Abstract
Objective
To characterize normative anti-Müllerian hormone (AMH) levels and ascertain which factors are associated with AMH in a large cohort of reproductive-aged women.
Design
Cross-sectional study.
Setting
Study of the Environment, Lifestyle and Fibroids (SELF), a longitudinal study performed by the National Institute of Environmental Health Sciences in conjunction with a major health care provider in Detroit, Michigan.
Patients
1,654 African-American women (AAW) aged 23-34 at recruitment.
Intervention
None
Main Outcome Measure
Serum AMH measured using an ultrasensitive ELISA.
Results
The median AMH was 3.18 ng/ml, and there was a significant, but nonlinear, relationship between age and AMH, with levels peaking at age 25. As AMH was not normally distributed, log transformation was performed and used for all analyses. In a multivariable age-adjusted model, body mass index, current use of hormonal contraception, and history of a thyroid condition were inversely associated with AMH, while history of abnormal menstrual bleeding and menstrual cycles longer than 35 days were positively associated with AMH.
Conclusions
While age is correlated with AMH, it accounts for only a portion of the variation seen. This study adds valuable information to the existing literature on normative AMH levels in young reproductive-aged women. While our findings fill a critical data gap for ovarian reserve in AAW, the insights gained will be of benefit for all women.
Keywords: AMH, ovarian reserve, African-American women
Introduction
Women in the United States are having children later in life, as demonstrated by the fact that the average age at first birth reached a record high of 26.3 years in 2014 (1). Concordantly, while birth rates have declined to a record low for women in their early twenties, birth rates have increased for women in their thirties and early forties (1). For some women who delay childbearing, infertility associated with aging presents a significant reproductive problem (2). In an attempt to circumvent the age related decline that occurs, some individuals are seeking options to preserve their fertility. The intention in more than 27,000 (14%) of the total assisted reproductive technology cycles performed in 2013 was to cryopreserve all retrieved eggs or embryos for future use (3). Given the trends in delayed childbearing along with the growing interest in and utilization of elective fertility preservation (4), it is important that clinicians are able to accurately counsel women on their reproductive potential and provide realistic information on personal fertile windows so that individuals are better prepared to make decisions regarding reproduction during their optimal fertile window.
Ovarian reserve testing has been proposed as a method to predict reproductive potential (5). Anti-Müllerian hormone (AMH), a member of the transforming growth factor-beta superfamily produced by ovarian granulosa cells (6), reflects the remaining follicular pool (7) and may be used as a marker of ovarian reserve (8). When compared with other known biomarkers of ovarian reserve, AMH appears to have the advantage of low inter- and intracycle variability (9). Serum AMH is now routinely used in clinical practice for multiple indications (10) (11) (12) including estimation of timing of menopause (13-16). The most recent of these studies focused on AMH from birth to menopause and suggest that AMH rises during childhood, peaks sometime between the late teens and early adulthood, and then plateaus and remains stable before beginning a continuous decline around age 25 (17, 18). While these studies provide a helpful overview of AMH trends, there is major individual variability in ovarian aging that can occur independently of a woman’s age (19). Although studies have suggested that various intrinsic as well as environmental factors, may be associated with AMH (20, 21), the data are overall inconclusive on the effects of these potential contributors to variability in AMH.
Several studies have suggested that race and/or ethnicity may also impact AMH (22, 23) and the timing of menopause (24, 25). Others have also demonstrated that racial differences exist when sex steroid levels in cycling women are compared (26, 27). These differences are particularly important to consider as many of the larger cohort studies that have established normative AMH levels have been based on data from women of primarily European descent (17, 18). Furthermore, some of the larger studies on normative AMH only included women with a certain diagnosis i.e. infertility or polycystic ovarian syndrome (PCOS) (28, 29). Consequently, these studies may not be generalizable to fertile women of all races and ethnicities.
Knowledge of normative AMH levels in a group of women in their prime reproductive years who are not selected based on fertility status, menstrual cycle regularity or other factors that can potentially influence markers of ovarian reserve may be useful in providing insight into the fertile window of individuals. Further, by studying such a group it may be possible to determine which women are at highest risk for a rapid decline in ovarian reserve and to identify modifiable risk factors that impact AMH. This large cross-sectional study in a well-defined cohort of African-American women (AAW) provides the unique opportunity to determine the impact of various health behaviors, medical conditions and environmental exposures on AMH in an otherwise unselected group of reproductive-aged women. The primary objective of the study was to characterize the distribution of AMH in over 1,600 AAW from the general population and to determine what specific lifestyle and reproductive factors are associated with AMH in these young women.
