Abstract
Cancer immunotherapy is becoming a standard approach to treat many cancers. However, shortcomings of current methods limit therapeutic benefit in many patients. Rationally designed biomaterial strategies to deliver immune modulatory drugs can potentially show improved safety profiles, while providing multifunctional and spatiotemporally controlled signals to immune cells to improve their anti-cancer activity. This brief review describes biomaterials-based strategies that enhance immune cell function at various tissue sites to improve anti-cancer immunity. Continued collaboration between bioengineers, immunologists, industry, and clinicians is required for biomaterial-based immunotherapy strategies to continue moving to the clinic.
Keywords: biomaterials, cancer immunotherapy, T cell, dendritic cell, vaccines
Introduction
While cancer cells use a number of strategies to avoid immune-mediated detection and killing during tumorigenesis, leveraging the immune system has become a viable strategy for treating human cancers within the last five years [1,2]. Optimal anti-tumor immune responses are specific to cancer cells, adaptable to changes in cancer cell gene expression, and provide durable disease control; these features are not readily achieved by conventional oncology strategies that aim to kill tumor cells directly. The recent FDA approval of a dendritic cell (DC)-based vaccine (Provenge) and checkpoint inhibitor antibodies against CTLA-4 and PD-1 have brought immunotherapy to the mainstream of clinical oncology.
Most immuno-oncology strategies aim to generate or unleash the potential of large numbers of functional, high avidity, cytotoxic T lymphocytes (CTL) to briskly infiltrate tumors and kill cancer cells. Cancer cells are recognized by CTL due to their possible expression of oncogenic virus proteins, unique mutated proteins, or abnormal expression of normal proteins. CTL recognize peptides derived from these proteins presented on major histocompatibility complex class I (MHC I) on the cancer cell surface and destroy the corresponding cell. A number of strategies aiming to induce CTL-mediated destruction of cancer cells have been tested clinically. Checkpoint inhibitor antibodies against the T cell inhibitory surface receptors CTLA-4 and PD-1 have shown remarkably durable responses in a number of cancers, but a large fraction of patients fail to respond to these agents, possibly due to a paucity of pre-existing anti-tumor T cells [3,4]. The number of anti-tumor CTL in a patient can be increased by adoptive cell therapy (ACT) with autologous ex vivo-expanded cancer-reactive T cells or chimeric-antigen receptor (CAR) T cells engineered against known tumor cell surface antigens [5,6]. These strategies produce durable clinical responses in some cancers, but associated toxicity, the substantial cost of ex vivo cell manipulation, and difficulties in maintaining cell survival and function after transfer into the patient pose challenges for their use. DC-based or synthetic therapeutic cancer vaccines containing irradiated whole tumor cells, tumor lysates, proteins, or peptides, often in combination with immunological adjuvants, have also been tested clinically, but with little durable survival benefit to date [7].
As only a minority of all cancer patients respond to current immuno-therapies, decades of research in the area of cell and drug delivery using biomaterials is now being adapted in efforts to develop more broadly efficacious immuno-oncology strategies. Biomaterials systems offer the ability to protect bioactive molecules or cells, control their spatiotemporal delivery profiles, and allow multiple agents to be delivered from a single platform [8,9]. These favorable properties potentially allow for reduced toxicity, dose sparing, and improved efficacy compared to conventional bolus delivered therapeutics. This brief review will highlight recently published in vivo biomaterials-based strategies for improving DC and T cell function at various tissue sites for cancer immunotherapy (Figure 1). For a more detailed overview of the field of immunoengineering for cancer therapy, the reader is directed to several comprehensive recent reviews [10–14].
Figure 1. Examples of biomaterial-based cancer immunotherapy strategies at various tissue sites.
i) Scaffolds delivered to peripheral tissues can be used as niches to program immune cell function. ii) Lymph node-draining nanoparticles (NPs) can efficiently traffic vaccine components to lymph nodes. (iii) Immune cells modified with drug-releasing nanoparticles can show enhanced function in the tumor microenvironment.
