Abstract
Objective
To evaluate if ICSI use and estradiol on the final day of ART stimulation are associated with adverse obstetric complications related to placentation.
Design
Retrospective cohort study.
Setting
Large private ART practice.
Patients
383 women who underwent ART resulting in a singleton live births.
Interventions
None.
Main outcome measures
Adverse placental outcomes composed of placental accreta, placental abruption, placental previa, intrauterine growth restriction, preeclampsia, gestational hypertension and small for gestational age infants.
Results
Patients with adverse placental outcomes had higher peak serum estradiol levels and were three times more likely to have used ICSI. Adverse placental outcomes were associated with increasing estradiol (OR 1.36, 95% CI 1.13–1.65) and ICSI (OR 3.86, 95%CI 1.61–9.27). Adverse outcomes increased when estradiol was over 3000 pg/ml and continued to rise in a linear fashion until estradiol was over 5000 pg/ml. The association of ICSI with adverse outcomes was independent of male factor infertility. Interaction testing suggested the adverse effect of estradiol was primarily seen in ICSI cycles, but not in conventional IVF cycles. Estradiol over 5000 pg/ml was associated with adverse placental events in 36% of all ART cycles and 52% of ICSI cycles.
Conclusion
ICSI and elevated estradiol on the day of hCG trigger were associated with adverse obstetric outcomes related to placentation. The finding of a potential interaction of estradiol and ICSI with adverse placental events is novel and warrants further investigation.
Keywords: elevated estradiol, adverse obstetrical outcomes, IVF, ICSI
Capsule
High serum estradiol on the last day of ART stimulation was associated with adverse obstetric outcomes related to placentation with ICSI, but not conventional IVF.
Introduction
Assisted Reproductive Technology (ART) use in the United States has continued to increase from 2004 to 2013, with the number of infants more than doubling. Over 66,000 infants were born in 2013 with the use of ART, approximately 1% of all live births in the United States (1). However, ART is not without risks. It is known that ART is associated with increased risk of chromosomal abnormalities, small for gestational age (SGA) and low birth weight (LBW) infants, preterm labor (PTL), preterm delivery (PTD), preterm premature rupture of membranes (PPROM), intrauterine growth restriction (IUGR), gestational hypertension, preeclampsia, placenta previa, placental abruption, gestational diabetes and cesarean delivery. The majority of these complications are primarily attributed to the increased risk of multiple gestations with ART (2 – 15). However, studies have shown that most of these risks remain elevated compared to spontaneously conceived pregnancies, even when there is a singleton pregnancy conceived through ART (12, 21, 33).
Many factors contribute to the success or failure of ART cycles, but most endpoints of studies on ART parameters in our current literature examine clinical pregnancy or live birth. Unfortunately, there are very few studies on the effect of ART parameters on clinical obstetrical complications as well as a dearth of knowledge at the molecular level of these parameters on the developing placenta. There are many ART parameters that could adversely affect implantation (all or nothing effect) or placentation (exaggerated or insufficient invasion of the trophectoderm).
Insufficient trophoblastic invasion at the time of implantation has been implicated as a possible cause of later obstetrical complications such as preeclampsia, premature preterm rupture of membranes (PPROM), preterm delivery (PTD), and intrauterine growth restriction (IUGR) resulting in small for gestational (SGA) infants (16). There is evidence that abnormal placentation occurs due to decreased trophoblastic invasion of the decidual and myometrial spiral arteries and aberrant cell survival and apoptosis (17, 18). If severe enough, the continued insult to the placenta can lead to IUGR, SGA, preeclampsia, and/or placental abruption (18). On the other hand, excessive trophoblastic invasion is thought to be associated with placenta accreta/increta/percreta through the decidua or the absence of an adequate decidua formation (16, 19).
One theory to explain the increased risks is that abnormal placentation is mediated by elevated estradiol levels at the time of implantation, thus adversely affecting trophoblastic invasion of the endometrium. Bonagura et al. demonstrated suppressed extravillous trophobastic spiral arterial invasion in the presence of elevated estradiol levels during the first trimester of baboon pregnancies (20). A study by Kalra et al. showed that supraphysiologic levels of sex steroid hormones prior to embryo implantation appeared to contribute to the risk of low birth weight and other disorders of abnormal placentation (10). Additionally, recent studies by Farhi et al. and Imudia et al. suggest that elevated peak estradiol levels during ART, even in singleton pregnancies, increased the risk of abnormal placentation without adversely affecting embryo implantation, pregnancy, or abortion risk (12, 21).
