi: 10.1016/j.febslet.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, Silverstein RL, Xie W. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 78.Bachmann K, Patel H, Batayneh Z, Slama J, White D, Posey J, Ekins S, Gold D, Sambucetti L. PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res. 2004;50:237–246. doi: 10.1016/j.phrs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Masson D, Lagrost L, Athias A, Gambert P, Brimer-Cline C, Lan L, Schuetz JD, Schuetz EG, Assem M. Expression of the pregnane X receptor in mice antagonizes the cholic acid-mediated changes in plasma lipoprotein profile. Arterioscler Thromb Vasc Biol. 2005;25:2164–2169. doi: 10.1161/01.ATV.0000183674.88817.fb. [DOI] [PubMed] [Google Scholar]
- 80.de Haan W, de Vries-van der Weij J, Mol IM, Hoekstra M, Romijn JA, Jukema JW, Havekes LM, Princen HM, Rensen PC. PXR agonism decreases plasma HDL levels in ApoE3-Leiden.CETP mice. Biochim Biophys Acta. 2009;1791:191–197. doi: 10.1016/j.bbalip.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 82.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, Thiebaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 83.Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, Marcovina SM, Glauser MP, Nicod P, Darioli R, Mooser V, Study SHC. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 84.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Costagliola D. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170:1228–1238. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 85.Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 86.Helsley RN, Sui Y, Ai N, Park SH, Welsh WJ, Zhou C. Pregnane X Receptor Mediates Dyslipidemia Induced by the HIV Protease Inhibitor Amprenavir in Mice. Mol Pharmacol. 2013;83:1190–1199. doi: 10.1124/mol.113.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 88.Feeney ER, Mallon PW. HIV and HAART-Associated Dyslipidemia. The open cardiovascular medicine journal. 2011;5:49–63. doi: 10.2174/1874192401105010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld GJ, Moen CJ, Muller M, Frants RR, Kasanmoentalib S, Post SM, Princen HM, Porter JG, Katan MB, Hofker MH, Moore DD. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- 90.Li T, Chen W, Chiang JY. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J Lipid Res. 2007;48:373–384. doi: 10.1194/jlr.M600282-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, Gonzalez FJ. Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther. 2007;322:391–398. doi: 10.1124/jpet.107.121913. [DOI] [PubMed] [Google Scholar]
- 92.Sui Y, Helsley RN, Park SH, Song X, Liu Z, Zhou C. Intestinal pregnane x receptor links xenobiotic exposure and hypercholesterolemia. Mol Endocrinol. 2015;29:765–776. doi: 10.1210/me.2014-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takeshita A, Igarashi-Migitaka J, Nishiyama K, Takahashi H, Takeuchi Y, Koibuchi N. Acetyl tributyl citrate, the most widely used phthalate substitute plasticizer, induces cytochrome p450 3a through steroid and xenobiotic receptor. Toxicol Sci. 2011;123:460–470. doi: 10.1093/toxsci/kfr178. [DOI] [PubMed] [Google Scholar]
- 94.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 95.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 96.Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011;73:239–259. doi: 10.1146/annurev-physiol-012110-142233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.I. Myocardial Infarction Genetics Consortium. Stitziel NO, Won HH, Morrison AC, Peloso GM, Do R, Lange LA, Fontanillas P, Gupta N, Duga S, Goel A, Farrall M, Saleheen D, Ferrario P, Konig I, Asselta R, Merlini PA, Marziliano N, Notarangelo MF, Schick U, Auer P, Assimes TL, Reilly M, Wilensky R, Rader DJ, Hovingh GK, Meitinger T, Kessler T, Kastrati A, Laugwitz KL, Siscovick D, Rotter JI, Hazen SL, Tracy R, Cresci S, Spertus J, Jackson R, Schwartz SM, Natarajan P, Crosby J, Muzny D, Ballantyne C, Rich SS, O’Donnell CJ, Abecasis G, Sunyaev S, Nickerson DA, Buring JE, Ridker PM, Chasman DI, Austin E, Ye Z, Kullo IJ, Weeke PE, Shaffer CM, Bastarache LA, Denny JC, Roden DM, Palmer C, Deloukas P, Lin DY, Tang ZZ, Erdmann J, Schunkert H, Danesh J, Marrugat J, Elosua R, Ardissino D, McPherson R, Watkins H, Reiner AP, Wilson JG, Altshuler D, Gibbs RA, Lander ES, Boerwinkle E, Gabriel S, Kathiresan S. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med. 2014;371:2072–2082. doi: 10.1056/NEJMoa1405386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wohl DA, Waters D, Simpson RJ, Jr, Richard S, Schnell A, Napravnik S, Keys J, Eron JJ, Jr, Hsue P. Ezetimibe alone reduces low-density lipoprotein cholesterol in HIV-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2008;47:1105–1108. doi: 10.1086/592116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chow D, Chen H, Glesby MJ, Busti A, Souza S, Andersen J, Kohrs S, Wu J, Koletar SL. Short-term ezetimibe is well tolerated and effective in combination with statin therapy to treat elevated LDL cholesterol in HIV-infected patients. AIDS. 2009;23:2133–2141. doi: 10.1097/QAD.0b013e32833068e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 103.Sieber MH, Thummel CS. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab. 2012;15:122–127. doi: 10.1016/j.cmet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson RA, Sando GN. Cloning and expression of cDNA encoding human lysosomal acid lipase/cholesteryl ester hydrolase. Similarities to gastric and lingual lipases. J Biol Chem. 1991;266:22479–22484. [PubMed] [Google Scholar]
- 105.Sheriff S, Du H, Grabowski GA. Characterization of lysosomal acid lipase by site-directed mutagenesis and heterologous expression. J Biol Chem. 1995;270:27766–27772. doi: 10.1074/jbc.270.46.27766. [DOI] [PubMed] [Google Scholar]
- 106.Carriere F, Barrowman JA, Verger R, Laugier R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 1993;105:876–888. doi: 10.1016/0016-5085(93)90908-u. [DOI] [PubMed] [Google Scholar]
- 107.Gargouri Y, Pieroni G, Riviere C, Lowe PA, Sauniere JF, Sarda L, Verger R. Importance of human gastric lipase for intestinal lipolysis: an in vitro study. Biochim Biophys Acta. 1986;879:419–423. doi: 10.1016/0005-2760(86)90234-1. [DOI] [PubMed] [Google Scholar]
- 108.Dubrac S, Elentner A, Ebner S, Horejs-Hoeck J, Schmuth M. Modulation of T lymphocyte function by the pregnane X receptor. J Immunol. 2010;184:2949–2957. doi: 10.4049/jimmunol.0902151. [DOI] [PubMed] [Google Scholar]
- 109.Albermann N, Schmitz-Winnenthal FH, Z’Graggen K, Volk C, Hoffmann MM, Haefeli WE, Weiss J. Expression of the drug transporters, MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol. 2005;70:949–958. doi: 10.1016/j.bcp.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 110.Owen A, Chandler B, Back DJ, Khoo SH. Expression of pregnane-X-receptor transcript in peripheral blood mononuclear cells and correlation with MDR1 mRNA. Antivir Ther. 2004;9:819–821. [PubMed] [Google Scholar]
- 111.Siest G, Jeannesson E, Marteau JB, Samara A, Marie B, Pfister M, Visvikis-Siest S. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab Dispos. 2008;36:182–189. doi: 10.1124/dmd.107.017228. [DOI] [PubMed] [Google Scholar]
- 112.Casey SC, Blumberg B. The steroid and xenobiotic receptor negatively regulates B-1 cell development in the fetal liver. Mol Endocrinol. 2012;26:916–925. doi: 10.1210/me.2011-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Casey SC, Nelson EL, Turco GM, Janes MR, Fruman DA, Blumberg B. B-1 cell lymphoma in mice lacking the steroid and xenobiotic receptor, SXR. Mol Endocrinol. 2011;25:933–943. doi: 10.1210/me.2010-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 115.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guy E, Kuchibhotla S, Silverstein R, Febbraio M. Continued inhibition of atherosclerotic lesion development in long term Western diet fed CD36o /apoEo mice. Atherosclerosis. 2007;192:123–130. doi: 10.1016/j.atherosclerosis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 118.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sui Y, Xu J, Rios-Pilier J, Zhou C. Deficiency of PXR decreases atherosclerosis in apoE-deficient mice. J Lipid Res. 2011;52:1652–1659. doi: 10.1194/jlr.M017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Jr, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 124.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, Galloway TS. Urinary bisphenol a concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- 126.Melzer D, Gates P, Osborn NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Schofield P, Mosedale D, Grainger D, Galloway TS. Urinary bisphenol a concentration and angiography-defined coronary artery stenosis. PLoS One. 2012;7:e43378. doi: 10.1371/journal.pone.0043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lind PM, Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 128.Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol a. Environ Health Perspect. 2010;118:1051–1054. doi: 10.1289/ehp.0901717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sui Y, Park SH, Helsley RN, Sunkara M, Gonzalez FJ, Morris AJ, Zhou C. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. Journal of the American Heart Association. 2014;3:e000492. doi: 10.1161/JAHA.113.000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 131.Moore Kathryn J, Tabas I. Macrophages in the Pathogenesis of Atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 133.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou C, Tabb MM, Nelson EL, Grun F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, Blumberg B. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 136.Xie W, Tian Y. Xenobiotic receptor meets NF-kappaB, a collision in the small bowel. Cell Metab. 2006;4:177–178. doi: 10.1016/j.cmet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 137.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 138.Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park SH, Sui Y, Gizard F, Xu J, Rios-Pilier J, Helsley RN, Han SS, Zhou C. Myeloid-Specific IkappaB Kinase beta Deficiency Decreases Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2012;32:2869–2876. doi: 10.1161/ATVBAHA.112.254573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sui Y, Park SH, Xu J, Monette S, Helsley RN, Han SS, Zhou C. IKKbeta links vascular inflammation to obesity and atherosclerosis. J Exp Med. 2014;211:869–886. doi: 10.1084/jem.20131281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension. 2007;49:328–333. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- 142.Swales KE, Moore R, Truss NJ, Tucker A, Warner TD, Negishi M, Bishop-Bailey D. Pregnane X receptor regulates drug metabolism and transport in the vasculature and protects from oxidative stress. Cardiovasc Res. 2012;93:674–681. doi: 10.1093/cvr/cvr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X, Fang X, Zhou J, Chen Z, Zhao B, Xiao L, Liu A, Li YS, Shyy JY, Guan Y, Chien S, Wang N. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc Natl Acad Sci U S A. 2013;110:13174–13179. doi: 10.1073/pnas.1312065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang S, Lei T, Zhang K, Zhao W, Fang L, Lai B, Han J, Xiao L, Wang N. Xenobiotic pregnane X receptor (PXR) regulates innate immunity via activation of NLRP3 inflammasome in vascular endothelial cells. J Biol Chem. 2014;289:30075–30081. doi: 10.1074/jbc.M114.578781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C, Rahmani R, Cavailles V, Daujat-Chavanieu M, Vivat V, Pascussi JM, Balaguer P, Bourguet W. Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds. Nat Commun. 2015;6:8089. doi: 10.1038/ncomms9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Acerini CL, Hughes IA. Endocrine disrupting chemicals: a new and emerging public health problem? Arch Dis Child. 2006;91:633–641. doi: 10.1136/adc.2005.088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bitter A, Rummele P, Klein K, Kandel BA, Rieger JK, Nussler AK, Zanger UM, Trauner M, Schwab M, Burk O. Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Arch Toxicol. 2015;89:2089–2103. doi: 10.1007/s00204-014-1348-x. [DOI] [PubMed] [Google Scholar]
- 149.Roth A, Looser R, Kaufmann M, Blaettler S, Rencurel F, Huang W, Moore DD, Meyer UA. Regulatory Crosstalk between Drug Metabolism and Lipid Homeostasis: Car and Pxr Increase Insig-1 Expression. Mol Pharmacol. 2008 doi: 10.1124/mol.107.041012. [DOI] [PubMed] [Google Scholar]
- 150.Yoshinari K, Ohno H, Benoki S, Yamazoe Y. Constitutive androstane receptor transactivates the hepatic expression of mouse Dhcr24 and human DHCR24 encoding a cholesterogenic enzyme 24-dehydrocholesterol reductase. Toxicol Lett. 2012;208:185–191. doi: 10.1016/j.toxlet.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 151.Sporstol M, Tapia G, Malerod L, Mousavi SA, Berg T. Pregnane X receptor-agonists down-regulate hepatic ATP-binding cassette transporter A1 and scavenger receptor class B type I. Biochem Biophys Res Commun. 2005;331:1533–1541. doi: 10.1016/j.bbrc.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 152.Gotoh S, Negishi M. Statin-activated nuclear receptor PXR promotes SGK2 dephosphorylation by scaffolding PP2C to induce hepatic gluconeogenesis. Sci Rep. 2015;5:14076. doi: 10.1038/srep14076. [DOI] [PMC free article] [PubMed] [Google Scholar]