Programming immune cell function in peripheral tissues
Non-lymphoid peripheral tissues are an initial site of tumor antigen acquisition by DCs, the most potent antigen-presenting cell (APC) [15]. If antigen exposure is accompanied by pro-inflammatory signals released by dying tumor cells, DCs will undergo phenotypic maturation, and migrate to lymph nodes (LN) where they present tumor antigens that stimulate naïve T cells to proliferate and mobilize to tumor sites. Thus, methods to optimally generate antigen-loaded mature DCs and support T cell trafficking and survival in peripheral tissues are of interest in immuno-oncology.
Biomaterials scaffolds delivering immunomodulatory factors can be used as controlled microenvironments to program DC function in situ, obviating the need for ex vivo cell manipulation in conventional DC-based vaccination protocols (Figure 2). A macroporous PLGA scaffold implanted subcutaneously in mice and releasing granulocyte macrophage colony-stimulating factor (GM-CSF), a cytokine that has been widely explored in oncology, was shown to enrich millions of DCs of multiple subsets within the scaffold [16,17]. Recruited DCs were simultaneously presented with tumor lysate as an antigen source, and CpG oligonucleotides as a danger signal, leading to their antigen-loading and maturation, respectively. In situ programmed DCs initiated CTL responses that provided therapeutic efficacy against the poorly immunogenic B16-F10 murine melanoma (~50% survival with two doses of scaffold vaccine vs 0% with irradiated tumor cells engineered to secrete GM-CSF). Subsequent work showed that a variety of DC recruitment factors and danger signals could be incorporated in these scaffolds with similar anti-tumor efficacy [18,19]. These promising results have led to an ongoing first-in-human biomaterials vaccine clinical trial (NCT01753089) that has shown acceptable patient safety and the feasibility of manufacturing and delivering patient-specific biomaterial devices in a hospital setting. To avoid the need for surgical implant of a scaffold, an injectable vaccine formed by in situ assembly of high surface area mesoporous silica rods was created [20**]. By acting as a release depot for GM-CSF, CpG, and tumor antigen, this approach improved CTL and dual Th1/Th2 antibody responses against the model antigen ovalbumin in comparison to the widely used alum adjuvant, leading to protective immunity against ovalbumin-expressing tumor cells. Recently, injectable, sponge-like cryogels were fabricated that allow for delivery via a needle [21,22], and used to deliver irradiated tumor cells as an antigen source while simultaneously releasing GM-CSF and CpG to recruit DCs and create an immunogenic microenvironment [23]. Significant therapeutic anti-tumor activity against B16-F10 tumors was observed by sustained co-localization of cellular antigen and immunomodulators using this strategy (~40% survival with two doses of cryogel vaccine vs 0% in unimmunized mice).
Figure 2. Porous scaffolds for dendritic cell programming in the periphery.
A subcutaneously-delivered porous biomaterial scaffold that releases a chemoattractant recruits naïve dendritic cells (DCs) into its void space. Scaffold-resident DCs are exposed to tumor antigens and adjuvants, resulting in increased presentation of peptides on major histocompatibility complex (MHC-peptide) and phenotypic maturation. Mature DCs traffic out of the scaffold to lymph nodes where they can stimulate anti-tumor immunity.
Biomaterial delivery strategies can overcome challenges associated with mucosal vaccination to more effectively treat mucosal tumors. Cancers frequently arise in mucosal tissues, and optimal vaccine-induced T cell homing to these tumors requires mucosal vaccination [24]. Particle-based vaccination at the mucosa can bypass hurdles associated with soluble vaccination such as dilution by mucosal secretions and degradation by mucosal enzymes, leading to improved antigen and adjuvant uptake by DCs at mucosal sites before clearance. For example, pulmonary vaccination with nanoparticle (NP)-bound antigens led to increased capture by pulmonary DCs and generated higher numbers of antigen-specific T cells that homed to the lungs relative to soluble vaccines [25]. Similarly, a multilayered liposomal vaccine encapsulating antigen and hydrophobic monophosphoryl lipid A (MPLA) as an adjuvant [26] was delivered intratracheally with soluble polyinosinic-polycytidylic acid (poly(I:C)) as an additional adjuvant [27]. Liposomal antigen delivery showed vastly improved uptake by lung-resident APCs, generated T cells with increased expression of mucosal homing receptors, and improved T cell trafficking to the lungs, relative to treatment with soluble vaccine.