However, a fundamental objective of controlled ovarian hyperstimulation is supraovulation with coincident supraphysiologic hormonal milieu. The highest ovarian responses are often seen in the most fertile females (e.g. male factor infertility and oocyte donors). We examined if estradiol was associated with obstetrical outcomes to better understand the mechanism of the elevated risks for adverse pregnancy outcomes.
Materials and Methods
Study Design
A retrospective cohort study was conducted at the largest United States military academic medical center with IRB approval. All fresh ART cycles from January 2010 to December 2013 were reviewed. Data was collected to include age, baseline levels for follicle stimulating hormone (FSH), peak estradiol on the final day of ART stimulation, infertility diagnosis, and the number of embryos transferred. Pregnancy outcomes were collected to include preterm delivery, birth weight, presence of gestational hypertension or preeclampsia, preterm premature rupture of membranes, intrauterine growth restriction, placental abruption, placenta previa and placenta accreta/increta/percreta. Adverse obstetric events were defined as:
-
–
Preterm delivery (PTD) was a birth <37 weeks gestation.
-
–
Small for gestational age (SGA) was birth weight < 10th percentile for gestational age using standard curves
-
–
Gestational hypertension (GHTN) was elevated systolic blood pressures ≥140 mmHg or diastolic blood pressures ≥90 mmHg after 20 weeks gestation
-
–
Preeclampsia was hypertension with presence of proteinuria or clinical features
-
–
Intrauterine growth restriction (IUGR) was an overall estimated fetal weight <10th percentile or abdominal circumference of fetus <10th percentile calculated from ultrasound evaluation using standard curve for gestational age
-
–
Placental abruption was diagnosed clinically from medical records and placental pathology
-
–
Placenta previa, accreta, increta or percreta was diagnosed by ultrasound and confirmed after delivery
The primary outcome was the composite risk of any adverse obstetric outcomes related to placentation. This included gestational hypertension, preeclampsia, IUGR/SGA, PPROM, placental abruption, placenta previa along with placenta accrete, increta or percreta. Secondary outcomes measures were rates of implantation, live birth rates, and preterm delivery rates.
Patients
ART records from 2010 – 2013 were evaluated for a singleton live birth. Patients with a singleton pregnancy noted by one gestational sac and fetal pole present during first trimester ultrasound were included after a fresh ART cycle with records available in the electronic medical record. Patients were only included one time during the study period. Exclusion criteria were:
Any factors that could potentially affect implantation such as a fibroid uterus;
Hydrosalpinx or any other congenital uterine anomalies;
Any chronic diseases that could affect the course of the pregnancy such as diabetes mellitus, autoimmune diseases, or hypertension;
Any history of pregnancy complications in previous pregnancies including gestational hypertension or preeclampsia, placental abruption, placenta previa, cesarean delivery, preterm delivery, and or placenta accrete, increta or percreta.
PGS cycles were excluded since all biopsied embryos are vitrified in our program.
Male factor infertility was assigned as a diagnosis if semen analysis on two separate occasions showed a sperm concentration <15 million/ml, motility <40%, or morphology <4%.
Stimulation Protocol
All patients undergoing ART underwent conventional IVF or ICSI. Ovarian stimulation was achieved using mixed FSH/LH protocols under pituitary suppression with either GnRH-antagonist or GnRH-agonist. Oral contraceptive treatment was typically started at least 21 days prior to the start of ovarian stimulation. During GnRH-antagonist (Ganirelix, Merck) cycles, Ganirelix was given when the lead follicle reached 14mm in mean diameter size. For GnRH-agonist cycles, 20 units (1 mg) of leuprolide acetate (Lupron, TAP Pharmaceuticals) was administered during the last 7 days of oral contraceptives. When ovarian suppression was confirmed, the Lupron dose was decreased to 5 units (0.25 mg).
Recombinant FSH (follitropin-alfa) (Gonal-F, EMD Serono) and human menopausal gonadotropin (Menopur, Ferring Pharmaceuticals) were used for ovarian stimulation. Final oocyte maturation was triggered with 10,000 international units of human chorionic gonadotropin (hCG) when the lead follicle was greater than or equal to 18 mm (mean) diameter. For those with GnRH-antagonist cycles at risk of ovarian hyperstimulation syndrome (OHSS), 4 mg of Lupron was administered for oocyte maturation. Ultrasound-guided transvaginal oocyte retrieval was carried out 36 hours later. ICSI was performed in couples with severe oligospermia <5 million total motile sperm, abnormal sperm morphology of <4%, unexplained infertility >2 years, 24 hour sperm survival of <50%, or prior poor fertilization with conventional IVF (22). Otherwise conventional IVF was performed. Embryos were transferred using transabdominal ultrasound guidance either 3 or 5 days after oocyte retrieval using the embryo afterload technique (23). If there were ≥3 high grade embryos on day 3, embryos were cultured to the blastocyst stage and transferred on day 5. When only 1 or 2 high grade embryos were available, a day 3 embryo transfer was performed. The number of embryos transferred was based on ASRM guidelines. Single embryo transferred was mandatory for patients <38 years old, with a good quality blastocysts, undergoing their 1st IVF cycle. Luteal support was provided with either 50 mg of progesterone-in-oil (Freedom Pharmacy, Bayfield, MA, USA) once daily starting on the night of oocyte retrieval or with 100mg of vaginal progesterone (Endometrin, Ferring Pharmaceuticals, Parsippany, NJ, USA) three-times daily starting the day after oocyte retrieval. Luteal support was provided until 10 weeks estimated gestational age.
