Abstract
Gamma-linolenic acid (GLA, 18:3n-6) is an omega-6 (n-6), 18 carbon (18C-) polyunsaturated fatty acid (PUFA) found in human milk and several botanical seed oils and is typically consumed as part of a dietary supplement. While there have been numerous in vitro and in vivo animal models which illustrate that GLA-supplemented diets attenuate inflammatory responses, clinical studies utilizing GLA or GLA in combination with omega-3 (n-3) PUFAs have been much less conclusive. A central premise of this review is that there are critical metabolic and genetic factors that affect the conversion of GLA to dihommo-gamma linolenic acid (DGLA, 20:3n-6) and arachidonic acid (AA, 20:4n-6), which consequently affects the balance of DGLA- and AA- derived metabolites. As a result, these factors impact the clinical effectiveness of GLA or GLA/n-3 PUFA supplementations in treating inflammatory conditions. Specifically, these factors include: 1) the capacity for different human cells and tissues to convert GLA to DGLA and AA and to metabolize DGLA and AA to bioactive metabolites; 2) the opposing effects of DGLA and AA metabolites on inflammatory processes and diseases; and 3) the impact of genetic variations within the fatty acid desaturase (FADS) gene cluster, in particular, on AA/DGLA ratios and bioactive metabolites. We postulate that these factors influence the heterogeneity of results observed in GLA supplement-based clinical trials and suggest that “one-size fits all” approaches utilizing PUFA-based supplements may no longer be appropriate for the prevention and treatment of complex human diseases.
Keywords: gamma-linolenic acid, dihommo gamma-linolenic acid, arachidonic acid, eicosanoid, inflammation
1. Introduction
Gamma-linolenic acid (GLA, 18:3n-6) is an omega-6 (n-6), 18 carbon (18C) polyunsaturated fatty acid (PUFA) found in human milk and several botanical seed oils (borage [~21% GLA], blackcurrant [~17%GLA] and evening primrose [~9%GLA]), and is typically consumed as a part of a dietary supplement. The scientific literature examining the clinical effects of GLA-containing supplements is both complex and confusing. While there have been numerous in vitro and in vivo animal models illustrating that GLA-supplemented diets attenuate various inflammatory responses, the clinical literature has been less conclusive (for a review, see (Fan and Chapkin, 1998)). The introduction of GLA supplementation strategies to achieve symptomatic relief of atopic dermatitis/eczema was historically preceded by the use of relatively large daily doses of oral linoleic acid (LA, 18:2n-6) containing oils. This was based on the premise that patients with atopic dermatitis/eczema had hallmark cutaneous signs of essential fatty acid deficiency and an impairment in PUFA biosynthesis at an early desaturase step (FADS2; Δ-6 desaturase) (Burr and Burr, 1929; Burr et al., 1932; Horrobin, 2000). It was hypothesized that GLA supplements could restore needed PUFAs and mitigate the disease.
Numerous studies primarily carried out in the 1980s and 1990s demonstrated that GLA-enriched botanical oils (evening primrose, borage, blackcurrant seed, and fungal-derived) had the capacity to relieve the signs and symptoms of several chronic inflammatory diseases, including rheumatoid arthritis (RA) and atopic dermatitis (Andreassi et al., 1997; Foolad et al., 2013; Kunkel et al., 1981; Leventhal et al., 1994; Leventhal et al., 1993; Lovell et al., 1981; Morse et al., 1989; Tate et al., 1989; Zurier et al., 1996). However, several more recent reviews and meta-analyses have questioned these earlier studies and raised doubts about the clinical effectiveness of GLA-enriched supplements particularly in the context of atopic dermatitis and RA (Bamford et al., 2013; Belch and Hill, 2000; Kitz et al., 2006; Macfarlane et al., 2011; Van Gool et al., 2004) (Table 1). A variety of issues complicate these studies including the fact that many of the trials have: 1) relatively low subject numbers; 2) less than ideal study designs (e.g. the absence of washout period in cross-over design trials); 3) variations in the types of GLA supplements and how they are administered (e.g. dose, duration); and 4) differences in selection/inclusion criteria (e.g. population demographics and disease states)(Foster et al., 2010; Van Gool et al., 2004).
Table 1.
Effect of GLA-enriched oil supplements on various human disease from meta-analyses and recent studies
| Study | Disease1 and Study Type2 |
Supplement3 | location | # subjects | # studies | duration | outcome | effect |
|---|---|---|---|---|---|---|---|---|
| Skin | ||||||||
| (Morse et al., 1989) | Atoptic dermatitis (CO, parallel) |
EPO (Epogam) |
UK, Italy, Finland |
311 | 9 (EPO) | 4, 8, or 12 wk |
Severity of symptoms |
reduced severity of symptoms |
| (Van Gool et al., 2004) | Atoptic dermatitis (RCT, CO, CCT) |
EPO, BO, BCO; 90–480mg GLA/d (children); 132–720mg GLA/d (adult) |
Germany, Italy, UK, Canada, USA, Finland, Sweden, Switzerland, |
1071 | 22 (total) BO (6) EPO (12) BCO ( 1) |
3–24wk | Severity of symptoms |
no effect |
| (Bamford et al., 2013) | Eczema (AE, AD, AEDS) adult, children (RCTs) |
EPO, BO | UK, Italy, Germany, India, NZ, Finland, Sweden, USA, Switzerland |
1596 | 27 (total) 19 (EPO) 8 (BO) |
3–24wk | Severity of symptoms |
no effect |
| (Morse and Clough, 2006) | Atopic eczema | EPO (Efamol®) |
1207 | 26 | 4–8wks | Severity of symptoms |
reduced severity of symptoms |
|
| (Fiocchi et al., 1994) | Atoptic dermatitis, infants |
EPO, 3g oil/d |
Italy | 10 | na | 4wk | Lesion number; Severity of Symptoms |
decrease number (trend); reduced severity of symptoms |
| (van Gool et al., 2003) | Atoptic dermatitis, infants (RCT) |
BO, 100mg/d | Netherlands | 118 | na | 6mo | Incidence in 1st yr; Severity of symptoms |
no prevention benefit; reduced severity of symptoms (trend) |
| (Kitz et al., 2006) | Atoptic dermatitis, infants | GLA, 40mg/d |
Germany | 131 | na | 6 mo | Prevention | no effect |
| (Kawamura et al., 2011) | Atoptic dermatitis, adult |
GLA, 200mg/d, oil of Mucor circinelloides in food |
Japan | 130 | na | 16wk | Trans-water loss; Nocturnal itching |
no effect; decreased |
| (Simon et al., 2014) | Atoptic dermatitis, children and adult (open study, non-controlled) |
EPA, 4–6g GLA/d |
Switzerland | 21 | na | 12wk | SCORAD4 index | plasma GLA content correlates with SCORAD |
| Arthritis | ||||||||
| (Cameron et al., 2011) (Macfarlane et al., 2011) |
Rheumatoid arthritis (RCT, parallel, placebo controlled) |
Herbal intervention 525–540mg GLA/d |
UK, USA | 286 (total) >90 (in 3 studies) |
22 (total) EPO (2) BCO (1) |
6mo | Morning stiffness; Pain |
decreased (2 of 3); no effect |
| (Cameron et al., 2011) (Macfarlane et al., 2011) |
Rheumatoid arthritis | 1400- 2800mg GLA/d |
USA, Finland | >111 | EPO (1) BO (2) BCO (1) |
6mo | Pain; Morning stiffness; Joint tenderness; Joint swelling; |
decreased; decreased; improvement; decreased; |
| Asthma | ||||||||
| (Arm et al., 2013) | Mild asthma, adults (randomized) |
BO+EO (GLA, 1.67g/d+ SDA, 0.88g/d) |
USA | 37 | na | 3wk | Basophil, Neutrophil leukotriene production (ex vivo) |
>50% decrease (basophil response); >35% decrease (neutrophil response) |
| (Ziboh et al., 2004) | Mild asthma, adults (randomized) |
BO (2g GLA/d) |
USA | 24 | na | 12mo | Neutrophil leukotriene production (ex vivo); Peak flow |
>20% decrease (p<0.05); no effect |
AD, atopic dermatitis; AE, atopic eczema; AEDS, atopic eczema/dermatitis syndrome;
RCT, randomized clinical trial; CO crossover; CCT, controlled clinical trial
BO, borage oil: BCO. Blackcurrant oil; EPO, evening primrose oil; EO, echium oil; GLA, gamma-linolenic acid; SDA, stearidonic acid
SCORAD, SCOing Atopic Dermatitis
Several studies have also investigated the effects of GLA when given in combination with botanical or marine omega-3 (n-3) enriched PUFA supplements. Enteral diets enriched with marine oils containing n-3 LC-PUFAs (i.e. eicosapentaenoic acid [EPA, 20:5n-3] and docosahexaenoic acid [DHA, 22:6n-3]) and GLA have been shown to reduce cytokine production and neutrophil recruitment into the lung resulting in fewer days on ventilation and shorter stays in the intensive care unit in patients with acute lung injury or acute respiratory distress syndrome (Gadek et al., 1999; Pontes-Arruda et al., 2006; Singer et al., 2006). Importantly, these dietary combinations of GLA and n-3 LC-PUFAs were also shown to reduce both morbidity and mortality of critically ill patients (Gadek et al., 1999; Li et al., 2015; Pontes-Arruda et al., 2006; Singer et al., 2006). However, as with the studies of GLA alone, the results combining GLA and n-3 LC-PUFAs have not been reproducible. Other clinical studies, such as the OMEGA trial, did not show a benefit of these GLA/n-3 LC-PUFA combinations on patient outcomes (Rice et al., 2011).