Biomaterials strategies can be used when delivering cells for ACT into peripheral tissues to improve their survival and proliferation. Ex vivo expanded anti-tumor T cells show poor persistence and function when reintroduced into the circulation. Systemic treatment with cytokines such as interleukin 2 (IL-2) can support the T cells, but also produces patient morbidity. However, ex vivo coupling of growth factor-releasing NPs to T cells has been shown to support their subsequent expansion and function after transfer in vivo [28]. Alternatively, efficient direct in vivo targeting of adoptively transferred T cells was achieved by PEGylated liposomes decorated with IL-2-Fc, as both a targeting and stimulatory ligand [29]. IL-2-Fc liposomes showed superior expansion of adoptively transferred T cells relative to free IL-2 and allowed multiple waves of in vivo T cell expansion with repeated dosing. Recently, a biomaterials scaffold was used to deploy CAR T cells in tumor resection beds and near sites of multifocal disease [30**]. This porous alginate scaffold was functionalized with integrin ligands for ex vivo T cell adhesion and loading, and contained embedded microparticles that presented immobilized stimulatory antibodies (anti- CD3, CD28, CD137) and released a soluble cue that supported T cell expansion in vivo (IL-15 superagonist). Scaffold-mediated cell delivery increased T cell expansion ~100-fold relative to freely injected T cells, leading to improved disease control in breast and ovarian cancer models relative to bolus delivered ACT.
Targeting lymph node antigen presenting cells
LNs are the primary site of initiation of adaptive immunity, and appropriate antigen presentation at this site after vaccination is critical to CTL generation [31]. In addition to direct antigen acquisition and trafficking to the LN by migratory peripheral DCs, LN-resident APCs screen lymph fluid for antigens and danger signals. Soluble vaccine components administered in the skin may bypass capture in the LN by APCs, and particulate formulations can be used to improve their retention. For example, intralymph node injection of adjuvant-releasing microparticles improves adjuvant accumulation in LNs, DC activation, and T cell priming compared to soluble intramuscular or intralymph node injection [32]. Alternatively, engineered NPs of the appropriate physical and surface properties, size range (10–100 nm), and targeting ligands can efficiently drain to LN and be captured by APCs after injection in the skin [33–38] (Figure 3). LN-draining NPs can deliver antigens and adjuvants to LN-resident APCs to prime anti-tumor T cell responses [39]. A recent study used LN-draining PEGylated liposomes to co-encapsulate model antigen and a cyclic dinucleotide (CDN) adjuvant [40]. LN-resident DCs and macrophages captured subcutaneously injected NPs, increasing LN accumulation of CDN 15-fold relative to soluble CDN injection. Co-delivery of CDN-containing NPs with ovalbumin led to a substantial antigen-specific T cell responses (ovalbumin-specific CD8+ T cells in peripheral blood: ~15% with NP-CDN and ovalbumin vs ~5% with soluble CDN and ovalbumin) and an improved median survival in an ovalbumin-expressing tumor EG.7 lymphoma model (29 days with NP-CDN and ovalbumin vs 17 days with soluble CDN and ovalbumin).