Statistical Analysis
Differences between groups for dichotomous outcomes were analyzed with Chi-Squared or Fisher’s exact tests as appropriate. Normality of data was assessed with Shapiro-Wilks test. Continuous variables were analyzed with Student’s t-test and Mann Whitney tests as appropriate. Generalized estimating equations (GEE) were used to evaluate the association of peak estradiol with obstetrical outcomes. Adjusted GEE modeling used to account for all significant confounders. GEE models were used to perform interaction tests on the relationship between peak estradiol with IVF vs. ICSI and adverse placental outcomes. Receiver operating characteristic (ROC) curves evaluated the ability of peak estradiol and ICSI to predict adverse obstetric outcomes. Greater than efficiency curves were generated to assess at what peak estradiol threshold adverse obstetric events began to increase. Data was expressed as the mean ± standard deviation for normally distributed data and median with interquartile range for non-normally distributed data. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, IBM, Armonk, NY). Statistical significance was defined as P< 0.05.
Results
During the study period, a total of 1595 consecutive ART cycles resulted in 466 singleton live births. Of these births, 383 patients met inclusion criteria. Of those patients, 283 patients underwent ICSI and 100 patients underwent conventional IVF. On average 1.7 embryos were transferred per cycle resulting in a pregnancy. The majority of cycles had two embryos transferred and 33% of cycles had a single embryo transfer. All included cycles had a single gestational sac on ultrasound. A total of 63 patients (16%) developed an adverse obstetric complication potentially related to the placenta. These outcomes occurred in 6 patients in the IVF cohort and 57 in the ICSI cohort. There were 8 preterm deliveries, 8 IUGR, 13 GHTN, 23 pre-eclampsia, and 32 SGA pregnancies. In 15 pregnancies, multiple complications were diagnosed. There was no difference in age or basal FSH between patients who had an adverse event versus those who did not (Table 1). Patients with adverse placental outcomes had a higher peak estradiol and use of ICSI (Table 1). A diagnosis of male factor infertility was similar between the two groups.
Table 1.
Comparison of baseline and ART cycle parameters between patients with a normal pregnancy and those who developed adverse pregnancy outcomes. Continuous data are expressed as media (IQR) and dichotomous data a percentage of the population.
| Normal Pregnancy (n=322) |
Adverse Outcome (n=63) |
P value | |
|---|---|---|---|
| Age | 34 (30–37) | 34 (31–37) | 0.70 |
| Male factor infertility | 39% | 45% | 0.40 |
| Basal FSH (IU/L) | 6.7 (5.0–8.3) | 6.7 (5.4–8.4) | 0.33 |
| Basal estradiol (pg/ml) | 38 (27–53) | 39 (31–57) | 0.30 |
| Days of stimulation | 11 (10–12) | 11 (10–12) | 0.63 |
| Total gonadotropins (IU) | 2550 (1800–4125) | 2625 (1800–3825) | 0.52 |
| Estradiol peak (pg/ml) | 3012 (2279–3878) | 3582 (2597–5121) | 0.02 |
| Oocytes retrieved | 7 (5–10) | 8 (6–11) | 0.27 |
| ICSI | 29% | 90% | <0.001 |
| Number Transferred | 2 (1–2) | 2 (1–2) | 0.86 |
GEE models demonstrated that adverse placental outcomes were significantly associated with peak estradiol (OR 1.36, 95% CI 1.13–1.65, P < 0.001) and ICSI (OR 3.86, 95%CI 1.61–9.27, P=0.002) (Table 2). These predictors remained significantly associated with adverse placental outcomes after adjustment with each other, age, and male factor infertility. A GEE model testing the interaction of estradiol and IVF versus ICSI on adverse placental outcomes was significant (P<0.001), indicating that estradiol interacts differently with outcomes in IVF versus ICSI cycles. No other variables in the analysis were associated with adverse outcomes (Table 2). In addition to being associated with composite placental outcomes, peak estradiol was also individually associated with SGA, GHTN, and pre-eclampsia (Supplemental Table 1). ICSI was associated with SGA (Supplemental Table 1). Adverse placental outcomes were not associated with male factor infertility (OR 1.27, 95%CI 1.27–2.20, P=0.38) or assisted hatching (OR 1.08, 95%CI 0.92–1.27, P=0.30). For every 1000 pg/ml increase in estradiol, birth weight decreased by 33 grams (95%CI 2 – 65 grams). Birth weight was similar in the IVF and ICSI groups (3288 grams versus 3243 grams, P=0.35)
Table 2.