Supplementation strategies providing GLA together with n-3 LC-PUFAs (i.e. EPA and DHA) have also been utilized in patients with atopic asthma (Surette et al., 2003a; Surette et al., 2003b; Surette et al., 2008) and have been shown to block ex vivo synthesis of leukotrienes from whole blood and isolated neutrophils. Importantly when provided as an emulsion, daily consumption of these combinations was associated with an improved quality of life in asthma patients and a decreased reliance on rescue medication (Surette et al., 2008). These results compared favorably with quality of life scores obtained in mild asthmatics treated with montelukast or zafirlukast (Riccioni et al., 2004). However, to our knowledge, no randomized, placebo-controlled trials have been conducted to investigate the effect of these combinations on the improved quality of life or other relevant clinical outcomes in asthma patients.
Alternatively, botanical oil combinations (e.g. borage and echium oils) containing GLA, the n-3 18C-PUFAs, alpha-linolenic acid (ALA, 18:3n-3) and stearidonic acid (SDA, 18:4n-3), have been shown to reduce leukotriene generation and forced expiratory volume in mild asthmatics (Arm et al., 2013; Kazani et al., 2014), improve glucose tolerance in insulin-resistant monkeys (Kavanagh et al., 2013) and reduce total and LDL cholesterol levels in patients with diabetes and metabolic syndrome (Lee et al., 2014). These botanical oil studies, however, have yet to be replicated in larger human clinical trials.
Together, these data indicate that the outcomes of clinical studies utilizing GLA supplementation, alone or in combination with other fatty acid-based supplements, while promising are highly inconsistent. These observations raise serious questions about our current understanding of the highly complex and dynamic nature of PUFA metabolism. More recent studies suggest that there are important metabolic and genetic factors within the human host that significantly impact the study of GLA or GLA/n-3 PUFAs combinations and reveal that a “one size fits all” model of supplementation may not be appropriate. Further, these studies suggest that it may be necessary to better understand key metabolic and genetic issues regarding GLA metabolism before GLA-enriched supplements can be effectively used to address human disease. This review will focus on potential key metabolic and genetic factors that may impact the use and clinical effectiveness of GLA or GLA/n-3 PUFA combinations.
2.0 Polyunsaturated Fatty Acid Metabolism
2.1 Long Chain Polyunsaturated Fatty Acid Biosynthesis
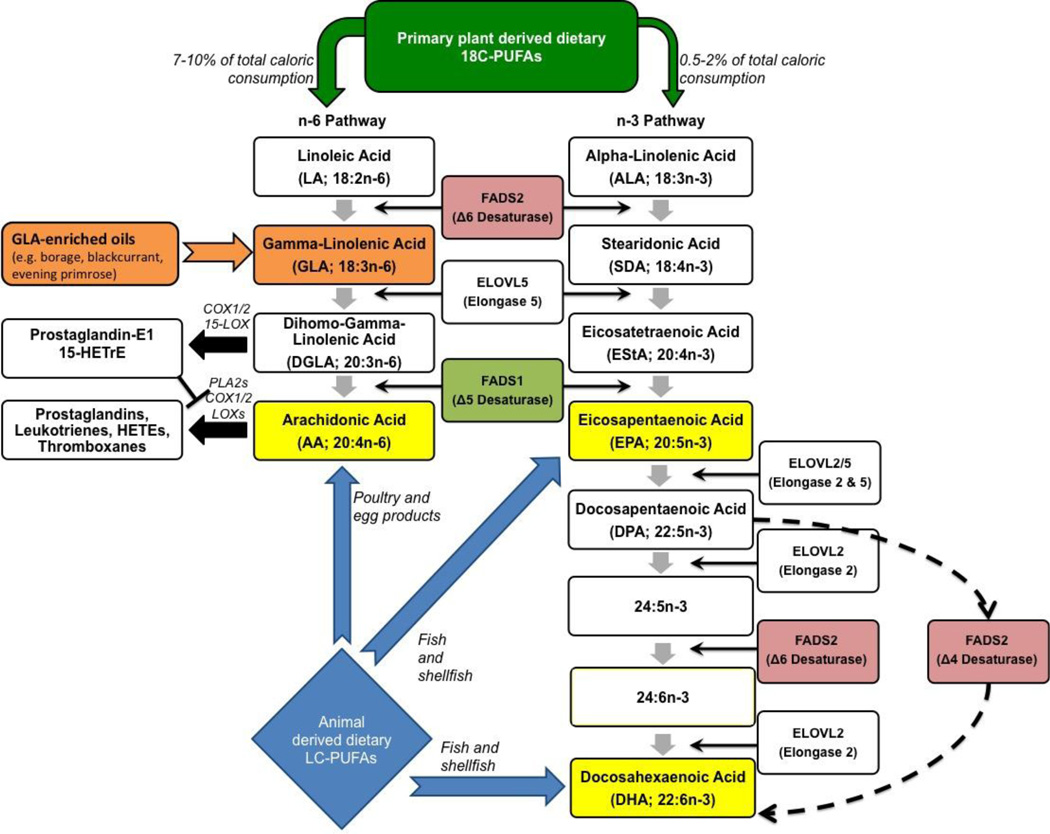
In mammals, n-6 and n-3, long chain (>20 carbons, LC) PUFAs such as arachidonic acid (AA, 20:4n-6), dihommo-gamma linolenic acid (DGLA, 20:3n-6), EPA (20:5n-3) and DHA (22:6n-3) can be synthesized from their respective precursors, n-6 and n-3 18C-PUFAs such as LA, GLA, ALA, and SDA. The PUFA pathways and attendant enzymes are illustrated in Fig. 1. Biologically important n-6 LC-PUFAs, DGLA and AA can be synthesized from LA using either two (one desaturation and one elongation step) or three (two desaturation and one elongation step) enzymatic steps, respectively (Sprecher, 1981). The desaturation reactions have long been recognized as the rate-limiting steps in this pathway (Bernert and Sprecher, 1975) and the enzymes that catalyze these reactions are encoded by the fatty acid desaturase 1 and 2 (i.e. FADS1 and FADS2) genes located on chromosome 11. This region is commonly referred to as the FADS cluster (11Q12.2-q13.1) (Glaser et al., 2011). These same enzymes are responsible for the rate-limiting steps in the conversion n-3 18C-PUFAs (ALA and SDA) to n-3 long chain-PUFAs including EPA. Recent studies suggest that the efficiency of several steps in the pathway, in particular the desaturase steps, is highly impacted by genetic variations within the FADS cluster (Chilton et al., 2014; Eaton, 1992). The potential impact of these genetic variations on GLA, DGLA and AA levels and their respective ratios will be discussed in greater detail in section 4. In addition, small quantities of LC-PUFAs can be obtained directly from the diet (Fig. 1). Dietary AA is obtained primarily from animal products such as, organ meats, eggs, poultry, and fish, whereas dietary EPA and DHA are found primarily in seafood, particularly cold-water fish (Hibbeln et al., 2006).
Fig. 1.