Materials and Methods
Study participants
Data for this study were collected as part of an ongoing prospective cohort study, the Study of Environment, Lifestyle, and Fibroids (SELF), which is supported by the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health. The study is being conducted in Detroit, Michigan in conjunction with the Henry Ford Health System (HFHS). The institutional review boards of the NIEHS and HFHS approved this study, and participants provided written informed consent.
The study design, methods, and recruitment results have previously been described in detail (30). Recruitment of premenopausal AAW who were aged 23-34 occurred between November 2010 and December 2012. To be eligible, women must have been residing in the United States, self-identified as African-American or Black and not pregnant at the time of enrollment. Women with a previous diagnosis of uterine fibroids were specifically excluded, as were those who had undergone a hysterectomy. Women who had taken medication to treat multiple sclerosis, Grave’s disease, scleroderma, lupus, or Sjogren’s were also excluded, as were women who had been treated with chemotherapy or radiation for a previous cancer diagnosis. Enrollment of each participant was completed only after collection of extensive questionnaire data and a clinic visit that included a blood draw and measurement of height, weight, blood pressure, and skin reflectance. Of the 1,696 women enrolled in SELF, serum was available for 1,654 individuals.
Assays
At enrollment, up to 55 ml of blood was drawn from each woman for storage of serum, plasma, whole blood, clot, and packed cells. Stored serum was assayed for AMH in each participant’s barcoded samples. To accomplish this, stored aliquots were sent to the Clinical Laboratory Research Core in the Pathology Department at Massachusetts General Hospital. Serum AMH was measured using an ultrasensitive enzyme linked immunosorbent assay (ELISA) (lower limit of detection 0.06 ng/mL, 1 ng/ml=7.14 pmol/l) with reflex testing using the picoAMH ELISA for undetectable samples (lower limit of detection of 0.0012 ng/ml) (Ansh Labs, Webster, TX) AMH values that were below the lower limit of detection (n=3) were assigned a value of 0.0008 ng/mL using an established formula (31). The AMH ELISA has intra- and interassay coefficients of variation (CVs) of less than 5%.
Covariates
BMI was calculated from the height and weight measured at the clinic. Obesity was defined as a BMI ≥30 kg/m2. All other variables were self-reported in computer assisted telephone interviews, computer assisted web-based interviews, self-administered hard-copy questionnaires, or responses to questions administered by the study staff at the clinic visit.
History of medical conditions were determined by asking participants whether they were told by a doctor or other health professional that they had a particular condition. Average menstrual cycle length was ascertained by asking how many days typically occurred between the first day of one menstrual cycle and the first day of the next menstrual cycle in the last 12 months. Irregular cycles were defined as cycles outside of the 25 to 35 day range. Only women who were not using any hormones at the time of enrollment or in the last 12 months were considered when analyzing data on cycle length.
Given that women were not excluded from SELF based on factors that are known to influence menstrual cycle regularity, a subset of women who were cycling regularly as defined above was created for additional analytic purposes. This group is referred to as the “regularly cycling cohort” (N=604) and excluded women who reported irregular cycles, presence of a thyroid condition, a diagnosis of PCOS or current breastfeeding, or had used hormones in the last 12 months, as these factors may affect menstrual regularity.
Statistical Analysis
The distribution of AMH, the outcome, and the covariates of interest are presented as means or medians (with standard deviations or interquartile ranges) for continuous variables and proportions for categorical variables. Because AMH levels are not normally distributed, they were log transformed for analysis in linear regression models. In our first model, we performed simple linear regression to evaluate the association between AMH and each covariate of interest. Given the inverse relationship between age and AMH that was identified with simple linear regression, as well as the established association between age and ovarian reserve, age-adjusted models were then created for each covariate; these models included age and a quadratic term for age. Quadratic age was included in addition to age to allow the shape of the relationship between log AMH and age to vary from being linear. Both factors were important so subsequent multivariable models adjusted for both age and quadratic age as well as any covariates that were significantly associated with log AMH in the age-adjusted models. Estimates of beta, confidence intervals, and P values were obtained from linear regression analysis.
All analyses were carried out using Statistical Analysis Software version 9.4 (SAS Institute, Cary, NC). Statistical significance was determined at P<0.05.