Evaluating association of variables with adverse placental outcomes by GEE model. Adjust model accounted for estradiol, ICSI, age, and male factor infertility.
| Variable | Unadjusted Odds Ratio (95%CI) |
P value | Adjusted Odds Ratio (95%CI) |
P value |
|---|---|---|---|---|
| Estradiol on hCG | 1.36 (1.13–1.65) | 0.001 | 1.32 (1.13–1.52) | 0.001 |
| ICSI | 3.86 (1.61–9.27) | 0.002 | 4.21 (1.73–10.25) | 0.002 |
| Female Age | 1.00 (0.99–1.01) | 0.37 | 1.03 (0.96–1.10) | 0.34 |
| Male Factor | 1.27 (0.73–2.20) | 0.38 | 1.30 (0.70–2.23) | 0.40 |
| BMI | 1.02 (0.96–1.10) | .43 | ||
| Days of Stimulation | 0.95 (0.81–1.11) | 0.35 | ||
| Gonadotropins used | 0.99 (0.98–1.01) | 0.33 | ||
| Follicles | 1.01 (0.98–1.05) | 0.39 | ||
| Oocytes Retrieved | 1.02 (0.98–1.06) | 0.22 | ||
| Mature Oocytes | 1.04 (0.99–1.09) | 0.07 | ||
| Assisted Hatching | 2.22 (0.28–17.58) | 0.45 | ||
| Blastocyst Transfer | 1.21 (0.70–2.09) | 0.47 | ||
| Embryos transferred | 0.99 (0.93–1.05) | 0.82 |
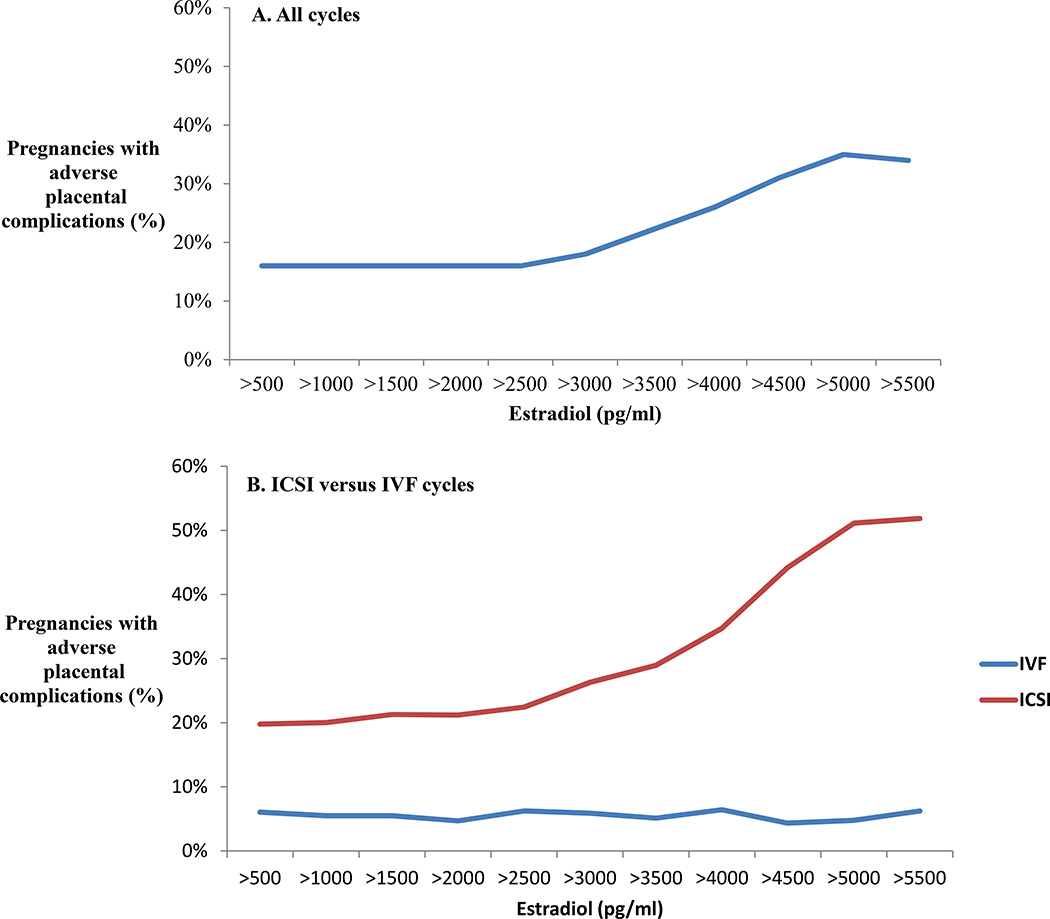
Greater than efficiency curves were plotted to determine estradiol thresholds at which adverse placental outcomes began to increase (Figure 1A). Adverse outcomes did not begin to rise in the overall cohort until estradiol was over 3000 pg/ml and there was a steady rise in adverse outcomes at each threshold above that point. Adverse outcomes peaked at 35% of cycles with an estradiol over 5000 pg/ml. The same efficiency curves were then generated separately for IVF and ICSI cycles (Figure 1B). Adverse outcomes remained rare and never increased over 6% in IVF cycles, regardless of estradiol levels. Conversely, adverse events began to increase in ICSI cycles when the estradiol was over 3000 pg/ml and were over 50% occurrence when estradiol was over 5000 pg/ml. To determine if estradiol thresholds affected different types of placental disorders