Illustration of PUFA pathway
GLA enters the n-6 pathway distal to the FADS2 enzymatic step and is efficiently converted to DGLA by an enzymatic activity, encoded for by a gene known as ELOVL5, in a wide range of cells (including several inflammatory cells) and tissues. Because of its rapid conversion, GLA is found in low levels in circulating lipids, cells or tissues. In contrast to GLA, the ELOVL5 product, DGLA is readily measured in circulating lipids and most cells, and levels of DGLA are consistently elevated after GLA supplementation (Chilton-Lopez et al., 1996; Johnson et al., 1997; Lee et al., 2014; Simon et al., 2014) (Table 2). Once formed, DGLA can be incorporated into cellular glycerolipids (primarily phospholipids). Upon cell activation, DGLA can be released as a free fatty acid by phospholipase A2(s) and enzymatically converted to several metabolites with predominantly anti-inflammatory properties. These pathways are described in further detail in section 3.3 below.
Table 2.
Effect of GLA supplementation on the DGLA content of immune cells
| Biochemical Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | design | supplement | dose | duration | cell type tested |
[DGLA]- pre |
[DGLA]- post |
p value |
unit |
| (Fletcher and Ziboh, 1990) |
In vivo fatty acid supplementation |
borage oil | 3% oil (by weight) in diet |
12wk | guinea pig neutrophil |
0.27 | 2.41 | p1 | mg /100mg total phospholipid |
| (Ziboh and Fletcher, 1992) |
In vivo fatty acid supplementation |
borage oil | 0.48g GLA/d | 6wk | human neutrophil |
1.35 | 1.96 | <0.05 | mg /100mg total phospholipid |
| (Ziboh and Fletcher, 1992) |
In vivo fatty acid supplementation |
blackcurrant oil | 0.48g GLA/d | 6wk | human neutrophil |
1.35 | 1.95 | <0.05 | mg /100mg total phospholipid |
| (Chilton-Lopez et al., 1996) |
In vitro fatty acid incorporation |
albumin-conjugated GLA |
200nmol | 24 hr | human neutrophil |
0.4 | 1.0 | p1 | nmol |
| (Chilton- Lopez et al., 1996) |
In vivo fatty acid supplementation |
borage oil | 3g GLA/d | 21d | human neutrophil |
0.45 | 0.80 | <0.05 | nmol/million cells |
| (Johnson et al., 1997) |
In vivo fatty acid supplementation |
borage oil + controlled diet |
1.5g GLA/d 3g GLA/d 6g GLA/d |
21d 21d 21d |
human neutrophil human neutrophil human neutrophil |
0.20 0.15 0.13 |
0.20 0.27 0.35 |
ns2 <0.05 <0.05 |
nmol/5 million cells nmol/5 million cells nmol/5 million cells |
| (Chapkin and Coble, 1991) |
In vitro fatty acid incorporation |
albumin-conjugated radiolabeled GLA |
tracer amounts |
3hr | murine peritoneal macrophage |
0 | 84 | p1 | % conversion to radiolabel product |
| (Ziboh et al., 2004) |
In vivo fatty acid supplementation |
borage oil | 2g GLA/d | 12mo | human neutrophil |
0.7 | 1.4 | <0.05 | mg /100mg total phospholipid |
p value not specified
ns, not significant
2.2 Metabolism of PUFAs to Lipid Meditators that Impact the Immune Response
DGLA and its metabolites have long been recognized to have potent inhibitory effects on platelet aggregation and inflammation. The impact of DGLA on platelet aggregation was first recognized in the early 1970s (Willis et al., 1974a), and Lagarde and colleagues showed that ten times more collagen and twice as much thrombin were necessary to obtain aggregation when platelets and endothelial cells were pretreated with DGLA as compared to untreated platelets and endothelial cells (Lagarde et al., 1981). Interestingly, DGLA was much more potent than EPA in inhibiting platelet aggregation.
The anti-inflammatory effects of DGLA have been attributed to both i) the anti-inflammatory properties of DGLA-derived metabolites and ii) the ability of DGLA to compete with AA in the synthesis of pro-inflammatory AA products (Billah et al., 1985; Chilton-Lopez et al., 1996; Iversen et al., 1991; Iversen et al., 1992; Vanderhoek et al., 1980) (Table 3). The synthesis of DGLA oxygenated metabolites and their impact is discussed in detail below in section 3.3.
Table 3.
Effect of GLA supplementation on functional endpoints of immune cells
| Reference | design | supplement | dose | duration | cells | Functional Outcomes |
|---|---|---|---|---|---|---|
| (Fletcher and Ziboh, 1990) |
In vivo fatty acid supplementation |
borage oil | 3% oil (by weight) in diet |
12wk | guinea pig neutrophil |
~25% inhibition of fMLP-stimulated superoxide production (vs control diet; p<0.005) No effect on PMA-stimulated superoxide production (vs control diet) |
| (Iversen et al., 1991) |
In vitro fatty acid Competition |
LA and DGLA conc. Curve |
0–100µM | 10m | human neutrophil |
50µM DGLA, 75% ↓ in LTB4 generation 50µM LA, 60% ↓ in LTB4 generation |
| (Iversen et al., 1992) |
In vitro fatty acid treatment |
DGLA conc. curve | 0–100µM | 10m | human PBMC1 |
50µM DGLA, 60% ↓ in LTB4 generation 50µM DGLA, >7-fold ↑ in PGE1 generation 50µm DGLA, significant ↑ in 15-HETrE generation (>250ng/10mil cells) |
| (Ziboh and Fletcher, 1992) |
In vivo fatty acid supplementation |
borage oil | 0.48g GLA/d | 6wk | human neutrophil |
50% ↓ LTB4 generation (vs olive oil control), ex vivo |
| (Ziboh and Fletcher, 1992) |
In vivo fatty acid supplementation |
black currant oil | 0.48g GLA/d | 6wk | human neutrophil |
50% ↓ LTB4 generation (vs olive oil control), ex vivo |
| (Chapkin et al., 1988a) | In vitro treatment | 15-HETrE | 0–30µM | 1hr | murine peritoneal macrophage |
10µM 15-HETrE, 90% ↓ in LTB4 generation |
| (Chilton- Lopez et al., 1996) | In vitro treatment | 15-HETrE | 0–20µM | human neutrophil |
10µM 15-HETrE, 75% ↓ in LTB4 generation | |
| (Johnson et al., 1997) |
In vivo fatty acid Supplementation |
borage oil + controlled diet |
3g GLA/day | 21d | human neutrophil |
58% ↓ LTB4 generation (vs baseline), ex vivo |
| (Barham et al., 2000) |
In vivo fatty acid supplementation |
borage + fish oils + controlled diet |
3g GLA + 3g EPA/day |
21d | human neutrophil |
30% ↓ LTB4 generation (vs baseline), ex vivo |
| (Amagai et al., 2015) |
In vitro fatty acid treatment |
DGLA conc. curve | 0–30µM | 48hr | RBL-2H3 cells |
30µM, significant (>30ng/ml) PGD1 formation |
| (Amagai et al., 2015) | In vivo fatty acid supplementation | DGLA | 11% of dietary fatty |
5wk | NC/Tnd mouse skin |
significant ↑ in PDG1, PDE1, PGD2, 8-HETrE, 15- HETrE (vs control diet) |
PBMC, peripheral blood mononuclear cells
Somewhat paradoxically from an inflammation perspective, AA can also be synthesized from DGLA utilizing an enzymatic activity originally known as the Δ-5 desaturase. As shown in Fig. 1, this activity is encoded for by FADS1 within the FADS cluster. AA and its metabolic products have long been known to play important roles in immunity and inflammation (Boyce, 2008; Calder, 2013; Schmitz and Ecker, 2008; Simopoulos, 2008) via their ability to impact normal and pathophysiologic responses through the conversion of AA to potent eicosanoid products (including prostaglandins [PG], thromboxanes [TX], leukotrienes [LT] and lipoxins). Additionally, AA and its oxidized products can regulate transcription and consequently a wide range of cellular activities via cellular and nuclear receptors (such NF-κB, PPAR and SREBP-1c (Berger et al., 2006; Caputo et al., 2011; Chinetti et al., 2000; Deckelbaum et al., 2006; Jump and Clarke, 1999; Jung et al., 2012; Schmitz and Ecker, 2008; Soberman and Christmas, 2003; Vanden Heuvel, 2012), thereby modulating the expression of numerous genes that impact immune responses. Therefore, dietary supplementation with GLA has the capacity to both increase levels of both DGLA, which can lead to several anti-inflammatory metabolites, and AA, whose metabolic products generally promote inflammation.