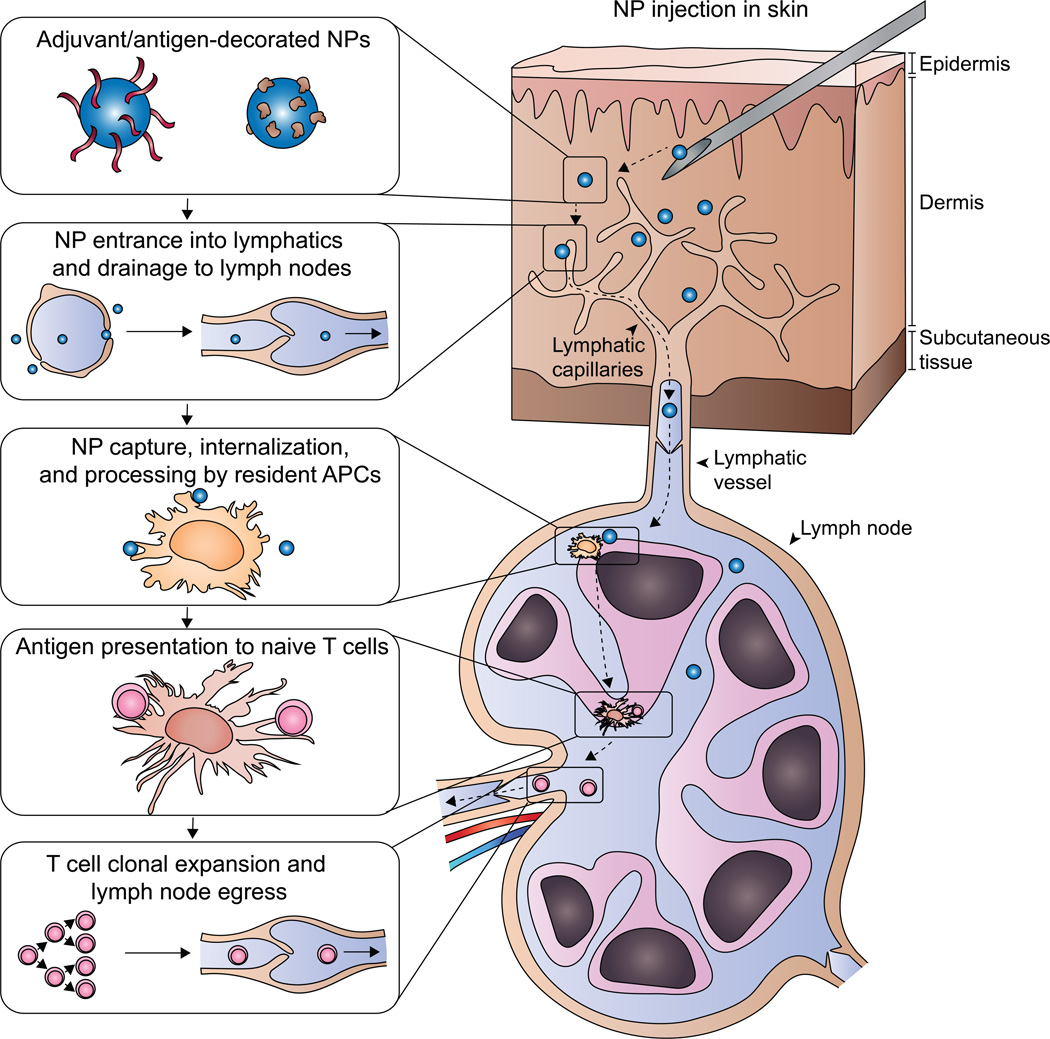
Figure 3. Lymph-node draining nanoparticles for improved vaccine delivery.
Nanoparticles (NPs) are decorated with tumor antigens and adjuvants. After injection in the skin, NPs of the appropriate physical properties enter into lymphatic capillaries and drain to the lymph node. Resident antigen presenting cells (APCs) efficiently take up and process the NPs and present tumor antigens to naïve T cells. Stimulation of naïve anti-tumor T cell clones by their cognate antigen results in clonal expansion and trafficking out of the lymph node to the tumor site.
The tumor-draining LN (TDLN), although bathed constantly in tumor antigen, can be a location of immune tolerance generation [41] and efforts have been made to deliver pro-inflammatory stimuli to APCs in the TDLN to instead generate antitumor immune response activation. Pluronic-stabilized poly(propylene sulfide)-core NPs loaded with adjuvants were injected intradermally and drained to the TDLN of subcutaneous B16-F10 tumors [42*]. CpG-loaded NPs increased the number of activated DCs in the TDLN and the quantity of favorable Th1 polarized CD4+ T cells and antigen-specific CD8+ T cells in the tumor, slowing tumor growth. Additional studies with this NP system have shown that the TDLN is a superior site for vaccination with exogenous tumor antigens, relative to non-TDLNs, further motivating investigation of TDLNs as a site for therapeutic intervention [43].