differently, greater than efficiency curves were also generated separately for all adverse events, hypertensive disorders, growth disorders, and placenta percreta or acreta or abruption (Supplemental Figure 1). Placenta percreta, accreta or abruption remained rare across all estradiol thresholds, suggesting increased placenta invasion is not related to the degree of ovarian stimulation. Hypertensive and growth disorders both became more frequent as estradiol rose above 3000 pg/ml and continued to rise as estradiol increased (Supplemental Figure 1). To further explore the effect of male factor infertility on abnormal placentation, greater than efficiency curves were also generated for ICSI patients with and without male factor infertility (Supplemental Figure 2). The curves demonstrated a rise in adverse events with rising estradiol in all ICSI patients, whether they had a diagnosis of male factor infertility or not.
Figure 1.
Greater than efficiency curves evaluating the effect of estradiol on adverse placental outcomes. The occurrence of adverse outcomes was calculated above each estradiol threshold in increments of 500 pg/ml. A) All cycles in the study. B) All cycles in the broken down into ICSI versus IVF.
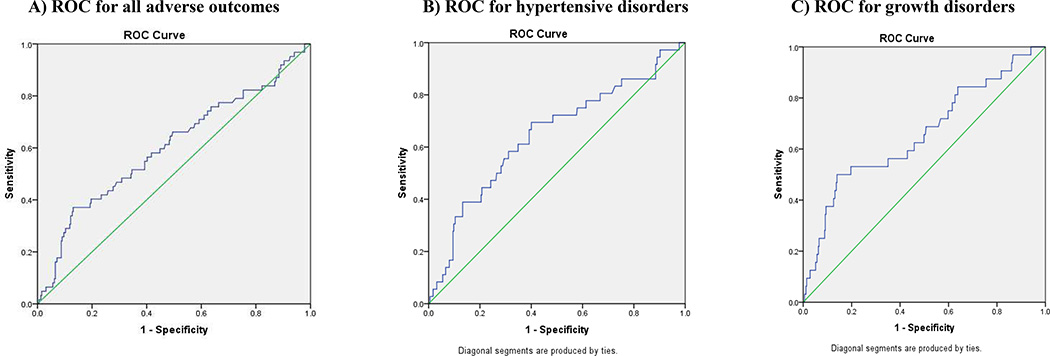
Receiver operating characteristic curve analysis evaluated the ability of peak estradiol measurements to predict adverse placental outcomes in all patients (Figure 2). Area under the curve (AUC) for hypertension in pregnancy was 0.65 (95%CI 0.54–0.74, P<0.05). For growth disorders, the AUC was 0.66 (95%CI 0.55–0.77, P < 0.05). For all adverse placental disorders, the AUC was 0.60 (95%CI 0.52–0.69).
Figure 2.
ROC characteristic curves evaluating the ability of peak estradiol measurements to predict adverse placental outcomes in A) all adverse outcomes B) hypertensive disorders C) growth disorders.