3. Factors that Determine the Balance of Pro- and Anti-inflammatory PUFAs and PUFA Metabolites after GLA Supplementation
3.1 Differential Metabolism of GLA to DGLA and AA in Human Cells and Tissues
Since metabolites of DGLA have predominantly anti-inflammatory effects and AA products generally enhance inflammation, it stands to reason that the balance of AA to DGLA (i.e. the ratio of AA/DGLA) in circulation, cells and tissues is a critical factor that impacts inflammatory processes. Several factors determine the levels of AA and DGLA and thus the ratio of AA metabolites and DGLA metabolites within cells and tissues. One is the differential capacities of cells or tissues to elongate GLA to DGLA and then to further desaturate it to AA. Differential expression of enzymatic activities is observed when comparing GLA metabolism within an inflammatory cell, such as the human neutrophil, and within a tissue bed or organ, such as the human liver. Both in vitro and in vivo studies demonstrate that human neutrophils contain the elongase (ELOVL5) but not the Δ-5 desaturase (i.e. FADS1) activity. In addition to human neutrophils, skin, murine peritoneal macrophages and platelets also appear to have high ELOVL5 elongase activity relative to FADS1 (Δ-5) desaturase activity (Chapkin and Coble, 1991; Chapkin et al., 1988b; Chapkin and Ziboh, 1984; de Bravo et al., 1985; Navarette et al., 1992; Ziboh et al., 2000). In contrast, several other tissues including liver, kidney, testes, brain and intestine appear to contain both activities (Bernert and Sprecher, 1975; Blond and Bézard, 1991; Hurtado de Catalfo et al., 1992; Irazu et al., 1993; Luthria and Sprecher, 1994; Pawlosky et al., 1994).
The PUFA pathway enzymatic portfolio of human neutrophils results in the accumulation of cellular DGLA upon, dietary GLA supplementation. DGLA formed within the human neutrophil generally resides in the same phospholipid pools as AA. For example, the largest pools of AA and DGLA within the neutrophil lipids are found in phosphatidylethanoamine (PE) molecular species. Additionally, there are significant increases in the amount of DGLA associated with PE after supplementation with GLA (Chilton-Lopez et al., 1996; Johnson et al., 1997). Thus, the AA/DGLA ratio in PE species markedly decreases after supplementation with GLA. Perhaps more importantly, both DGLA and AA are released from membrane phospholipids, particularly PE, after neutrophil stimulation indicating that DGLA is located in membrane phospholipids that are readily accessible to hydrolysis by phospholipase(s).An altered AA/DGLA ratio has functional implications for immune cells (Table 3).
In contrast to these observations made in several inflammatory cell types, dietary GLA supplementation markedly increases circulating levels of both DGLA and AA in humans, suggesting that ingested GLA is both elongated to DGLA and subsequently desaturated to AA in vivo by tissues such as the liver (Barham et al., 2000; Johnson et al., 1997; Surette et al., 2003a). Examination of circulating lipoproteins reveals that DGLA and AA are almost exclusively localized in phospholipid pools, and GLA supplementation further enriches phospholipid pools with both DGLA and AA, but does not cause any appreciable change in other serum lipid classes (Johnson et al., 1997). In vitro studies utilizing Hep-G2 liver cells confirm that these transformed liver cells contain the enzymatic capacities to both elongate GLA to DGLA and then to further desaturate it to form AA (Barham et al., 2000).
Thus, in vivo GLA metabolism in humans is extremely complex since all cellular compartments do not metabolize it in a uniform manner due to the differential expression of PUFA metabolizing enzymes. GLA supplementation leads to elevated DGLA and has no effect on AA levels in certain inflammatory cells (e.g. neutrophils), but also increases both DGLA and AA levels in circulating lipids. The biological ramification of AA accumulation in circulation of humans is controversial. Some studies suggest that dietary AA has no influence on immune responses, blood lipids, lipoproteins or health in general (Kelley et al., 1997; Nelson et al., 1997). However, other studies show a strong association between elevated levels of AA and the formation of platelet-aggregating endoperoxides and thromboxanes (Hamberg and Samuelsson, 1974; Hamberg et al., 1975; Smith and Lands, 1972; Willis et al., 1974b). Additionally, high levels of AA in humans have also been shown to result in an increased tendency for the secondary irreversible phase of platelet aggregation (Seyberth et al., 1975).
3.2 Impact of n-3 PUFAs on GLA Metabolism
As mentioned in the Introduction, GLA-enriched supplements have also been given in combination with marine n-3 long chain-PUFA supplements. These supplementation strategies often provide GLA together with the n-3 long chain-PUFAs, EPA and DHA. There are three primary rationales for using these combinations. First, addition of n-3 long chain-PUFAs inhibits the conversion of GLA-derived DGLA to AA. In vitro experiments show that EPA blocks FADS1 activity in cultured HEP-G2 cells (Barham et al., 2000). Additionally, in vivo studies that demonstrate that the addition of fish oil (with EPA and DHA) to GLA-enriched diets prevents the accumulation of serum AA in response to GLA without inhibiting accumulation of DGLA in neutrophils (Barham et al., 2000). Other studies show that inclusion of as little as 0.25 g/d EPA + DHA can block GLA-induced elevations in plasma AA levels (Surette et al., 2003a; Surette et al., 2003b).
Secondly, like GLA alone, supplementation with borage + fish oil combinations inhibit leukotriene generation (Barham et al., 2000; Surette et al., 2003a; Surette et al., 2003b) and attenuate the expression of early steps in signal transduction, as well as the expression of genes for pro-inflammatory cytokines (Weaver et al., 2009). Finally, addition of fish oil to GLA supplemented diets enriches cells and tissues with EPA, DPA and DHA and their metabolites. Many of these metabolites have potent anti-inflammatory effects (Ariel and Serhan, 2007; Serhan et al., 2004; Serhan et al., 2002). Consequently, GLA/n-3 long chain-PUFA combinations theoretically would induce a powerful combination of anti-inflammatory metabolites from DGLA, EPA and DHA.
Botanical oil combinations that contain borage oil, enriched in GLA, and echium oil, (from Echium plantagineum L.) enriched in n-3 PUFAs (ALA and SDA), also markedly increase circulating levels of DGLA and have little impact on circulating AA levels (Arm et al., 2013; Lee et al., 2014). These studies suggest that botanical n-3 18C-PUFAs not only enhance the conversion of dietary GLA to DGLA but also inhibit further conversion of that DGLA to AA.
3.3 Differential Function and Impact of DGLA- and AA-Derived Eicosanoids
Free (unesterified) DGLA and AA released by phospholipases A2(s) are substrates for cyclooxygenases (COX) and lipoxygenases (LOX), leading to the synthesis of a variety of eicosanoid products including PGs, TXs, LTs and hydroxyl epoxides. As mentioned above (section 2.2), there are many eicosanoid derivatives of AA. In particular, the 2-series PGs and TXs, and the 4-series LTs, are by far the most commonly studied and are very well characterized. These lipid mediators tend to exhibit pro-inflammatory activities in numerous cell types and disease states. Additionally, there is emerging scientific literature revealing that free AA and oxidized products of AA can regulate gene expression, and consequently a wide range of cellular activities via cellular and nuclear receptors (Berger et al., 2006; Caputo et al., 2011; Chinetti et al., 2000; Deckelbaum et al., 2006; Jump and Clarke, 1999; Jung et al., 2012; Schmitz and Ecker, 2008; Soberman and Christmas, 2003; Vanden Heuvel, 2012).
Depending on the cell type, DGLA can be metabolized by COX 1/2 (prostaglandin endoperoxide H synthase-1 and −2, PGHS1/2) to 1-series PGs, particularly PGE1, and by 15-lipoxygenase into 15-(S)-hydroxy-8,11,13-eicosatrienoic acid (15-HETrE) (Borgeat et al., 1976). These two metabolites of DGLA have been shown to suppress inflammation, promote vasodilation, lower blood pressure, inhibit smooth muscle cell proliferation, and exert anti-neoplastic activities (Fan et al., 1995; Tabolacci et al., 2010; Zurier, 1991). DGLA supplementation has also been observed to regulate PGD1 and PGD2 levels (Amagai et al., 2015) (Table 3).