Results
Baseline Characteristics
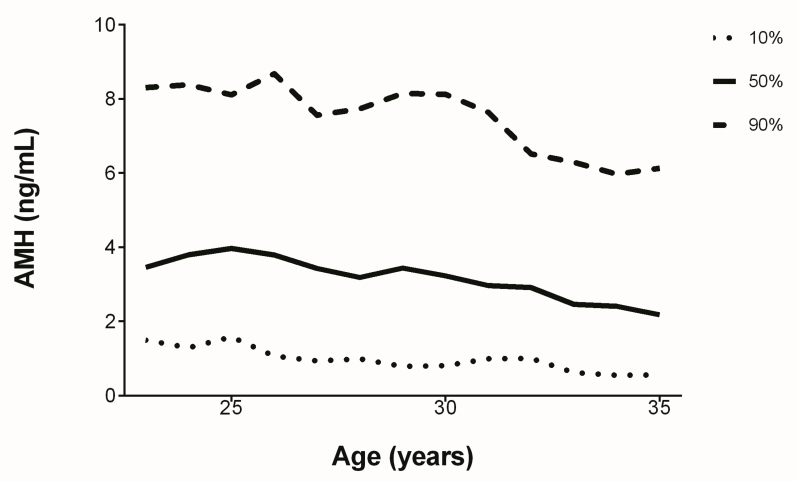
Demographic characteristics for the entire cohort are described in Table 1. The mean age was 28.7 years and the median AMH was 3.18 ng/ml. There were three participants who had AMH levels below the lower detection limit of the assay. Figure 1 demonstrates AMH at the tenth, fiftieth, and ninetieth percentiles as a function of age. The majority of the cohort was obese (59.5%). Most of the women were educated beyond high school, with more than a quarter having attained at least a bachelor’s degree. The majority of these women had been pregnant previously, and more than half had experienced a live birth. Many of these individuals had used some form of hormonal contraception in the past, but less than one third reported current use.
Table 1. Participant Characteristics.
| Entire Cohort (n=1654) |
Regularly Cycling Cohort (n=604) |
|
|---|---|---|
| Age, y (mean ±SD; range)a | 28.7 ± 3.5; 23-35 | 29.1 ± 3.4; 23-35 |
| 23-25 | 371 (22.4%) | 108 (17.9%) |
| 26-28 | 417 (25.2%) | 153 (25.3%) |
| 29-31 | 444 (26.8%) | 174 (28.8%) |
| 32-35 | 422 (25.5%) | 169 (28.0%) |
| Body mass index, kg/m2 (median, IQR; range) | 32.4, 26.3-39.5; 15.9-79.4 |
32.9, 26.4-40.0; 15.9-72.9 |
| AMH, ng/mL (median, IQR; range) | 3.18, 1.68-5.33; <0.002-39.4 |
3.35, 1.89-5.49; 0.02-30.4 |
| Education (%) | ||
| High school or less | 22.2 | 23.8 |
| Some college, but no degree | 37.7 | 36.9 |
| Associate or technical degree | 12.3 | 11.6 |
| Bachelor’s degree | 19.4 | 20.2 |
| Graduate degree | 8.4 | 7.5 |
| Gross annual household income (%) | ||
| Less than $20,000 | 46.1 | 45.8 |
| $20,001-50,000 | 36.8 | 37.4 |
| $50,001-100,000 | 14.6 | 15.1 |
| Over $100,000 | 2.5 | 1.7 |
| Currently married or living as though married (%) | 26.8 | 25.7 |
| Age at menarche, y (mean ±SD; range) | 12.0 ± 1.8; 7-19 | 11.9 ± 1.7; 7-18 |
| Pregnancy history | ||
| Never pregnant (%) | 26.7 | 28.0 |
| Previous pregnancy without history of a live birth (%) |
14.0 | 15.2 |
| Previous live birth but no history of breastfeeding (%) |
21.3 | 22.7 |
| Previous live birth with history of breastfeeding (%) |
38.0 | 34.1 |
| Number of pregnancies (mean ±SD range) | 3.1 ± 2.0; 1-15 b | 3.2 ± 2.1; 1-13 c |
| Current hormonal contraception use (%) | 27.5 | --- |
| History of hormonal contraception use (%) | 85.9 | 76.0 |
| Any hormone use in the last 12 months (%) | 39.2 | --- |
| Irregular menses in last 12 months (%)d | 31.6 | --- |
| Menstrual cycle length in last 12 monthsd | ||
| <25 days (%) | 23.4 | --- |
| 25-35 days (%) | 68.4 | 100.0 |
| >35 days (%) | 8.2 | ---- |
| History of polycystic ovarian syndrome (%) | 3.2 | --- |
| History of abnormal menstrual bleeding (%) | 11.5 | 8.5 |
| History of amenorrhea (%) | 4.2 | --- |
| History of endometriosis (%) | 1.8 | 1.0 |
| History of seeking care for difficulty conceiving (%) |
5.8 | 5.5 |
| History of a thyroid condition (%) | 2.9 | --- |
| Smoking status (%) | ||
| Current | 19.2 | 22.5 |
| Former | 7.4 | 7.5 |
| Never | 73.3 | 70.0 |
| Alcohol consumption in last 12 months (%) | ||
| At least weekly | 24.9 | 24.3 |
| Less than weekly | 46.6 | 43.5 |
| Never | 29.5 | 32.1 |
Women ages 23-34 were recruited, but some women had turned 35 by the time that all baseline activities and enrollment were completed.