Discussion
This study aimed at determining the effects of specific ART parameters prior to embryo transfer on obstetrical complications experienced later during pregnancy in patients undergoing ART. These data demonstrated a strong association of estradiol and ICSI with adverse outcomes. These findings support previous studies performed by Imudia et al. and Farhi et al. showing a relationship between elevated estradiol levels and subsequent pregnancy events (12, 21). Also similarly, no association was identified between implantation rate, SAB, PTD, or placenta accrete, increta or percreta. However, our study is the first to differentiate the relationship between estradiol and placentation disorder by conventional IVF vs ICSI. We did not observe a relationship between estradiol and adverse outcomes in IVF cycles.
Intrauterine growth restriction is a result of several factors, all leading to one major cause: compromised nutrient and oxygen supply from the placenta to the fetus. Compromised implantation and subsequent placental growth and maintenance, is associated with altered circulating levels of estradiol at the time of implantation by the trophectoderm. This study is consistent with data from Kalra et al. showing lower birth weights following fresh embryo transfer cycles (which are associated with exceedingly higher estradiol levels) compared to spontaneous conceptions or frozen embryo transfers (10). Increased rates of gestational hypertension and preeclampsia following ART cycles were also found to be associated with elevated estradiol levels in retrospective studies as well (12, 21). Our study found a 2-fold higher odds of developing severe preeclampsia in patients with elevated estradiol levels compared to those with estradiol levels <90th percentile. Our data and others’ clearly demonstrate a higher risk of developing disorders related to abnormal placentation in patients with elevated estradiol levels undergoing controlled ovarian hyperstimulation for ART (21).
When looking at the composite placental obstetrical outcomes, for every 1000 pg/ml increase in serum estradiol levels, there was a 36% increase in the likelihood for any adverse placental outcome (SGA, gestational hypertension, and or severe preeclampsia). By analyzing efficiency curves, a threshold for increasing adverse pregnancy outcomes began when serum estradiol was >3000 pg/ml. Additionally, this rise in adverse outcomes at the 3000 pg/ml cutoff occurred only in ICSI and not conventional IVF. An interaction test was also performed to compare estradiol with ICSI or conventional IVF on the effect of placental abnormalities. This result indicated that elevated estradiol levels may interact with the embryo and/or trophectoderm differently during ICSI than conventional IVF. It is uncertain why such a difference existed in these patients. This differential interaction may arise from the mechanical disruption of the zona pellucida during sperm insertion, alterations in paternal genes expressed in the placenta in sperm injected immobilized sperm compared to sperm which spontaneously fertilize the egg or altered embryonic expression of L–Selectin or cytokines at the time of embryo implantation. These changes may cause abnormal placentation with oxidative stress, causing failure or delay of cytotrophoblastic differentiation leading to shallow interstitial extravillous cytrotrophoblastic invasion and reduced endovascular invasion, culminating with intrauterine growth restriction for the fetus or preeclampsia for the mother (24, 25).
There are many mediators of endometrial implantation regulated by estradiol and progesterone. Estradiol has been shown to be a crucial modifier in villous trophoblastic development and uteroplacental blood flow (33). The low levels of estradiol present in early gestation as compared with the later part of pregnancy are required to permit normal progression of uterine spiral artery invasion by cytotrophoblasts (20, 21) This study exhibited less adverse outcomes with lower levels of estradiol on the final day of ART stimulation, which may be attributed to normal remodeling of spiral artery and trophoblast invasion. However, the corpus luteum is known to express many hormonal substances (e.g. androgens, inhibins, activins, progesterones) in addition to estrogens, and they remain present throughout pregnancy. It is possible that elevated estradiol is a marker of global corpus luteum hormonal secretion, and that another hormone that is not routinely measured during ART contributes to placentation.
Rajesh et al. compared obstetric and perinatal outcomes in IVF versus ICSI conceived pregnancies and noted a significant decrease in mean fetal birth weight with the ICSI group (26). Similarly, while comparing conventional IVF versus ICSI, Nouri et al. found significant differences in the live birth rates. This finding was attributed to the manipulation of the oocytes during ICSI having a long-term deleterious effect (27). A meta-analysis demonstrated ICSI was associated with a higher risk of antepartum hemorrhage, congenital anomalies, hypertensive disorders of pregnancy, premature rupture of membranes, cesarean delivery, low birth weight infants, preterm delivery, and overall perinatal mortality (28). Others have postulated that adverse obstetrical outcomes associated with ART may be influenced by the cause of infertility (i.e. combining relatively healthy oocytes with sperm from patients with male factor infertility vs. combining relatively healthy sperm with oocytes from patients with female infertility), as noted in the meta-analysis by Pinborg et al. (29). ICSI may result in oocyte alterations during injection of the immobilized spermatozoa through breakage of the oolemma, or during aspiration of the oocyte cytoplasm. It is still unknown whether these mechanical alterations of the oocyte during ICSI causes an increase in adverse obstetrical complications later in pregnancy.