To better understand the capacity of PGHS1/2 to synthesize PGE1 and PGE2, it is important to understand 1) the cells that contain PGHS1/2 activities, 2) conditions in which PGHS1 and PGHS2 genes are expressed, 3) whether PGHS1 and PGHS2 can equally utilize DGLA and AA as substrates for prostaglandin biosynthesis, and 4) the capacity of PGE1 and PGE2 to bind E-type prostanoid receptors and induce biological responses. A great deal remains unknown in all of these areas. Levin and colleagues compared the DGLA and AA affinities and reaction rates for PGHS1 and PGHS2. DGLA and AA had similar affinities (Km) and maximal reaction rates (Vmax) for PGHS2 (Levin et al., 2002). In contrast, AA was metabolized preferentially by PGHS1. PGHS2 (Levin et al., 2002) is thought to be the dominant source for prostaglandin formation during inflammation (Ricciotti and FitzGerald, 2011). Together, these data suggest that both DGLA and AA can be efficiently converted to PGE1 and PGE2 during inflammatory responses.
Prostaglandins, including PGE1 and PGE2, exert their effects by binding to rhodopsin-like seven transmembrane spanning G protein-coupled receptors (Ricciotti and FitzGerald, 2011). The prostanoid receptor family has several members including EP1 (E prostanoid receptor 1), EP2, EP3, and EP4 subtypes and while it has been suggested that certain biological properties of PGE1 are ~20 times stronger than PGE2 (Fan and Chapkin, 1998), much remains to be learned about the affinity of PGE1 compared to PGE2 for PGE receptor subtypes and their subsequent biological activities.
Both PGE1 and 15-HETrE have been shown to antagonize the synthesis of AA-derived eicosanoids. When exogenously provided, 15-HETrE reduces A23187-stimulated LT generation in a dose-dependent manner in human neutrophils (Chilton-Lopez et al., 1996), peripheral blood mononuclear cells (Iversen et al., 1992) and murine peritoneal macrophages (Chapkin et al., 1988a) by 75–90% (Table 3). The inhibitory effect of 15-HETrE on neutrophil LTB4 generation is reversible and required only short (5min) exposure time (Chilton-Lopez et al., 1996). Interestingly, 15-HETrE is a much more potent inhibitor (90% inhibition at 20µM) of 5-lipoxygenase than 15-lipoxygenase derivatives of AA (15-HETE; 25% at 20µM), EPA (15-HEPE; 33% at 20µM) and DHA (17-HODHE; 33% at 20µM) (Iversen et al., 1992; Ziboh, 1996). PGE1 appears to also contribute to the inhibitory impact of GLA supplementation on LT synthesis. In mouse dendritic cells, both PGE1 and PGE2 suppress LTB4 production, the latter by an IL-10 dependent mechanism that interferes with the 5-LO activating protein (FLAP) expression (Harizi et al., 2003). Taken together, the isomeric series of the lipid mediators synthesized from AA and DGLA are functionally distinct and typically have opposing actions.
While all putative mechanisms have not been elucidated, the functional consequence of elevated DGLA content in neutrophils is a dramatic reduction in LTB4 generation in response to stimulation (Chilton-Lopez et al., 1996; Johnson et al., 1997; Ziboh and Fletcher, 1992). Cysteinyl LTs are important in the pathology of asthma and utilize receptors (CysLT1T and CysLT2R) localized in bronchial smooth muscle, vascular endothelium and secretory cells (Heise et al., 2000; Lynch et al., 1999). Basophil cysteinyl LT generation is reduced up to 50% in mild asthmatics by supplementation with botanical oil (borage + echium) combinations that contain both GLA and SDA (Arm et al., 2013). The generation of other potent lipid mediators including platelet activating factor is also inhibited as a result of dietary supplementation with GLA (Johnson et al., 1997).
4. The Impact of Genetic Variation in the Fatty Acid Desaturase (FADS) Gene Cluster on AA/DGLA Ratios and Eicosanoid Production
Until recently, the conversion of LA and ALA to AA and DHA, respectively, via the pathway shown in Fig. 1 was thought to be inefficient and uniform for all populations. However, mounting evidence indicates that common genetic and epigenetic variations in close proximity to and within the FADS cluster markedly affect the rate of conversion of 18C-PUFAs, including GLA, to LC-PUFAs and thus affecting the amount of circulating and tissue LC-PUFA levels. Specifically, single nucleotide polymorphisms (SNPs) and the methylation status of CpG sites in the FADS gene cluster are strongly associated with DGLA, AA and DHA levels in plasma and liver tissues (Chilton et al., 2014; Hoile et al., 2014; Howard et al., 2014; Mathias et al., 2014). As discussed in section 2, the human FADS gene cluster is located on chromosome 11q12-q13.1, comprised of 91.9kb and has nearly 500 SNPs annotated to this region with exon/intron organization (Fig. 2) (Glaser et al., 2010).
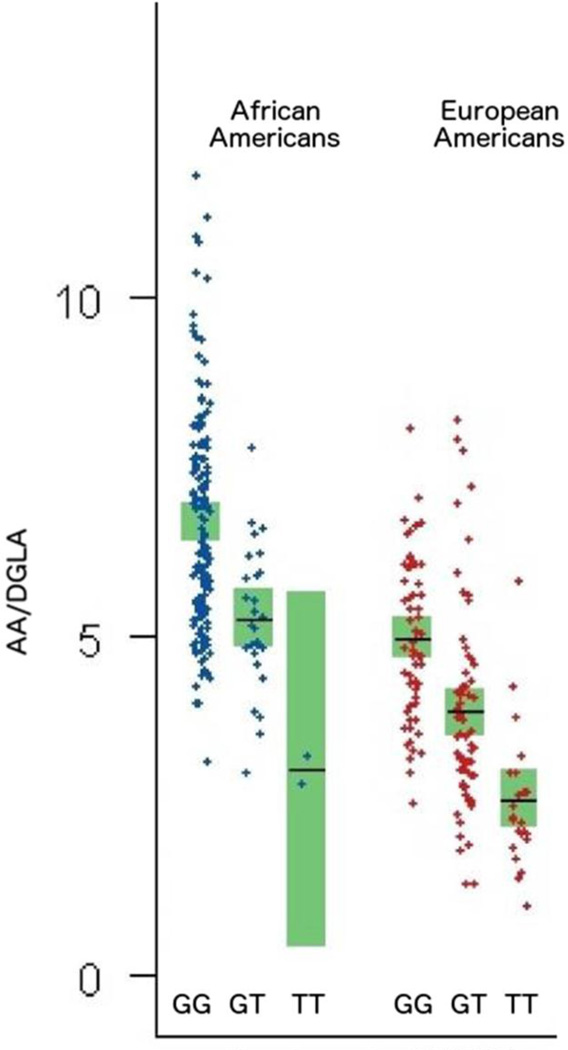
Fig. 2.
Plasma AA/DGLA ratios vary by genotype at rs174537 in both African American and European American populations (Adapted from (Mathias et al., 2011))
Recent studies from our lab have revealed dramatic population-based differences in the frequency of genetic variations that impact long chain (LC; >20 carbons) PUFAs and their metabolites (e.g. eicosanoids) (Hester et al., 2014; Mathias et al., 2011; Sergeant et al., 2012). These studies clearly support that associations between genetic variants and PUFA levels are strongly related to ethnicity. For example ~80% of African Americans carry two copies of the alleles associated with increased levels of AA and DHA and reduced levels of DGLA, compared to only ~45% of European Americans. Together these studies raise important questions of whether gene-PUFA interactions induced by a modern western diet are differentially driving the risk of diseases of inflammation in diverse populations, and are these interactions leading to health disparities. They also suggest that “one size fits all” dietary recommendations and supplementation strategies may not be appropriate for all populations or even individuals within specific populations.