Among those with at least 1 previous pregnancy, n=1212
Among those with at least 1 previous pregnancy, n=435
Among those not using hormonal contraception at any time in the last 12 months, n=953
Figure 1.
Median AMH by age, with tenth and ninetieth percentiles.
There were 953 women who had not taken hormones in the last 12 months and who provided data on their cycle length. Although 8.2% of these women reported menstrual cycles that were longer than 35 days, only 3.2% of the entire cohort reported a diagnosis of PCOS.
AMH as a Function of Age
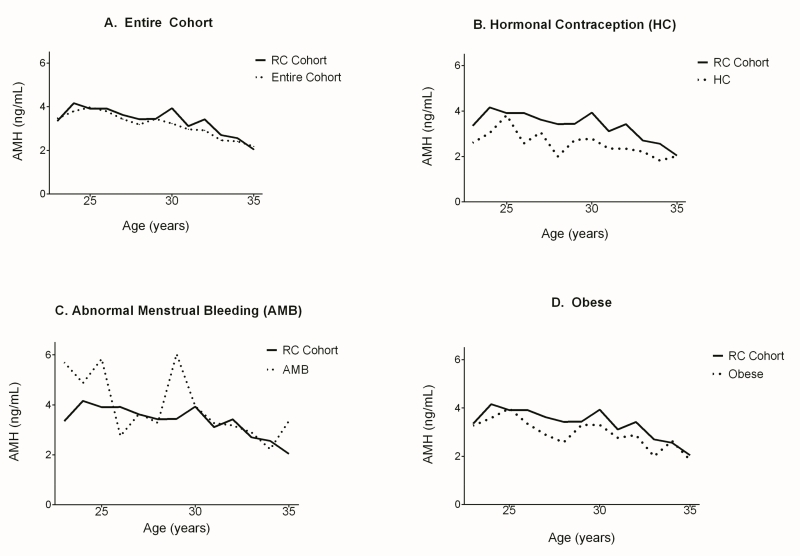
Simple linear regression confirmed a significant association between age and AMH (Supplemental Table 1). This relationship between age and AMH was nonlinear, as median AMH increased from 3.46 ng/ml at age 23 to 3.80 ng/ml at age 24, peaking at age 25 with a median AMH of 3.97 ng/ml. At age 35 AMH reached a low of 2.18 ng/ml. There was a generally similar age association for women who were regularly cycling (Supplemental Table 1). Figure 2a demonstrates AMH as a function of age in the regularly cycling cohort compared to the entire cohort. Median AMH peaked at age 24 with a value of 4.16 ng/ml. The decline after age 25 in the regularly cycling cohort appeared to be slower and more variable than in the entire cohort.
Figure 2.
Median AMH of the regularly cycling (RC) cohort (N=604) compared to (A) the entire cohort (N=1654), (B) women using hormonal contraception (N=454), (C) women with abnormal menstrual bleeding (N=188), and (D) obese women (N=978). The regularly cycling cohort excluded women who reported irregular menstrual cycles, any hormone use in the last 12 months, current use of hormonal contraception, presence of a thyroid condition, a history of PCOS, or current breastfeeding.
The age-specific AMH patterns are shown in Figure 2b, c, and d for the subsets of women who were using hormonal contraception, who reported a history of abnormal menstrual bleeding, and who were obese, respectively. At every age the median AMH was lower in the women who were currently taking hormonal contraception (N=454) compared to the regularly cycling cohort (Figure 2b). When compared to the median AMH of the regularly cycling cohort, AMH of the women with a history of abnormal menstrual bleeding (N=188) appeared higher at the majority of ages (Figure 2c). AMH appeared lower in the obese group compared to the AMH of the regularly cycling cohort at every age except 25 and 34 (Figure 2d).
Linear Regression Modeling
Supplemental Table 1 demonstrates the covariates that were associated with AMH when simple linear regression was performed. Table 2 reveals that after adjusting for age and quadratic age, there were significant associations between AMH and BMI, current use of hormonal contraception, history of abnormal menstrual bleeding, menstrual cycle length, history of a thyroid condition, and history of seeking care for difficulty conceiving. In a subsequent model (Table 2) that simultaneously examined all of the significant terms from the previous age-adjusted model (with the exception of menstrual cycle length which was examined only on the subset of women not using hormonal medication), history of seeking care for difficulty conceiving was weaker and no longer significant. However, all other factors (BMI, current hormonal contraception use, history of abnormal menstrual bleeding, and history of a thyroid condition) were still significant and the strength of the associations was generally stronger than in the simple age-adjusted model. In a separate multivariable model designed to examine menstrual cycle length that was limited to women not using hormonal contraception in the last 12 months, long cycles remained significantly associated with elevated AMH. As shown in Supplemental Table 2, in the regularly cycling cohort, BMI was the only covariate significantly associated with AMH in an age-adjusted model.