A few retrospective studies have suggested that elevated estradiol levels and ICSI are associated with adverse placental outcomes including SGA and hypertensive disorders. For most patients, extremely high levels of estradiol do not prevent a successful pregnancy or live-birth, but providers should be aware of the possible adverse pregnancy outcomes associated with supraphysiologic estradiol levels at the time of final oocyte maturation (21). While high responder ART patients often have their day 5 embryos cryopreserved to reduce the risk of OHSS, it may also reduce the risks of obstetric disorders associated with abnormal placentation. While these data suggest risk factors associated with fresh embryo transfer, we did not evaluate if frozen-thawed embryo transfer ameliorate that effect. In the absence of randomized controlled data demonstrating such a benefit, it is difficult to recommend frozen-thawed embryo transfer purely for improvement in obstetric outcomes.
Strengths of this study include the military setting, where the ART cycle, obstetric care and delivery were managed in the same health care system, allowing complete ART and obstetrics records to be evaluated. GEE models, efficiency curves, and ROC curves allowed for thorough analyses across the spectrum of estradiol levels and the varying tests yielded consistent results. Weaknesses of the study include the retrospective nature of the design. We cannot exclude the possibility of unidentified confounding variables. We did not have data available on NICU admissions or neonatal mortality, so effects cannot be estimated on these outcomes. There was a smaller sample size of patients receiving IVF and it is possible that the lack of association of estradiol with abnormal placentation in the IVF cohort was a type II error. In a post hoc power analysis, with 100 patients in the IVF group, if the true rate of abnormal placentation was 6% (as detected in the IVF group), we were powered to detect an increase in abnormal placentation from 6% at baseline to 26% with estradiol > 3000 pg/ml. The results did not indicate any trend in this direction, with a 6% rate both above and below this threshold. Additionally, if the effect in the IVF group had been similar to the ICSI group, our sample size was adequate to detect it. Finally, the working hypothesis for the study was that estradiol and ICSI were associated with adverse placental complications. However, the suggestion that estradiol was only associated with adverse events in ICSI and not IVF cycles was not an a priori hypothesis. Based on these limitations, these findings should be interpreted as investigational and not definitive. They highlight the need for greater understanding into the underlying mechanisms of implantation and trophoblast invasion and how ART may alter those events. An ideal study design would be a paired analysis of sibling single embryo transfer pregnancies resulting from a single fresh retrieval and giving rise to both a fresh and a frozen transfer pregnancy. This would control for potential confounders in the method of insemination, culture conditions, and maternal factors and could help isolate the effect of estradiol.
In conclusion, this study demonstrated a relationship between the ART parameters estradiol and ICSI with adverse obstetric outcomes. Further studies are needed to confirm and clarify the interaction of high estradiol and ICSI and abnormal placentation events.
Supplementary Material
Acknowledgments
The only disclosure for any author is that Dr. Wolff received an unrelated grant from OvaScience.
This research was supported, in part, by Intramural research program of the Program in Reproductive and Adult Endocrinology, NICHD, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of Defense, Department of Health and Human Services or the United States Government.
References
- 1.Assisted Reproductive Technology 2013 Report. [Accessed 18 November 2015]; External link http://www.cdc.gov/ART.