Our laboratory has focused on numerous SNPs and epigenetic sites within the FADS cluster but particularly the SNP, rs174537. Rs 174537, located 13–15kb downstream of FADS1, was originally chosen because it was shown in a large GWAS to be the strongest genetic determinant associated circulating plasma AA levels (p = 5.95 × 10−46) (Tanaka et al., 2009) Additionally, there are marked frequency differences in genotypes at rs174537 between African-Americans and European-Americans. Our studies, across several cohorts, show that this SNP in particular, is robustly associated with ratios of AA to DGLA and thus the enzymatic efficiency of FADS1. Fig. 2 shows the relationships between genotypes at rs174537 and serum AA/DGLA ratios in African American and European American populations (Mathias et al., 2011). These data indicate that there is a greater than 3-fold difference (e.g. African American GG versus European American TT) in the AA/DGLA ratio between all genotypes in both populations and a greater than 2-fold difference between genotypes within either population. African Americans with the GG genotype have a mean ratio of AA/DGLA approaching 7.5 to 1, with some individuals well over 10 to 1. Studies have not been performed to determine how genetic variation within the FADS cluster impacts AA/DGLA in tissue or inflammatory cell lipids. These observations indicate the critical need for studies that are focused on the impact of gene variations, such as rs174537, on AA/DGLA ratios after supplementation with GLA-enriched oils. However, findings to date suggest that variation within the FADS cluster is likely to have significant impact on how individuals respond to GLA supplementation.
5. Discussion and Future Directions
This review emphasizes that the study of GLA and DGLA metabolism and its relationship to eicosanoid biosynthesis and inflammatory processes is a complex area of research. On the one hand, there are promising studies that suggest that supplementation with GLA and particularly combinations of GLA with n-3 long chain-PUFAs have great potential to dampen inflammatory processes and improve signs and symptoms of several inflammatory diseases. However, as a whole, this field of study is currently riddled with confusion. Much of the perplexity arises from many of the issues raised in this review including a limited knowledge about how genetic variation affects PUFA supplementation and subsequent metabolism.
A critical question is where does the field go from here? First, even in its current state, we feel the clinical studies indicate that this is an important area of research that should continue to be emphasized. Currently, the clinical effectiveness of a wide variety of supplementation strategies (with fish, flaxseed and GLA-containing oils) is being questioned. For example, recent clinical trials and meta-analyses have challenged the efficacy of supplementation with fish oils containing n-3 long chain-PUFA (Chen et al., 2011; Filion et al., 2010; Rizos et al., 2012; Zhao et al., 2009). Similarly, a meta-analysis of 27 studies (Pan et al., 2012) showed higher ALA exposure was associated with a moderately lower risk of CVD, but found “high unexplained heterogeneity” that warranted further studies. A central issue in all of these studies is the fact that large, diverse clinical trials inevitably have sizeable subsets of individuals with markedly different circulating and tissue levels of 18C- and LC-PUFAs, and great variability in how individuals respond to PUFA-based supplements. It seems clear that in light of such host-related complexities, study approaches that provide complex n-6 or n-3 dietary supplements to diverse groups of individuals using a “one size fits all” model, are unlikely to yield conclusive results.
In contrast, when genetic diversity is taken into consideration and the resultant marked differences in n-6 to n-3 LC-PUFA levels and ratios are recognized, then complex n-6 or n-3 dietary supplementation strategies can be used to correct critical diet-gene interactions in a targeted manner for individuals that need them. Moreover, in vitro, animal and human studies have demonstrated the benefits of balancing n-6 and n-3 metabolic pathways to reduce inflammatory processes, prevent disease and improve human health. It seems that understanding and recognizing individual and population differences provides this field with a great opportunity to optimize the use of PUFA-based supplements (including GLA-enriched supplements) as we move into the era of individualized medicine.
Acknowledgments
This work was supported by NIH P50 AT002782 (FHC, PI) and NIH AT008621-01A1 (FHC, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Floyd H. Chilton is a paid consultant for Eagle Wellness, LLC. This information has been revealed to Wake Forest Baptist Medical Center and is institutionally managed. Other authors have no conflict of interest.
References
- Amagai Y, et al. Dihomo-γ-linolenic acid prevents the development of atopic dermatitis through prostaglandin D1 production in NC/Tnd mice. J Dermatol Sci. 2015;79:30–37. doi: 10.1016/j.jdermsci.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Andreassi M, et al. Efficacy of gamma-linolenic acid in the treatment of patients with atopic dermatitis. J Int Med Res. 1997;25:266–274. doi: 10.1177/030006059702500504. [DOI] [PubMed] [Google Scholar]
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Arm J, et al. Impact of botanical oils on polyunsaturated fatty acid metabolism and leukotriene generation in mild asthmatics. Lipids Health Dis. 2013;12:141. doi: 10.1186/1476-511X-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JT, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database System Rev. 2013 doi: 10.1002/14651858.CD004416.pub2. Art. No. CD004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham JB, et al. Addition of eicosapentaenoic acid to g-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr. 2000;130:1925–1931. doi: 10.1093/jn/130.8.1925. [DOI] [PubMed] [Google Scholar]
- Belch JJ, Hill A. Evening primrose oil and borage oil in rheumatologic conditions. Am J Clin Nutr. 2000;71:352s–356s. doi: 10.1093/ajcn/71.1.352s. [DOI] [PubMed] [Google Scholar]
- Berger A, et al. How dietary arachidonic- and docosahexaenoic- acid rich oils differentially affect the murine hepatic transcriptome. Lipids Health Dis. 2006;5:10. doi: 10.1186/1476-511X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert J, John T, Sprecher H. Studies to determine the role rates of chain elongation and desaturation play in regulating the unsaturated fatty acid composition of rat liver lipids. Biochim Biophys Acta. 1975;398:354–363. doi: 10.1016/0005-2760(75)90186-1. [DOI] [PubMed] [Google Scholar]
- Billah MM, et al. Lipoxygenase products of arachidonic acid modulate biosynthesis of platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) by human neutrophils via phospholipase A2. J Biol Chem. 1985;260:6899–6906. [PubMed] [Google Scholar]
- Blond JP, Bézard J. Δ5-desaturation of dihomogammalinolenic acid (20:3(n-6)) into arachidonic acid (20 : 4(n-6)) by rat liver microsomes and incorporation of fatty acids in microsome phospholipids. Biochim Biophys Acta. 1991;1084:255–260. doi: 10.1016/0005-2760(91)90067-r. [DOI] [PubMed] [Google Scholar]
- Borgeat P, et al. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976;251:7816–7820. [PubMed] [Google Scholar]
- Boyce J. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8:335–349. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- Burr GO, Burr MM. A new deficiency disease produced by the ridid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. [Google Scholar]
- Burr GO, et al. On the fatty acids essential in nutrition. III. J Biol Chem. 1932;97:1–9. [Google Scholar]
- Calder PC. Long chain fatty acids and gene expression in inflammation and immunity. Cur Opin Clin Nutr Metab Care. 2013;16:425–433. doi: 10.1097/MCO.0b013e3283620616. [DOI] [PubMed] [Google Scholar]
- Cameron M, et al. Herbal therapy for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews Art No: CD002948. 2011 doi: 10.1002/14651858.CD002948.pub2. [DOI] [PubMed] [Google Scholar]
- Caputo M, Zirpoli H, Torino G, Tecce MF. Selective regulation of UGT1A1 and SREBP-1c mRNA expression by docosahexaenoic, eicosapentaenoic, and arachidonic acids. J Cell Physiol. 2011;226:187–193. doi: 10.1002/jcp.22323. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Coble KJ. Utilization of gammalinolenic acid by mouse peritoneal macrophages. Biochim Biophys Acta. 1991;1085:365–370. doi: 10.1016/0005-2760(91)90141-4. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, et al. Ability of 15-hydroxyeicosatrienoic acid (15-OH-20:3) to modulate macrophage arachidonic acid metabolism. Biochem Biophys Res Comm. 1988a;153:799–804. doi: 10.1016/s0006-291x(88)81166-5. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, et al. Inability of murine peritoneal macrophages to convert linoleic acid into arachidonic acid. Evidence of chain elongation. J Immunol. 1988b;140:2350–2355. [PubMed] [Google Scholar]