Table 2. Age and Multivariable Adjusted Associations of Demographic, Reproductive, Medical, and Lifestyle Factors with AMH in the Entire Cohort.
| Predictor | βa (95% CI) |
P value |
βb (95% CI) |
P value |
|---|---|---|---|---|
| Body mass index (BMI) | −0.010 (−0.016, −0.005) | 0.0003 | −0.014 (−0.019, −0.008) | <.0001 |
| Current hormonal contraception (HC) use |
−0.277 (−0.394, −0.159) | <.0001 | −0.290 (−0.408, −0.171) | <.0001 |
| History of abnormal menstrual bleeding |
0.222 (0.055, 0.388) | 0.0091 | 0.286 (0.117, 0.455) | 0.0009 |
| Menstrual cycle length in the last 12 monthsc (Reference group: 25-35 days) |
--- | 0.0144 | --- | 0.0026 d |
| <25 days | −0.108 (−0.261, 0.045) | 0.1680 | −0.115 (−0.267, 0.037) | 0.1371 |
| >35 days | 0.278 (0.042, 0.513) | 0.0212 | 0.356 (0.112, 0.599) | 0.0042 |
| History of a thyroid condition |
−0.380 (−0.694, −0.065) | 0.0180 | −0.449 (−0.760, −0.138) | 0.0047 |
| History of seeking care for difficulty conceiving |
0.227 (0.001, 0.453) | 0.0492 | 0.197 (−0.031, 0.425) | 0.0901 |
| Number of previous pregnancies |
−0.011 (−0.036, 0.014) | 0.3931 | --- | --- |
| Education (Reference group: high school or less) |
--- | 0.2716 | --- | --- |
| Some college | −0.009 (−0.150, 0.132) | 0.8992 | --- | --- |
| Associate or technical degree |
0.028 (−0.160, 0.216) | 0.7706 | --- | --- |
| Bachelor’s degree | 0.094 (−0.071, 0.257) | 0.2632 | --- | --- |
| Graduate degree | −0.154 (−0.371, 0.062) | 0.1627 | --- | --- |
| Household income (Reference group: <$20,000) |
--- | 0.4847 | --- | --- |
| $20,001-50,000 | 0.018 (−0.100, 0.136) | 0.7658 | --- | --- |
| >$50,000 | −0.076 (−0.228, 0.076) | 0.3266 | --- | --- |
| Marital status | 0.019 (−0.102, 0.140) | 0.7611 | --- | --- |
| Pregnancy history (Reference group: never pregnant) |
--- | 0.5439 | --- | --- |
| Previous pregnancy without history of a live birth |
−0.115 (−0.289, 0.060) | 0.1987 | --- | --- |
| Previous live birth but without breastfeeding history |
−0.057 (−0.213, 0.100) | 0.4796 | --- | --- |
| Previous live birth with history of breastfeeding |
−0.083 (−0.219, 0.053) | 0.2291 | --- | --- |
| Age at menarche | 0.027 (−0.003, 0.056) | 0.0809 | --- | --- |
| Irregular menses in the last 12 monthsc |
−0.008 (−0.146, 0.130) | 0.9143 | --- | --- |
| History of polycystic ovarian syndrome |
0.244 (−0.058, 0.547) | 0.1135 | --- | --- |
| History of amenorrhea | −0.208 (−0.470, 0.054) | 0.1196 | --- | --- |
| History of hormonal contraception use |
−0.090 (−0.42, 0.062) | 0.2462 | --- | --- |
| History of endometriosis | −0.024 (−0.427, 0.378) | 0.9056 | --- | --- |
| Current smoking | 0.047 (−0.087, 0.181) | 0.4900 | --- | --- |
| History of smoking | 0.056 (−0.063, 0.176) | 0.3537 | --- | --- |
| Frequency of alcohol consumption in the last 12 months (Reference group: never) |
--- | 0.8362 | --- | --- |
| Less than weekly | −0.019 (−0.144, 0.106) | 0.7671 | --- | --- |
| At least weekly | 0.021 (−0.123, 0.165) | 0.775 7 |
--- | --- |
The age-adjusted model adjusted for age and quadratic age.
The multivariable model adjusted for BMI, HC use, history of abnormal menstrual bleeding, menstrual cycle length, history of a thyroid condition and history of seeking care for difficulty conceiving.
Among those not using hormonal contraception at any time in the last 12 months, n=953.