Recently, a molecular vaccine approach exploited the ability of serum albumin to effectively traffic bound proteins to LNs [44**]. By creating conjugates of antigen or adjuvant with lipid domains that bind serum albumin, subcutaneously injected vaccine components were efficiently delivered to LNs and accumulated within resident APCs. This approach produced antigen-specific CD8+ T cell responses of unprecedented magnitude for a molecular vaccine (e.g. HPV E7 protein-specific T cells in peripheral blood: ~30% with E7 long peptide lipid conjugate and CpG lipid conjugate vs ~5% with E7 long peptide and CpG), and showed significantly delayed tumor growth in a number of tumor models tested.
Immunomodulation in the tumor microenvironment
The immunosuppressive tumor microenvironment (TME) limits the function of tumor-infiltrating APCs and T cells [45], but biomaterials may allow one to modify this microenvironment. Mechanisms of normal tumor immune evasion include changes in antigen repertoire, downregulation of surface MHC I-peptide complexes, restriction of T cell infiltration, expression of T cell inhibitory ligands, recruitment of immunosuppressive cells, maintenance of metabolically unfavorable conditions, and the elaboration of immunosuppressive cytokines and enzymes that promote APC and T cell dysfunction. Therapeutic delivery of immunomodulators to the TME can potentially be used to overcome these mechanisms.
Biomaterials strategies can improve the activity of pro-inflammatory drugs that act on APCs in the TME, relative to systemic or locally injected soluble formulations (Figure 4-A). Improved local retention of such agents increases their effect on tumor-infiltrating APCs and reduces toxicity resulting from leakage into systemic circulation from the injection site. For example, while intratumorally injected CpG rapidly leaked from the TME, CpG that was modified with a diacyllipid inserted into cell membranes and remained localized in the TME, resulting in improved anti-tumor activity [46]. In another approach, liposomes were designed to present an APC-stimulating CD40 antibody in combination with CpG when injected at tumors [47]. This produced an increase in median survival in a B16-F10 model (45 days with anti-CD40/CpG liposomes vs 35 days with soluble anti-CD40/CpG vs 19 days with PBS treatment), while diminishing systemic dissemination of these immunostimulants and reducing toxicity compared to soluble formulations. Recently, cationized protein or peptide antigens was electrostatically associated with a DNA hydrogel containing CpG sequences and delivered to the TME [48]. The intrinsic adjuvant activity of the CpG sequences in the hydrogel and controlled release of cationic peptide antigen at the site of ovalbumin-expressing EG.7 lymphoma tumors resulted in an increased fraction of surviving mice (~65% with cationized peptide/CpG-gel vs. ~15% with native peptide/CpG-gel vs. 0% with cationized peptide/GpC-gel).
Figure 4. Modulating T cell and dendritic cell function in the tumor microenvironment.
A) Adoptively transferred T cells can have drug-releasing nanoparticles (NPs) conjugated to their cell membrane. Local release of inhibitors of T cell suppressive pathways by the NPs can provide autocrine signaling that improves T cell activity in the tumor microenvironment (TME). B) Nanoparticles decorated with dendritic cell (DC)-stimulating ligands can convert tumor-antigen loaded DCs in the TME from an immature state to a mature phenotype capable of stimulating anti-tumor T cells.