- Chapkin RS, Ziboh VA. Inability of skin enzyme preparations to biosynthesize arachidonic acid from linoleic acid. Biochem Biophys Res Comm. 1984;124:784–792. doi: 10.1016/0006-291x(84)91026-x. [DOI] [PubMed] [Google Scholar]
- Chen Q, et al. Effects of omega-3 fatty acid for sudden cardiac death prevention in patients with cardiovascular disease: A contemporary meta-analysis of randomized, controlled trials. Cardiovas Drugs Therap. 2011;25:259–265. doi: 10.1007/s10557-011-6306-8. [DOI] [PubMed] [Google Scholar]
- Chilton-Lopez T, et al. Metabolism of gammalinolenic acid in human neutrophils. J Immunol. 1996;156:2941–2947. [PubMed] [Google Scholar]
- Chilton FH, et al. Diet-gene interactions and PUFA metabolism: A potential contributor to health disparities and human diseases. Nutrients. 2014;6:1993–2022. doi: 10.3390/nu6051993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti G, et al. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflam Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- de Bravo MM, et al. Metabolism of gammalinolenic acid by human blood platelet microsomes. Biochem Int. 1985;10:889–896. [PubMed] [Google Scholar]
- Deckelbaum RJ, et al. n-3 Fatty acids and gene expression. Am J Clin Nutr. 2006;83:S1520–1525S. doi: 10.1093/ajcn/83.6.1520S. [DOI] [PubMed] [Google Scholar]
- Eaton SB. Humans, lipids and evolution. Lipids. 1992;27:814–820. doi: 10.1007/BF02535856. [DOI] [PubMed] [Google Scholar]
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411–1414. doi: 10.1093/jn/128.9.1411. [DOI] [PubMed] [Google Scholar]
- Fan Y-Y, et al. Dietary γ-linolenic acid modulates macrophage-vascular smooth muscle cell interactions: Evidence for a macrophage-derived soluble factor that downregulates DNA synthesis in smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:1397–1403. doi: 10.1161/01.atv.15.9.1397. [DOI] [PubMed] [Google Scholar]
- Filion K, et al. Omega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Dis. 2010;10:24. doi: 10.1186/1471-2261-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi A, et al. The efficacy and safety of γ-linolenic acid in the treatment of infantile atopic dermatitis. J Int Med Res. 1994;22:24–32. doi: 10.1177/030006059402200103. [DOI] [PubMed] [Google Scholar]
- Fletcher MP, Ziboh VA. Effects of dietary supplementation with eicosapentaenoic acid or gamma-linolenic acid on neutrophil phospholipid fatty acid composition and activation responses. Inflammation. 1990;14:585–597. doi: 10.1007/BF00914278. [DOI] [PubMed] [Google Scholar]
- Foolad N, et al. Effect of nutrient supplementation on atopic dermatitis in children: A systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350–355. doi: 10.1001/jamadermatol.2013.1495. [DOI] [PubMed] [Google Scholar]
- Foster RH, et al. Borage oil in the treatment of atopic dermatitis. Nutrition. 2010;26:708–718. doi: 10.1016/j.nut.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Gadek JE, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Glaser C, et al. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism. 2010;59:993–999. doi: 10.1016/j.metabol.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Glaser C, et al. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Maternal Child Nutr. 2011;7:27–40. doi: 10.1111/j.1740-8709.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Nat Acad Sci. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, et al. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Nat Acad Sci. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harizi H, et al. Prostaglandins inhibit 5-lipoxygenase-activating protein expression and leukotriene B4 production from dendritic cells via an IL-10-dependent mechanism. J Immunol. 2003;170:139–146. doi: 10.4049/jimmunol.170.1.139. [DOI] [PubMed] [Google Scholar]
- Heise CE, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- Hester AG, et al. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J Biol Chem. 2014;289:22482–22489. doi: 10.1074/jbc.M114.579557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR, et al. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:S1483–S1493. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Hoile SP, et al. Supplementation with n-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS ONE. 2014;9:e109896. doi: 10.1371/journal.pone.0109896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. Am J Clin Nutr. 2000;71:367s–372s. doi: 10.1093/ajcn/71.1.367s. [DOI] [PubMed] [Google Scholar]
- Howard TD, et al. DNA methylation in an enhancer region of the FADS cluster is associated with FADS activity in human liver. PLoS One. 2014;9:e97510. doi: 10.1371/journal.pone.0097510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado de Catalfo GE, et al. Arachidonic acid biosynthesis in non-stimulated and adrenocorticotropin-stimulated Sertoli and Leydig cells. Lipids. 1992;27:593–598. doi: 10.1007/BF02536116. [DOI] [PubMed] [Google Scholar]
- Irazu CE, et al. Delta 5 desaturase activity in rat kidney microsomes. Mol Cell Biochem. 1993;129:31–37. doi: 10.1007/BF00926573. [DOI] [PubMed] [Google Scholar]
- Iversen L, et al. Linoleic acid and dihommogammalinolenic acid inhibit leukotriene B4 formation and stimulate the fomation of their 15-lipoxygenase poducts by human neutrohils in vitro. Evidence of formation of antiinflammatory compounds. Agents Actions. 1991;33:286–291. doi: 10.1007/BF01986575. [DOI] [PubMed] [Google Scholar]
- Iversen L, et al. Effect of dihomogammalinolenic acid and its 15-lipoxygenase metabolite on eicosanoid metabolism by human mononuclear leukocytes in vitro: selective inhibition of the 5-lipoxygenase pathway. Arch Dermatol Res. 1992;284:222–226. doi: 10.1007/BF00375798. [DOI] [PubMed] [Google Scholar]
- Johnson MM, et al. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr. 1997;127:1435–1444. doi: 10.1093/jn/127.8.1435. [DOI] [PubMed] [Google Scholar]
- Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Ann Rev Nutr. 1999;19:63–90. doi: 10.1146/annurev.nutr.19.1.63. [DOI] [PubMed] [Google Scholar]
- Jung UJ, et al. Fatty acids regulate endothelial lipase and inflammatory markers in macrophages and in mouse aorta: A role for PPARγ. Arterioscler Thromb Vasc Biol. 2012;32:2929–2937. doi: 10.1161/ATVBAHA.112.300188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, et al. Stearidonic and γ-linolenic acids in echium oil improves glucose disposal in insulin resistant monkeys. Prostaglandin Leukotr Essent Fatty Acid. 2013;89:39–45. doi: 10.1016/j.plefa.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura A, et al. Dietary supplementation of gamma-linolenic acid improves skin parameters in subjects with dry skin and mild atopic dermatitis. J Oleo Sci. 2011;60:597–607. doi: 10.5650/jos.60.597. [DOI] [PubMed] [Google Scholar]
- Kazani S, et al. LTC4 synthase polymorphism modifies efficacy of botanical seed oil combination in asthma. SpringerPlus. 2014;3:1–8. doi: 10.1186/2193-1801-3-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DS, et al. Effects of dietary arachidonic acid on human immune response. Lipids. 1997;32:449–456. doi: 10.1007/s11745-997-0059-3. [DOI] [PubMed] [Google Scholar]
- Kitz RJ, et al. Impact of early dietary gamma-linolenic acid supplementation on atopic eczema in infancy. Pediatr Allergy Immunol. 2006;17:112–117. doi: 10.1111/j.1399-3038.2005.00369.x. [DOI] [PubMed] [Google Scholar]
- Kunkel S, et al. Suppression of chronic inflammation by evening primrose oil. Prog Lipid Res. 1981;20:885–888. doi: 10.1016/0163-7827(81)90165-x. [DOI] [PubMed] [Google Scholar]
- Lagarde M, et al. Human platelet PGE1 and dihomogammalinolenic acid. Comparison to PGE2 and arachidonic acid. Prog Lipid Res. 1981;20:439–443. doi: 10.1016/0163-7827(81)90078-3. [DOI] [PubMed] [Google Scholar]
- Lee TC, et al. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014;13:196. doi: 10.1186/1476-511X-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal L, et al. Treatment of rheumatoid arthritis with blackcurrant seed oil. Br J Rheumatol. 1994;33:847–852. doi: 10.1093/rheumatology/33.9.847. [DOI] [PubMed] [Google Scholar]
- Leventhal L, et al. Treatment of rheumatoid arthritis with gammalinolenic acid. Ann Intern Med. 1993;119:867–873. doi: 10.7326/0003-4819-119-9-199311010-00001. [DOI] [PubMed] [Google Scholar]
- Levin G, et al. Differential metabolism of dihomo-γ-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002;365:489–496. doi: 10.1042/BJ20011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. Enteral immunomodulatory diet (omega-3 fatty acid, γ-linolenic acid and antioxidant supplementation) for acute lung injury and acute respiratory distress syndrome: An updated systematic review and meta-analysis. Nutrients. 2015;7:5239. doi: 10.3390/nu7075239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C, et al. Treatment of atopic eczema with evening primrose oil. Lancet. 1981;1:278. doi: 10.1016/s0140-6736(81)92119-x. [DOI] [PubMed] [Google Scholar]