The analysis of cycle length was performed in a separate multivariable model limited to those not using hormonal contraception at any time in the last 12 months
Discussion
In this study, we describe the distribution of serum AMH in a large study of unselected reproductive-aged women. Although the study is restricted to AAW, we anticipate that insights gained from this group have the potential to provide useful information regarding AMH in similarly aged women of other races and ethnicities. We expect that these data will aid clinicians in identifying women who may be at a higher risk for a more rapid decline in ovarian reserve and counseling women on their fertile window. Our data indicate that for the entire cohort, AMH rises from age 23 to a peak at age 25, and decline thereafter. Other studies also show a decline in AMH beginning at approximately 25 years of age (17, 18) but a peak in the mid to late teens. Further, given that participants were not selected based on cycle regularity or fertility status, we were able to determine that in this large group of women, AMH was associated with lifestyle factors, health history, and menstrual cycle dynamics. Amongst the entire cohort, age, BMI, current hormonal contraception use, and history of a thyroid condition were inversely associated with AMH, whereas menstrual cycles longer than 35 days and history of abnormal menstrual bleeding were positively associated with AMH. Age and BMI remained significantly inversely associated with AMH when the sample was restricted to regularly cycling women not using hormonal contraception.
We identified a significant association between menstrual cycle length and AMH, whereby those with cycles longer than 35 days had a higher AMH. Women with PCOS tend to have higher AMH levels (32, 33) and those not using hormones will commonly have long cycle intervals. Women on hormonal contraception were excluded when examining the relationship between menstrual cycle length and AMH. Given that some of these individuals, particularly those with PCOS, would likely have had long cycles if they had not been taking hormonal contraception, there is a possibility that this relationship may have been even stronger if these women had been off of hormones and able to be included in the analysis. Interestingly, women with PCOS in our sample had similarly elevated AMH as those with long cycles, though the association was not significant, most likely due to the small number of women who reported this diagnosis. The prevalence of PCOS in our sample is lower than the reported population prevalence (3.7% in our sample versus 6-15% depending on the criteria that are applied to define the disorder) (34). The positive association that was demonstrated between abnormal menstrual bleeding and AMH may reflect undiagnosed PCOS.
The existing literature regarding lifestyle and environmental factors that are associated with AMH is inconsistent. While earlier studies suggested that hormonal contraception does not influence AMH (35, 36), more recent studies have demonstrated lower AMH levels among users (20, 21), with an increase in AMH after hormonal contraception is discontinued (37). Similarly, several studies have concluded that no relationship exists between BMI and AMH (21, 38) while others have identified an inverse association between these two factors (39-41). Our findings of lower AMH amongst obese women in this large cohort of women with significant heterogeneity in BMI (range 15.9-79.4 kg/m2) may offer clarity to existing conflicts in the literature. While a substantial proportion of our study population was overweight or obese, these proportions are reflective of BMI distributions of AAW in the United States (42).
Although there is evidence that smoking decreases ovarian reserve (21, 43), our study and others have not found a relationship between current smoking and AMH (44, 45). Given the relatively young age of our cohort, it is possible that smoking has less of an impact on ovarian reserve in young women. Overall, the relationship between smoking and AMH must be understood in a more nuanced manner.
Nearly 3% of SELF participants reported a history of a thyroid condition, and those with a history of thyroid disorder were found to have significantly lower AMH. These findings appear to agree with those of Michalakis et al, which examined TSH levels in women undergoing assisted reproductive technologies, and found that those with diminished ovarian reserve had higher TSH levels compared to women with normal ovarian reserve (46), but does not address the question of whether the association is due to changes in thyroid function per se or is a reflection of thyroid autoimmunity which is known to be associated with primary ovarian insufficiency (47). More recently, in a population of approximately 5,000 women attending a reproductive medicine center, Polyzos and colleagues examined the relationship between AMH and TSH as well as anti-thyroperoxidase antibodies. The authors concluded that in this large cohort of Belgian women hypothyroidism and thyroid autoimmunity disorders are not associated with low ovarian reserve (48). As there were differences in participant characteristics, as well as inconsistent findings between studies, additional prospective studies are clearly necessary to further elucidate the relationship between thyroid disorders and markers of ovarian reserve.
This study has a number of significant strengths. The major strength is that unlike most previous large studies of AMH, the women in this study were not selected for inclusion based on menstrual cycle dynamics or fertility criteria. Because women were not excluded due to hormone use, cycle regularity, a history of PCOS, BMI, or other factors that may impact AMH, such factors could all be examined. Although a single racial group was studied, participants were recruited by broadly by advertising the study throughout the community (30). As a result, our findings are likely to be generalizable and helpful in providing valuable information applicable to reproductive-aged women of other races and ethnicities. Other strengths of this study include the large size of the cohort and the fact that the women were of African descent; thus, addressing a major gap in the literature. Although other investigators have analyzed AMH in AAW (22, 23), our study included more than three times the number of AAW that the largest previous study examined.