T cell function within the TME can be improved by locally delivering stimulatory cues and blocking immunosuppressive pathways using biomaterials (Figure 4-B). Liposome tethering of IL-2-Fc and an agonistic antibody against CD137 allowed their retention at the tumor and TDLN after intratumoral injection, preventing fatal systemic toxicity associated with bolus injection of these agents [49*]. Remarkably, this approach caused CD8+ T cells to infiltrate both treated and contralateral untreated B16-F10 melanoma tumors, inhibiting tumor growth. The ability of biomaterials to be multifunctional has been demonstrated with core-shell particles that released water-soluble IL-2 and a hydrophobic small molecule inhibitor of the immunosuppressive TGF-β pathway [50]. When injected intravenously, these NPs accumulated in lung tumors, presumably due to leaky tumor vasculature, and increased the number of activated CD8+ T cells and natural killer (NK) cell-mediated tumor destruction. Ex vivo conjugation of T cells with drug-releasing NPs that provide locally high concentrations of immunomodulators at the cell surface can also protect their function in the TME [51]. T cells coupled with NPs releasing an inhibitor of a T cell suppressive pathway (Shp1/Shp2) showed increased proliferation in the TME and reduced tumor growth. Recently, an alginate hydrogel was used to locally deliver anti-PD-1 directly to its presumed site of action in the TME [52]. Combination delivery of anti-PD-1 and the anti-inflammatory drug celecoxib at the tumor using a gel resulted in increased effector T cell infiltration, a reduction in inhibitory immune cells, and improved overall survival in mice bearing B16-F10 tumors (~55% with drug-releasing gel vs ~10% with soluble drugs) or 4T1 breast cancer tumor (~30% with drug-releasing gel vs 0% with soluble drugs).
Conclusions and outlook
Biomaterials-based strategies show tremendous promise for improving the spatiotemporal delivery of immunomodulators to enhance their safety and efficacy in cancer immunotherapy. Additionally, the multifunctionality provided by biomaterials can be used to impact immune signaling programs at many stages. A number of biomaterials strategies tested in murine models are grounded in principles that apply to the human immune system, warranting non-human primate and clinical testing. Biomaterials approaches will also likely be tested for their ability to enhance the efficacy of clinically validated checkpoint inhibitor therapies. Strategies that use raw materials previously approved by the FDA for medical applications will likely be translated more readily, and will be the focus of initial research. Further, advances in cancer immunology will continue to inform novel immunoengineering strategies. For example, recently developed methods to determine patient-specific mutated antigens can feed back directly into the design of personalized biomaterials-based vaccines with increased safety and efficacy [53]. Improved collaboration between bioengineers, industry, immunologists and clinicians will accelerate the pace of engineered immunotherapy development and translation.
Highlights.
Biomaterials can potentially improve the safety and efficacy of cancer immunotherapy
Biomaterials scaffolds can program immune cell function in peripheral tissues
Lymph node-draining nanoparticles provide potent vaccines
Biomaterial strategies can improve immune cell function within tumors
Acknowledgments
The authors acknowledge funding from NIH R01 EB015498 to DJM and an HHMI ISRF to STK, and thank Rajiv Desai and Alexander Cheung for their helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Allison J. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian S, Drake C, Pardoll D. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melief CJM, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest. 2015;125:3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearney CJ, Mooney DJ. Macroscale delivery systems for molecular and cellular payloads. Nat Mater. 2013;12:1004–1017. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 9.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 10.Singh A, Peppas NA. Hydrogels and scaffolds for immunomodulation. Adv Mater. 2014;26:6530–6541. doi: 10.1002/adma.201402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung AS, Mooney DJ. Engineered materials for cancer immunotherapy. Nano Today. 2015 doi: 10.1016/j.nantod.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta NK, Moynihan KD, Irvine DJ. Engineering new approaches to cancer vaccines. Cancer Immunol Res. 2015;3:836–843. doi: 10.1158/2326-6066.CIR-15-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Moon JJ. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines. 2015;3:662–685. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali OA, Tayalia P, Shvartsman D, Lewin S, Mooney DJ. Inflammatory cytokines presented from polymer matrices differentially generate and activate DCs in situ. Adv Funct Mater. 2013;23:4621–4628. doi: 10.1002/adfm.201203859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali OA, Verbeke C, Johnson C, Sands RW, Lewin SA, White D, Doherty E, Dranoff G, Mooney DJ. Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants. Cancer Res. 2014;74:1670–1681. doi: 10.1158/0008-5472.CAN-13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72. doi: 10.1038/nbt.3071. ** Showed the ability to create in vivo assembled porous scaffolds by subcutaneous injection of mesoporous silica rods. These scaffolds were able to recruit and program immune cells to generate potent cellular and humoral immune responses.