- Luthria DL, Sprecher H. A comparison of the specific activities of linoleate and arachidonate in liver, heart and kidney phospholipids after feeding rats ethyl linoleate-9,10,12,13-d4. Biochim Biophys Acta. 1994;1213:1–4. doi: 10.1016/0005-2760(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Lynch KR, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- Macfarlane G, et al. Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: a systematic review. Rheumatol. 2011;50:1672–1683. doi: 10.1093/rheumatology/ker119. [DOI] [PubMed] [Google Scholar]
- Mathias R, et al. The impact of FADS genetic variants on omega-6 polyunsaturated fatty acid metabolism in African Americans. BMC Genetics. 2011;12:50. doi: 10.1186/1471-2156-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RA, et al. Genetic variants in the FADS gene: Implications for dietary recommendations for fatty acid intake. Cur Nutr Rep. 2014;3:139–148. doi: 10.1007/s13668-014-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse NL, Clough PM. A meta-analysis of randomized, placebo-controlled clinical trials of Efamol®; evening primrose oil in atopic eczema. Where do we go from here in light of more recent discoveries? Cur Pharmaceut Biotech. 2006;7:503–524. doi: 10.2174/138920106779116946. [DOI] [PubMed] [Google Scholar]
- Morse PF, et al. Meta-analysis of placebo-controlled studies of the efficacy of Epogam in the treatment of atopic eczema. Relationship between plasma essential fatty acid changes and clinical response. Br J Dermatol. 1989;121:75–90. doi: 10.1111/j.1365-2133.1989.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Navarette R, et al. Dietary intake of concentrated gamma-linolenic acid (GLA)-enriched oil suppresses cutaneous level of dihomo-gamma-linolenic acid (DGLA): possible in vivo inhibition of microsomal elongation of GLA to DGLA. Prostaglandins Leukot Essent Fatty Acids. 1992;46:139–144. doi: 10.1016/0952-3278(92)90220-d. [DOI] [PubMed] [Google Scholar]
- Nelson GJ, et al. The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 1997;32:421–425. doi: 10.1007/s11745-997-0055-7. [DOI] [PubMed] [Google Scholar]
- Pan A, et al. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:1262–1273. doi: 10.3945/ajcn.112.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlosky R, et al. Essential fatty acid metabolism in the feline: relationship between liver and brain production of long-chain polyunsaturated fatty acids. J Lipid Res. 1994;35:2032–2040. [PubMed] [Google Scholar]
- Pontes-Arruda, et al. Effects of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- Riccioni G, et al. Effect of the two different leukotriene receptor antagonists, montelukast and zafirlukast, on ouality of life: A 12-week randomized study. Allergy Asthma Proc. 2004;25:445–448. [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscle Thromb Vascul Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice TW, et al. Enteral omega-3 fatty acid, γ-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos EC, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Sergeant S, et al. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br J Nutr. 2012;107:547–555. doi: 10.1017/S0007114511003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C, et al. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- Serhan CN, et al. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyberth HW, et al. Increased arachidonate in lipids after administration to man: effects on prostaglandin biosynthesis. Clin Pharmacol Therap. 1975;18:521–529. doi: 10.1002/cpt1975185part1521. [DOI] [PubMed] [Google Scholar]
- Simon D, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Therapy. 2014;31:180–188. doi: 10.1007/s12325-014-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Singer P, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- Smith WL, Lands WE. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972;11:3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- Soberman RJ, Christmas P. The organization and consequences of eicosanoid signaling. J Clin Invest. 2003;111:1107–1113. doi: 10.1172/JCI18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher H. Biochemistry of essential fatty acids. Prog Lipid Res. 1981;20:13–22. doi: 10.1016/0163-7827(81)90009-6. [DOI] [PubMed] [Google Scholar]
- Surette ME, et al. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin Therap. 2003a;25:948–971. doi: 10.1016/s0149-2918(03)80116-9. [DOI] [PubMed] [Google Scholar]
- Surette ME, et al. Inhibition of leukotriene biosynthesis by a novel dietary fatty acid formulation in patients with atopic asthma: A randomized, placebo-controlled, parallel-group, prospective trial. Clin Therap. 2003b;25:972–979. doi: 10.1016/s0149-2918(03)80117-0. [DOI] [PubMed] [Google Scholar]
- Surette ME, et al. The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and the quality of life of adult asthma patients. Cur Medl Res Opin. 2008;24:559–567. doi: 10.1185/030079908x273011. [DOI] [PubMed] [Google Scholar]
- Tabolacci C, et al. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16-F10 murine melanoma cells. Melanoma Res. 2010;20:273–279. doi: 10.1097/CMR.0b013e328339d8ac. [DOI] [PubMed] [Google Scholar]
- Tanaka T, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI study. PLoS Genet. 2009:e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate G, et al. Suppression of acute and chronic inflammation by dietary gamma linolenic acid. J Rheumatol. 1989;16:729–734. [PubMed] [Google Scholar]
- van Gool CJAW, et al. γ-Linolenic acid supplementation for prophylaxis of atopic dermatitis—a randomized controlled trial in infants at high familial risk. Am J Clin Nutr. 2003;77:943–951. doi: 10.1093/ajcn/77.4.943. [DOI] [PubMed] [Google Scholar]
- Van Gool CJAW, et al. Oral essential fatty acid supplementation in atopic dermatitis—a meta-analysis of placebo-controlled trials. Br J Dermatol. 2004;150:728–740. doi: 10.1111/j.0007-0963.2004.05851.x. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP. Nutrigenomics and nutrigenetics of ω3 polyunsaturated fatty acids. In: Bouchard C, Ordovas JM, editors. Progress in Molecular Biology and Translational Science. Academic Press; 2012. pp. 75–112. [DOI] [PubMed] [Google Scholar]
- Vanderhoek JY, et al. Inhibition of leukotriene biosynthesis by the leukocyte product 15-hydroxy-5,8,11,13-eicosatetraenoic acid. J Biol Chem. 1980;255:10064–10066. [PubMed] [Google Scholar]
- Weaver KL, et al. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J Biol Chem. 2009;284:15400–15407. doi: 10.1074/jbc.M109.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AL, et al. Dihomo-gamma-linolenate suppresses platelet aggregation when administered in vitro or in vivo. Prostaglandins. 1974a;8:509–519. doi: 10.1016/0090-6980(74)90063-x. [DOI] [PubMed] [Google Scholar]
- Willis AL, et al. An endoperoxide aggregator (Lass), formed in platelets in response to thrombotic stimuli: purification, identification and unique biological significance. Prostaglandins. 1974b;8:453–507. doi: 10.1016/0090-6980(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Zhao Y-T, et al. Prevention of sudden cardiac death with omega-3 fatty acids in patients with coronary heart disease: A meta-analysis of randomized controlled trials. Ann Med. 2009;41:301–310. doi: 10.1080/07853890802698834. [DOI] [PubMed] [Google Scholar]
- Ziboh VA. The significance of polyunsaturated fatty acids in cutaneous biology. Lipids. 1996;31:S249–S253. doi: 10.1007/BF02637085. [DOI] [PubMed] [Google Scholar]
- Ziboh VA, Fletcher MP. Dose-response effects of dietary gamma-linolenic acid-enriched oils on human polymorphonuclear-neutrophil biosynthesis of leukotriene B4. Am J Clin Nutr. 1992;55:39–45. doi: 10.1093/ajcn/55.1.39. [DOI] [PubMed] [Google Scholar]
- Ziboh VA, et al. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and antiproliferative metabolites. Am J Clin Nutr. 2000;71:361s–366s. doi: 10.1093/ajcn/71.1.361s. [DOI] [PubMed] [Google Scholar]
- Ziboh VA, et al. Suppression of leukotriene B(4) generation by ex-vivo neutrophils isolated from asthma patients on dietary supplementation with gammalinolenic acid-containing borage oil: Possible implication in asthma. Clin Devel Immunol. 2004;11:13–21. doi: 10.1080/10446670410001670445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. Role of prostaglandins E in inflammation and immune responses. Adv Prostaglandin Thromboxane Leukot Res. 1991;21B:947–953. [PubMed] [Google Scholar]
- Zurier RB, et al. Gamma-linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheumatism. 1996;39:1808–1817. doi: 10.1002/art.1780391106. [DOI] [PubMed] [Google Scholar]