A limitation of this analysis is that all medical history data were based on self-report. Specifically, questions that required participants to report on historical facts (i.e. medical history and reproductive history) may be subject to recall bias. In addition we used a cross-sectional approach that did not allow for assessment of all factors that may be associated with AMH. However, in clinical practice physicians are often required to formulate assessments and recommendations based on a single AMH value. In these situations, it is important that clinicians understand which factors may affect an AMH at a single point in time.
Conclusions
Our findings add valuable information to the existing literature on normative AMH in the general reproductive-aged population. These data may aid clinicians in identifying women who are potentially at an increased risk for a more rapid decline in ovarian reserve and may allow for improved counseling on individual fertile windows. As this study was performed in a group of women who were not selected based on cycle regularity, fertility status, or other conditions, such as underlying disease, certain factors that were excluded from other large studies could be fully examined to accurately determine whether associations with AMH exists, and relationships with BMI, current use of hormonal contraception, history of a thyroid condition, abnormal menstrual bleeding and menstrual cycle length were present. Although further studies are needed to clarify these associations, these powerful observations suggest that careful consideration to these factors should be given when interpreting AMH and counseling women on their reproductive potential. This study also provides information that had been lacking on markers of ovarian reserve in AAW.
Supplementary Material
Acknowledgements
We thank Dr. Patrick Sluss and the Clinical Laboratory Research Core in the Pathology Department at Massachusetts General Hospital for performing the assays used in this study. We thank Dr. Janet Hall and Dr. Freya Kamel for reviewing earlier drafts of the manuscript. We thank our research coordinator, Meera Tavathia, for assistance in manuscript preparation and submission. We also thank the study manager and talented staff, women who took part in this study, and the many others who made this study possible.
Support:
The National Institutes of Health (grants P01HD57877 and R21 HD077479-01) (EEM), NIH K12HD050121 Women’s Reproductive Health Research Scholar Program at Northwestern (EEM), the RWJ Harold Amos Medical Faculty Development Award (EEM), the Friends of Prentice Women’s Health Research Award (EEM), the Evergreen Invitational Women’s Health Grants Initiative (EEM), and the Woman’s Board of Northwestern Memorial Hospital Grant (LAB). In addition, this research was supported in part (ongoing salary for DDB) by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: LAB, MLS, PJD, JAV, MRC, and DDB have no conflicts of interest.
EEM attended an advisory board meeting for AbbVie.
References
- 1.Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final Data for 2014. Natl Vital Stat Rep. 2015;64:1–64. [PubMed] [Google Scholar]
- 2.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention [Accessed January 21, 2016];ART Fertility Success Rates. 2013 Available at: http://www.cdc.gov/art/pdf/2013-report/art_2013_national_summary_report.pdf.
- 4.Cobo A, Garcia-Velasco JA, Coello A, Domingo J, Pellicer A, Remohi J. Oocytes vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2015 doi: 10.1016/j.fertnstert.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Practice Committee of the American Society for Reproductive M Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103:e9–e17. doi: 10.1016/j.fertnstert.2014.12.093. [DOI] [PubMed] [Google Scholar]
- 6.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–9. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 7.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 8.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–62. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 9.La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-Mullerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–71. doi: 10.1093/humrep/del421. [DOI] [PubMed] [Google Scholar]
- 10.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–85. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 12.Hvidman HW, Petersen KB, Larsen EC, Macklon KT, Pinborg A. Nyboe Andersen A. Individual fertility assessment and pro-fertility counselling; should this be offered to women and men of reproductive age? Hum Reprod. 2015;30:9–15. doi: 10.1093/humrep/deu305. [DOI] [PubMed] [Google Scholar]
- 13.van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, et al. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–34. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- 14.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–80. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolleman M, Faddy MJ, van Disseldorp J, van der Schouw YT, Messow CM, Leader B, et al. The relationship between anti-Mullerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metab. 2013;98:1946–53. doi: 10.1210/jc.2013-3105. [DOI] [PubMed] [Google Scholar]
- 16.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96:2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 17.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-mullerian hormone from conception to menopause. PloS one. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–5. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 20.Bentzen JG, Forman JL, Pinborg A, Lidegaard O, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25:612–9. doi: 10.1016/j.rbmo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Dolleman M, Verschuren WM, Eijkemans MJ, Dolle ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Mullerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98:2106–15. doi: 10.1210/jc.2012-3995. [DOI] [PubMed] [Google Scholar]