- 21.Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, Mooney DJ. Injectable preformed scaffolds with shape-memory properties. Proc Natl Acad Sci U S A. 2012;109:19590–19595. doi: 10.1073/pnas.1211516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshy ST, Ferrante TC, Lewin SA, Mooney DJ. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35:2477–2487. doi: 10.1016/j.biomaterials.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bencherif SA, Sands RW, Ali OA, Li WA, Lewin SA, Braschler TM, Shih T-Y, Verbeke CS, Bhatta D, Dranoff G, Mooney DJ. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun. 2015 doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandoval F, Terme M, Nizard M, Badoual C, Bureau M-F, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013;5:172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc Natl Acad Sci U S A. 2011;108:E989–E997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Ho **Um S, Khant H, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, Seidman MA, Yen M, Im EJ, Foley MH, Barouch DH, Irvine DJ. Generation of effector memory T cell–based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci Transl Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J Controlled Release. 2013;172:426–435. doi: 10.1016/j.jconrel.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat Biotechnol. 2015;33:97–101. doi: 10.1038/nbt.3104. ** Used a multifunctional alginate scaffold system to simultaneously deliver and stimulate CAR T cells, improving their proliferation and overall anti-tumor activity in adoptive cell therapy.
- 31.Pal I, Ramsey JD. The role of the lymphatic system in vaccine trafficking and immune response. Adv Drug Delivery Rev. 2011;63:909–922. doi: 10.1016/j.addr.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Jewell CM, López SCB, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci U S A. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 34.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 35.John ALS, Chan CY, Staats HF, Leong KW, Abraham SN. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat Mater. 2012;11:250–257. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang Y, Ma Y, Wang C, Hai L, Yan C, Zhang Y, Liu F, Cai L. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: Role of lymphatic trafficking and biodistribution. J Controlled Release. 2012;159:135–142. doi: 10.1016/j.jconrel.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Cruz LJ, Rosalia RA, Kleinovink JW, Rueda F, Löwik CW, Ossendorp F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: A comparative study. J Controlled Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 38.Mueller SN, Tian S, DeSimone JM. Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol Pharm. 2015;12:1356–1365. doi: 10.1021/mp500589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc Natl Acad Sci U S A. 2013;110:19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J Clin Invest. 2015;125:2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 42. Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–824. doi: 10.1016/j.biomaterials.2013.10.003. ** Lymph node-draining nanoparticles conjugated with adjuvants were targeted to the tumor draining lymph node. This strategy matured antigen-bathed dendritic cells in the tumor draining lymph node, resulting in a shift towards productive anti-tumor immunity.
- 43.Jeanbart L, Ballester M, de Titta A, Corthésy P, Romero P, Hubbell JA, Swartz MA. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res. 2014;2:436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- 44. Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. ** Design and testing of novel molecular conjugates of vaccine components with albumin-binding moieties In vivo administration resulted in lymph node retention of vaccine components, potent T cell response generation, and inhibited tumor growth.
- 45.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Kwong B, Irvine DJ. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew Chem, Int Ed. 2011;50:7052–7055. doi: 10.1002/anie.201101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwong B, Liu H, Irvine DJ. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32:5134–5147. doi: 10.1016/j.biomaterials.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umeki Y, Mohri K, Kawasaki Y, Watanabe H, Takahashi R, Takahashi Y, Takakura Y, Nishikawa M. Induction of potent antitumor immunity by sustained release of cationic antigen from a DNA-based hydrogel with adjuvant activity. Adv Funct Mater. 2015;25:5758–5767. [Google Scholar]
- 49. Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ. Localized immunotherapy via liposome-anchored anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 2013;73:1547–1558. doi: 10.1158/0008-5472.CAN-12-3343. ** Intratumorally delivered liposomes decorated with T cell stimulatory anti-CD137 and IL-2-Fc, resulting in systemic anti-tumor immunity and an improved safety profile compared to soluble components.
- 50.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, Li W, Wu G, Ren J, Wang Z, et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. OncoImmunology. 2015 doi: 10.1080/2162402X.2015.1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]