- 2.Retzloff MG, Hornstein MD. Is intracytoplasmic sperm injection safe? Fertil Steril. 2003;80:851–859. doi: 10.1016/s0015-0282(03)01014-8. [DOI] [PubMed] [Google Scholar]
- 3.Shieve LA, Rasmussen SA, Buck GM, Schendel DE, Reynolds MA, Wright VC. Are children born after assisted reproductive technology at increased risk for adverse health outcomes? Obstet Gynecol. 2004;103:1154–1163. doi: 10.1097/01.AOG.0000124571.04890.67. [DOI] [PubMed] [Google Scholar]
- 4.Allen VM, Wilson RD, Cheung A. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can. 2006;28:220–250. doi: 10.1016/S1701-2163(16)32112-0. [DOI] [PubMed] [Google Scholar]
- 5.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Perri T, Chen R, Yoeli R, Merlob P, Orvieto R, Shalev Y, et al. Are singleton assisted reproductive technology pregnancies at risk of prematurity? J Assist Reprod Genet. 2001;18:245–249. doi: 10.1023/A:1016614217411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 9.Tan SL, Doyle P, Campbell S, Beral V, Rizk B, Brinsden P, et al. Obstetric outcome of in vitro fertilization pregnancies compared with normally conceived pregnancies. Am J Obstet Gynecol. 1992;167:778–784. doi: 10.1016/s0002-9378(11)91589-0. [DOI] [PubMed] [Google Scholar]
- 10.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118:863–871. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozinszky Z, Zadori J, Orvos H, Katona M, Pal A, Kovacs L. Risk of cesarean section in singleton pregnancies after assisted reproductive techniques. J Reprod Med. 2003;48:160–164. [PubMed] [Google Scholar]
- 12.Farhi J, Haroush AB, Andrawus N, Pinkas H, Sapir O, Fisch B, Ashkenazi J. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online. 2010;21:331–337. doi: 10.1016/j.rbmo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Zadori J, Kozinszky Z, Orvos H, Katona M, Kaali SG, Pal A. The incidence of major birth defects following in vitro fertilization. J Assist Reprod Genet. 2003;20:131–132. doi: 10.1023/A:1022682908307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2007;109:967–977. doi: 10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- 15.Bower C, Hansen M. Assisted reproductive technologies and birth outcomes: overview of recent systematic reviews. Reprod Fertil Dev. 2005;17:329–333. doi: 10.1071/rd04095. [DOI] [PubMed] [Google Scholar]
- 16.Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13:591–599. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placenta complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 18.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercrysse L, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoozemans DA, Schats R, Lambalk CB, et al. Human embryo implantation: current knowledge and clinical implications in assisted reproductive technology. Reproductive BioMedicine Online. 2004;9:692–715. doi: 10.1016/s1472-6483(10)61781-6. [DOI] [PubMed] [Google Scholar]
- 20.Bonagura TW, Pepe GJ, Enders AC, Albrecht E. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology. 2008;149:5078–5087. doi: 10.1210/en.2008-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374–1379. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 22.Hill MJ, Royster GD, IV, Healy MW, Richter KS, Levy G, DeCherney AH, Levens ED, Suthar G, Widra E, Levy MJ. Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril. 2015;103:1477–1484. doi: 10.1016/j.fertnstert.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neithardt AB, Segars JH, Hennessy S, James AN, McKeeby JL. Embryo afterloading: a refinement in embryo transfer technique that may increase clinical pregnancy. Fertil Steril. 2005;83:710–714. doi: 10.1016/j.fertnstert.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12(6):731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 25.Cha J, Sun X, Dey S. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajesh H, Yap HAC, Wu YJ. Pregnancy outcomes form in-vitro fertilisation and intracytoplasmic sperm injection: a comparison. Singapore Med J. 2006;47:309–314. [PubMed] [Google Scholar]
- 27.Nouri K, Ott J, Stoegbauer L, Pietrowski D, Frantal S, Walch K. Obstetric and perinatal outcomes in IVF versus ICSI-conceived pregnancies at a tertiary care center - a pilot study. Reprod Bio Endo. 2013;11:84. doi: 10.1186/1477-7827-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Human Reprod Update. 2012;18:485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 29.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, Nygren KG, Hazekamp J, Bergh C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Human Reprod Update. 2012;19:87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 30.Pinborg A, Loft A, Nyboe Andersen A. Neonatal outcome in a Danish national cohort of 8602 children born after in vitro fertilization or intracytoplasmic sperm injection: the role of twin pregnancy. Acta Obstet Gynecol Scand. 2004;83:1071–1078. doi: 10.1111/j.0001-6349.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 31.Fauque P, Jouannet P, Davy C, Guibert J, Viallon V, Epelboin S, et al. Cumulative results including obstetrical and neonatal outcome of fresh and frozen-thawed cycles in elective single versus double fresh embryo transfers. Fertil Steril. 2010;94:927–935. doi: 10.1016/j.fertnstert.2009.03.105. [DOI] [PubMed] [Google Scholar]
- 32.Veleva Z, Karinen P, Tomas C, Tapanainen JS, Martikainen H. Elective single embryo transfer with cryopreservation improves the outcome and diminishes the costs of IVF/ICSI. Hum Reprod. 2009;24:1632–1639. doi: 10.1093/humrep/dep042. [DOI] [PubMed] [Google Scholar]
- 33.Imudia AN, Goldman RH, Awonuga AO, Wright DL, Styer AK, Toth TL. The impact of supraphysiologic serum estradiol levels on peri-implantation embryo development and early pregnancy outcome following in vitro fertilization cycles. J Assist Reprod Genet. 2014;31:65–71. doi: 10.1007/s10815-013-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.