- 22.Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI. Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril. 2014;101:199–207. doi: 10.1016/j.fertnstert.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seifer DB, Golub ET, Lambert-Messerlian G, Benning L, Anastos K, Watts DH, et al. Variations in serum mullerian inhibiting substance between white, black, and Hispanic women. Fertil Steril. 2009;92:1674–8. doi: 10.1016/j.fertnstert.2008.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol. 2008;167:1287–94. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 25.McKnight KK, Wellons MF, Sites CK, Roth DL, Szychowski JM, Halanych JH, et al. Racial and regional differences in age at menopause in the United States: findings from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Am J Obstet Gynecol. 2011;205:353, e1–8. doi: 10.1016/j.ajog.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh EE, Shaw ND, Klingman KM, Tiamfook-Morgan TO, Yialamas MA, Sluss PM, et al. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab. 2011;96:3199–206. doi: 10.1210/jc.2011-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw ND, Srouji SS, Welt CK, Cox KH, Fox JH, Adams JM, et al. Evidence that increased ovarian aromatase activity and expression account for higher estradiol levels in African American compared with Caucasian women. J Clin Endocrinol Metab. 2014;99:1384–92. doi: 10.1210/jc.2013-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–50. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Nelson SM, Messow MC, Wallace AM, Fleming R, McConnachie A. Nomogram for the decline in serum antimullerian hormone: a population study of 9,601 infertility patients. Fertil Steril. 2011;95:736–41. e1–3. doi: 10.1016/j.fertnstert.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Baird DD, Harmon QE, Upson K, Moore KR, Barker-Cummings C, Baker S, et al. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. J Womens Health (Larchmt) 2015 doi: 10.1089/jwh.2015.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 32.Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum mullerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2002;77:141–6. doi: 10.1016/s0015-0282(01)02944-2. [DOI] [PubMed] [Google Scholar]
- 33.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–62. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 34.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Li HW, Wong CY, Yeung WS, Ho PC, Ng EH. Serum anti-mullerian hormone level is not altered in women using hormonal contraceptives. Contraception. 2011;83:582–5. doi: 10.1016/j.contraception.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Mullerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39:574–80. doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 37.van den Berg MH, van Dulmen-den Broeder E, Overbeek A, Twisk JW, Schats R, van Leeuwen FE, et al. Comparison of ovarian function markers in users of hormonal contraceptives during the hormone-free interval and subsequent natural early follicular phases. Hum Reprod. 2010;25:1520–7. doi: 10.1093/humrep/deq071. [DOI] [PubMed] [Google Scholar]
- 38.Halawaty S, ElKattan E, Azab H, ElGhamry N, Al-Inany H. Effect of obesity on parameters of ovarian reserve in premenopausal women. J Obstet Gynaecol Can. 2010;32:687–90. doi: 10.1016/s1701-2163(16)34573-x. [DOI] [PubMed] [Google Scholar]
- 39.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87:101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 40.Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause. 2008;15:857–61. doi: 10.1097/gme.0b013e318165981e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buyuk E, Seifer DB, Illions E, Grazi RV, Lieman H. Elevated body mass index is associated with lower serum anti-mullerian hormone levels in infertile women with diminished ovarian reserve but not with normal ovarian reserve. Fertil Steril. 2011;95:2364–8. doi: 10.1016/j.fertnstert.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 42.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plante BJ, Cooper GS, Baird DD, Steiner AZ. The impact of smoking on antimullerian hormone levels in women aged 38 to 50 years. Menopause. 2010;17:571–6. doi: 10.1097/gme.0b013e3181c7deba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dafopoulos A, Dafopoulos K, Georgoulias P, Galazios G, Limberis V, Tsikouras P, et al. Smoking and AMH levels in women with normal reproductive history. Arch Gynecol Obstet. 2010;282:215–9. doi: 10.1007/s00404-010-1425-1. [DOI] [PubMed] [Google Scholar]
- 45.La Marca A, Spada E, Grisendi V, Argento C, Papaleo E, Milani S, et al. Normal serum anti-Mullerian hormone levels in the general female population and the relationship with reproductive history. Eur J Obstet Gynecol Reprod Bio. 2012;163:180–4. doi: 10.1016/j.ejogrb.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Michalakis KG, Mesen TB, Brayboy LM, Yu B, Richter KS, Levy M, et al. Subclinical elevations of thyroid-stimulating hormone and assisted reproductive technology outcomes. Fertil Steril. 2011;95:2634–7. doi: 10.1016/j.fertnstert.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goswami R, Marwaha RK, Goswami D, Gupta N, Ray D, Tomar N, et al. Prevalence of thyroid autoimmunity in sporadic idiopathic hypoparathyroidism in comparison to type 1 diabetes and premature ovarian failure. J Clin Endocrinol Metab. 2006;91:4256–9. doi: 10.1210/jc.2006-1005. [DOI] [PubMed] [Google Scholar]
- 48.Polyzos NP, Sakkas E, Vaiarelli A, Poppe K, Camus M, Tournaye H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Human Reprod. 2015;30:1690–6. doi: 10.1093/humrep/dev089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.